The Immunomodulatory Effects of Fluorescein-Mediated Sonodynamic Treatment Lead to Systemic and Intratumoral Depletion of Myeloid-Derived Suppressor Cells in a Preclinical Malignant Glioma Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Apoptosis Assay
2.3. RNA Extraction and Real-Time PCR
- Rae1b fw CAGCAAATGCCACTGAAGTGAA rev GGTCTTGTGAGTGTCCACTTTG
- Rae1d fw CTCCTACCCCAGCAGATGAAG rev CCCTGGGTCACCTGAAGTC
- Rae1e fw GACCCACAGACCAAATGGCA rev CTCTGTCCTTTGAGCTTCTTGC
- MICA fw CCACCTGTGGATAGTGTACCTG rev GCCACCAGTCTTTGGTTGTC
- MICB fw GTTTCTGGCTGACGTGGAG rev ATAGCGCAGAGTGTGGGTTC
- MULT fw TGAAGTCACCTGTGTTTATGCAG rev CACTGTCAAAGAGTCATCCAACA
- β-actin fw GATGTGGATCAGCAAGCAGGA rev AGCTCAGTAACAGTCCGCCTA
2.4. Mice
2.5. Sonodynamic and Other Treatments
- In vitro experiment—After seeding a total of 0.3 × 106 GL261 in 35 mm2-plates and adding FL (0.5 mg/mL), FUS was applied continuously for 20 min at a frequency of 0.983 MHz, 100 kPa of peak positive pressure, 10% duty cycle (300 cycles, 10 ms period). Then cells were incubated for 12, 24, and 48 h, and then analyzed for immunogenicity and apoptosis.
- In vivo experiment—In the FL-SDT group, after anesthesia, sodium fluorescein 10 mg/kg was injected intraperitoneally, and mice were sonicated 20 min later. To perform low-intensity-focused ultrasound, we used a single-element planar transducer with 0.485 Freq./MHz, 100 kPa of peak positive pressure, 10% duty cycle (350 cycles, 10 ms period) for 20 min. These parameters were maintained for the FUS-only group and the same FL dose was used in the FL-only group. The SDT was performed using a plane wave source transducer TRA08 (Istituto Nazionale di Ricerca Metrologica, Turin, Italy) based on a lithium-niobate piezoelectric transducer (Boston Piezo-Optics Inc., Bellingham, MA, USA), connected to a signal generator (Model 33250A, Agilent Technologies, CA, USA) through a power amplifier (Model AR 100A250A, Amplifier Research, PA, USA). The animal was anesthetized using tribromoethanol and positioned prone on a soft pad. The transducer was positioned close to the mouse’s skull, at the level of the tumor injection point. A US aqueous coupling medium was used to reduce air interference to a minimum. Images of the setup are reported in the Supplementary Materials.
2.6. MRI
2.7. Isolation of CNS-Infiltrating Lymphocytes
2.8. Flow-Cytometer Analysis
3. Results
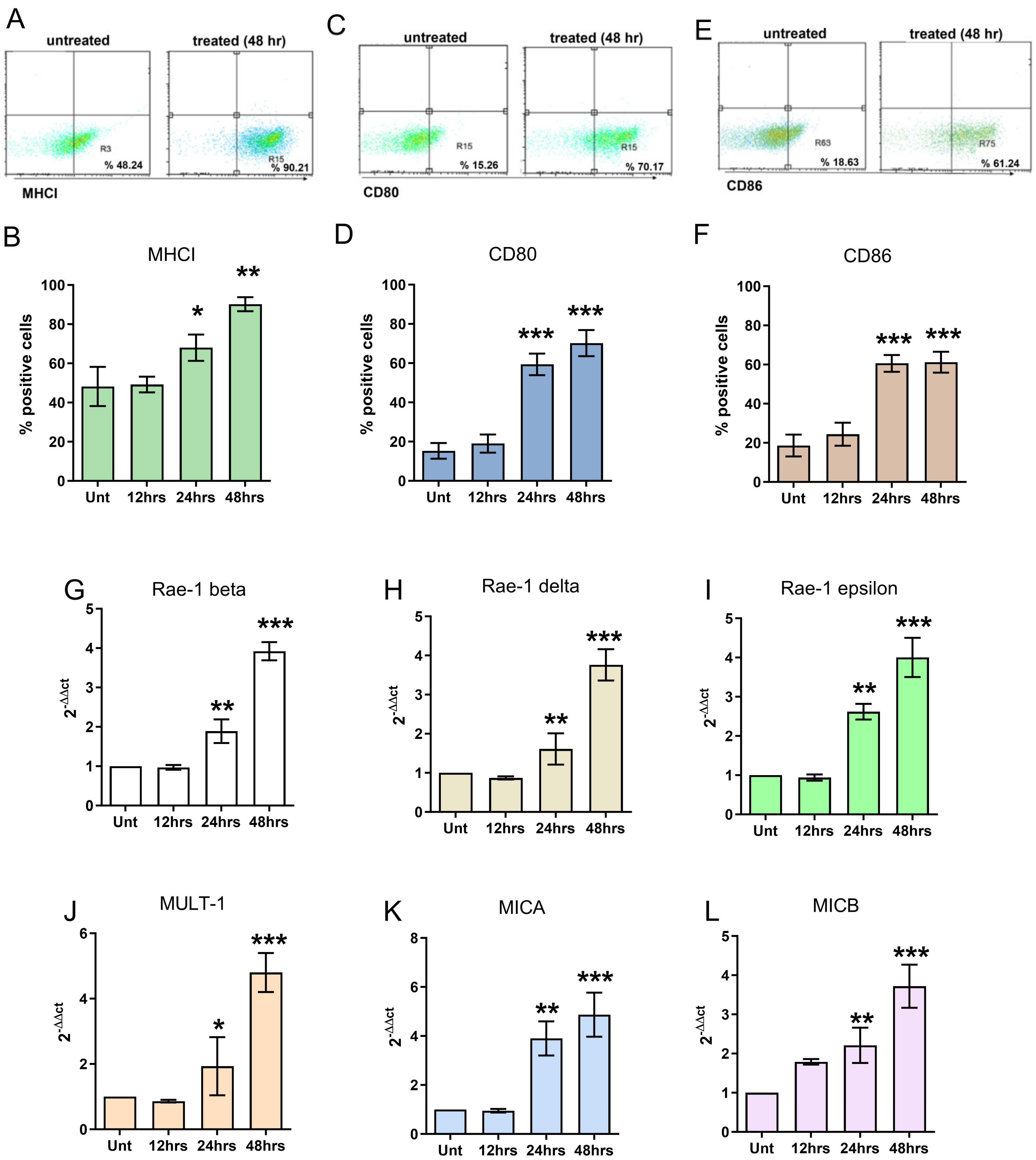
3.1. In Vitro Effects of FL and FL-SDT Treatment on GL261 Glioma Cells
3.2. In Vivo Effects of FL and FL-SDT Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.-X.; Cao, H.; Zhai, Y.; Deng, S.-Z.; Chao, M.; Hu, Y.; Mou, Y.; Guo, S.; Zhao, W.; Li, C.; et al. Immune gene signatures and immunotypes in immune microenvironment are associated with glioma prognose. Front. Immunol. 2022, 13, 823910. [Google Scholar] [CrossRef]
- Najem, H.; Khasraw, M.; Heimberger, A.B. Immune microenvironment landscape in CNS tumors and role in responses to immunotherapy. Cells 2021, 10, 2032. [Google Scholar] [CrossRef] [PubMed]
- Musca, B.; Russo, M.G.; Tushe, A.; Magri, S.; Battaggia, G.; Pinton, L.; Bonaudo, C.; Della Puppa, A.; Mandruzzato, S. The immune cell landscape of glioblastoma patients highlights a myeloid-enriched and immune suppressed microenvironment compared to metastatic brain tumors. Front. Immunol. 2023, 14, 1236824. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.E.; Anderson, D.E.; Elder, J.B.; Brown, M.D.; Mandigo, C.E.; Parsa, A.T.; Goodman, R.R.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N. Lack of B7 expression, not human leukocyte antigen expression, facilitates immune evasion by human malignant gliomas. Neurosurgery 2007, 60, 1129–1136; discussion 1136. [Google Scholar] [CrossRef] [PubMed]
- Roesch, S.; Rapp, C.; Dettling, S.; Herold-Mende, C. When immune cells turn bad—tumor-associated microglia/macrophages in glioma. Int. J. Mol. Sci. 2018, 19, 436. [Google Scholar] [CrossRef]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; Coccè, V.; Bonomi, A.; Buccarelli, M.; Pascucci, L.; Alessandri, G.; Pessina, A.; et al. Human mesenchymal stromal cells inhibit tumor growth in orthotopic glioblastoma xenografts. Stem Cell Res. Ther. 2017, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, S.; Ohnishi, T.; Yamashita, D.; Kohno, S.; Inoue, A.; Nishikawa, M.; Ohue, S.; Tanaka, J.; Kunieda, T. Enhancement of antitumor activity by using 5-ALA-mediated sonodynamic therapy to induce apoptosis in malignant gliomas: Significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J. Neurosurg. 2018, 129, 1416–1428. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Y.; Huang, Y.; Zeng, X.; Huang, L.; Diao, X.; Chen, S.; Chen, X. An in vitro study on the antitumor effect of sonodynamic therapy using sinoporphyrin sodium on human glioblastoma cells. Ultrasonics 2021, 110, 106272. [Google Scholar] [CrossRef]
- Bonosi, L.; Marino, S.; Benigno, U.E.; Musso, S.; Buscemi, F.; Giardina, K.; Gerardi, R.; Brunasso, L.; Costanzo, R.; Iacopino, D.G.; et al. Sonodynamic therapy and magnetic resonance-guided focused ultrasound: New therapeutic strategy in glioblastoma. J. Neurooncol. 2023, 163, 219–238. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, J.; Huang, W.; Gong, P.; Shi, F.; Xu, X.; Fu, C.; Wang, X.; Wong, Y.K.; Long, Y.; et al. Antitumor Effects of a Distinct Sonodynamic Nanosystem through Enhanced Induction of Immunogenic Cell Death and Ferroptosis with Modulation of Tumor Microenvironment. JACS Au 2023, 3, 1507–1520. [Google Scholar] [CrossRef]
- Wang, T.; Peng, W.; Du, M.; Chen, Z. Immunogenic sonodynamic therapy for inducing immunogenic cell death and activating antitumor immunity. Front. Oncol. 2023, 13, 1167105. [Google Scholar] [CrossRef] [PubMed]
- Bilmin, K.; Kujawska, T.; Grieb, P. Sonodynamic therapy for gliomas. perspectives and prospects of selective sonosensitization of glioma cells. Cells 2019, 8, 1428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiao, J.; Yang, R.; Wen, B.; Wu, Q.; Xu, L.; Tong, X.; Yan, H. Temozolomide-based sonodynamic therapy induces immunogenic cell death in glioma. Clin. Immunol. 2023, 256, 109772. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, P.; Shah, N.H.; Cui, Y.; Wang, Y. A comprehensive review of inorganic sonosensitizers for sonodynamic therapy. Int. J. Mol. Sci. 2023, 24, 12001. [Google Scholar] [CrossRef]
- Ahrens, L.C.; Krabbenhøft, M.G.; Hansen, R.W.; Mikic, N.; Pedersen, C.B.; Poulsen, F.R.; Korshoej, A.R. Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis. Cancers 2022, 14, 617. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, L.S.; Sepúlvida, R.; Abbud, R.; Leal, R.T.M.; Acioly, M.A. Fluorescein application in spinal ependymomas: Have we come so far? Neurosurg. Rev. 2022, 45, 3467–3468. [Google Scholar] [CrossRef]
- Roberts, J.W.; Powlovich, L.; Sheybani, N.; LeBlang, S. Focused ultrasound for the treatment of glioblastoma. J. Neuro-Oncol. 2022, 157, 237–247. [Google Scholar] [CrossRef]
- Folaron, M.; Strawbridge, R.; Samkoe, K.S.; Filan, C.; Roberts, D.W.; Davis, S.C. Elucidating the kinetics of sodium fluorescein for fluorescence-guided surgery of glioma. J. Neurosurg. 2019, 131, 724–734. [Google Scholar] [CrossRef]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188.e7. [Google Scholar] [CrossRef]
- Naik, A.; Smith, E.J.; Barreau, A.; Nyaeme, M.; Cramer, S.W.; Najafali, D.; Krist, D.T.; Arnold, P.M.; Hassaneen, W. Comparison of fluorescein sodium, 5-ALA, and intraoperative MRI for resection of high-grade gliomas: A systematic review and networkmeta-analysis. J. Clin. Neurosci. 2022, 98, 240–247. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef]
- Prada, F.; Sheybani, N.; Franzini, A.; Moore, D.; Cordeiro, D.; Sheehan, J.; Timbie, K.; Xu, Z. Fluorescein-mediated sonodynamic therapy in a rat glioma model. J. Neurooncol. 2020, 148, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Musca, B.; Bonaudo, C.; Tushe, A.; Battaggia, G.; Russo, M.G.; Silic-Benussi, M.; Pedone, A.; Della Puppa, A.; Mandruzzato, S. Sodium fluorescein uptake by the tumor microenvironment in human gliomas and brain metastases. J. Neurosurg. 2023; epub ahead of printing. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Yang, I.; Kane, A.J.; Rutkowski, M.J.; Fang, S.; James, C.D.; Parsa, A.T. Immunological considerations of modern animal models of malignant primary brain tumors. J. Transl. Med. 2009, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.; Fakurnejad, S.; Sayegh, E.T.; Clark, A.J.; Ivan, M.E.; Sun, M.Z.; Safaee, M.; Bloch, O.; James, C.D.; Parsa, A.T. Immunocompetent murine models for the study of glioblastoma immunotherapy. J. Transl. Med. 2014, 12, 107. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Y.; Yu, N.; Zhou, J.; Zhu, L.; Wang, X.; Li, J. Augmenting Immunogenic Cell Death and Alleviating Myeloid-Derived Suppressor Cells by Sono-Activatable Semiconducting Polymer Nanopartners for Immunotherapy. Adv. Mater. Weinheim 2023, 35, e2302508. [Google Scholar] [CrossRef] [PubMed]
- Friedmann-Morvinski, D.; Hambardzumyan, D. Monocyte-neutrophil entanglement in glioblastoma. J. Clin. Investig. 2023, 133, e163451. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, W.; Wang, J.; Liu, Q.; Qian, M.; Wang, S.; Zhang, Z.; Gao, X.; Chen, Z.; Guo, Q.; et al. Glioma exosomes mediate the expansion and function of myeloid-derived suppressor cells through microRNA-29a/Hbp1 and microRNA-92a/Prkar1a pathways. Int. J. Cancer 2019, 144, 3111–3126. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Peterson, T.E.; Balgeman, A.; Chen, S.; Zhang, L.; Renner, D.N.; Johnson, A.J.; Parney, I.F. Increasing glioma-associated monocytes leads to increased intratumoral and systemic myeloid-derived suppressor cells in a murine model. Neuro. Oncol. 2015, 17, 978–991. [Google Scholar] [CrossRef]
- Prada, F.; Franzini, A.; Moosa, S.; Padilla, F.; Moore, D.; Solbiati, L.; DiMeco, F.; Legon, W. In vitro and in vivo characterization of a cranial window prosthesis for diagnostic and therapeutic cerebral ultrasound. J. Neurosurg. 2020; epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegatta, S.; Corradino, N.; Zingarelli, M.; Porto, E.; Gionso, M.; Berlendis, A.; Durando, G.; Maffezzini, M.; Musio, S.; Aquino, D.; et al. The Immunomodulatory Effects of Fluorescein-Mediated Sonodynamic Treatment Lead to Systemic and Intratumoral Depletion of Myeloid-Derived Suppressor Cells in a Preclinical Malignant Glioma Model. Cancers 2024, 16, 792. https://doi.org/10.3390/cancers16040792
Pellegatta S, Corradino N, Zingarelli M, Porto E, Gionso M, Berlendis A, Durando G, Maffezzini M, Musio S, Aquino D, et al. The Immunomodulatory Effects of Fluorescein-Mediated Sonodynamic Treatment Lead to Systemic and Intratumoral Depletion of Myeloid-Derived Suppressor Cells in a Preclinical Malignant Glioma Model. Cancers. 2024; 16(4):792. https://doi.org/10.3390/cancers16040792
Chicago/Turabian StylePellegatta, Serena, Nicoletta Corradino, Manuela Zingarelli, Edoardo Porto, Matteo Gionso, Arianna Berlendis, Gianni Durando, Martina Maffezzini, Silvia Musio, Domenico Aquino, and et al. 2024. "The Immunomodulatory Effects of Fluorescein-Mediated Sonodynamic Treatment Lead to Systemic and Intratumoral Depletion of Myeloid-Derived Suppressor Cells in a Preclinical Malignant Glioma Model" Cancers 16, no. 4: 792. https://doi.org/10.3390/cancers16040792
APA StylePellegatta, S., Corradino, N., Zingarelli, M., Porto, E., Gionso, M., Berlendis, A., Durando, G., Maffezzini, M., Musio, S., Aquino, D., DiMeco, F., & Prada, F. (2024). The Immunomodulatory Effects of Fluorescein-Mediated Sonodynamic Treatment Lead to Systemic and Intratumoral Depletion of Myeloid-Derived Suppressor Cells in a Preclinical Malignant Glioma Model. Cancers, 16(4), 792. https://doi.org/10.3390/cancers16040792






