Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT)
Abstract
Simple Summary
Abstract
1. Introduction
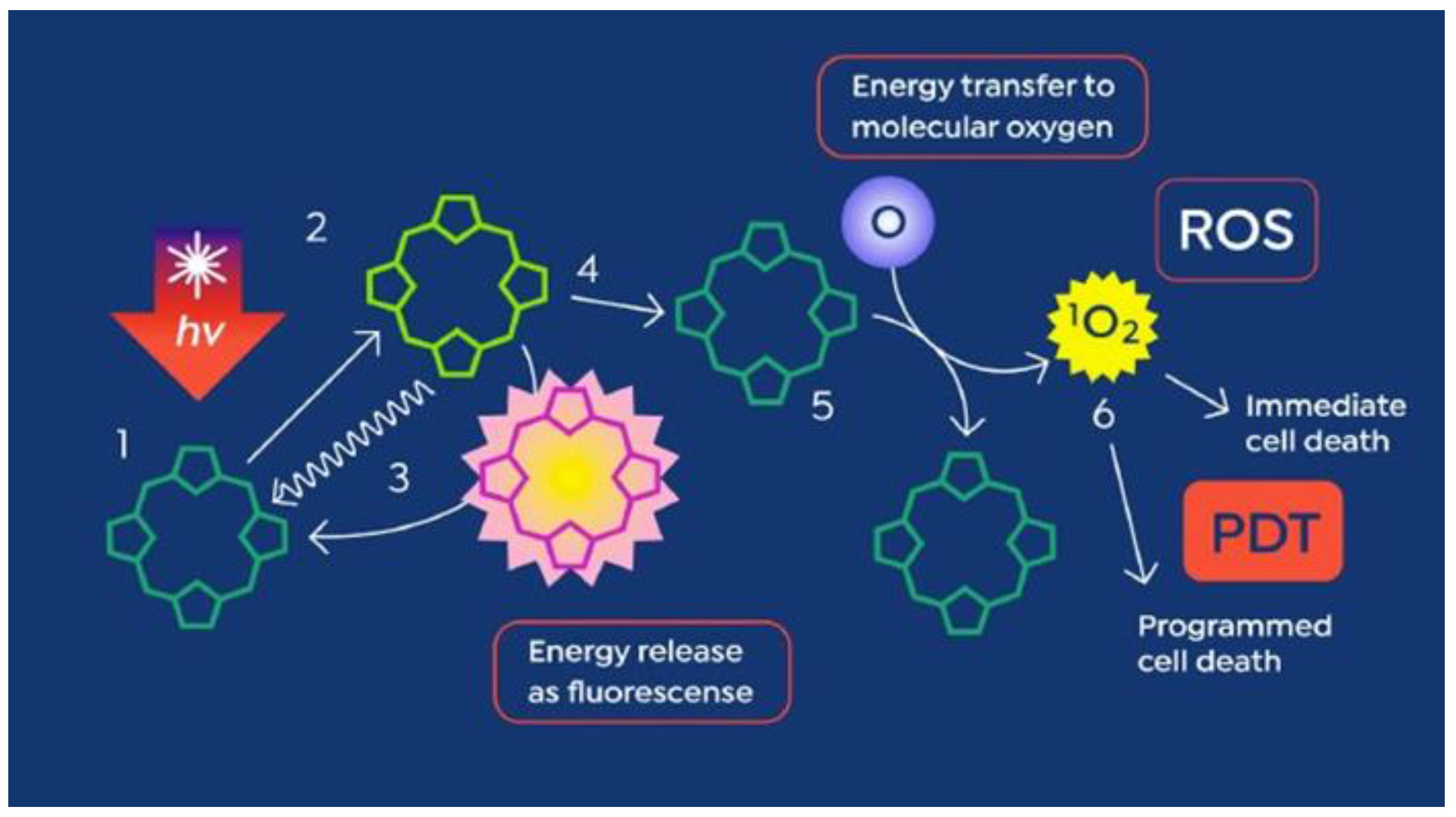
1.1. The Heme Pathway—Introducing PpIX
1.2. Erythropoietic Protoporphyria (EPP): Nature’s Experiment in Photodynamic Phototoxicity and Its Use as a Model for ALA PDT
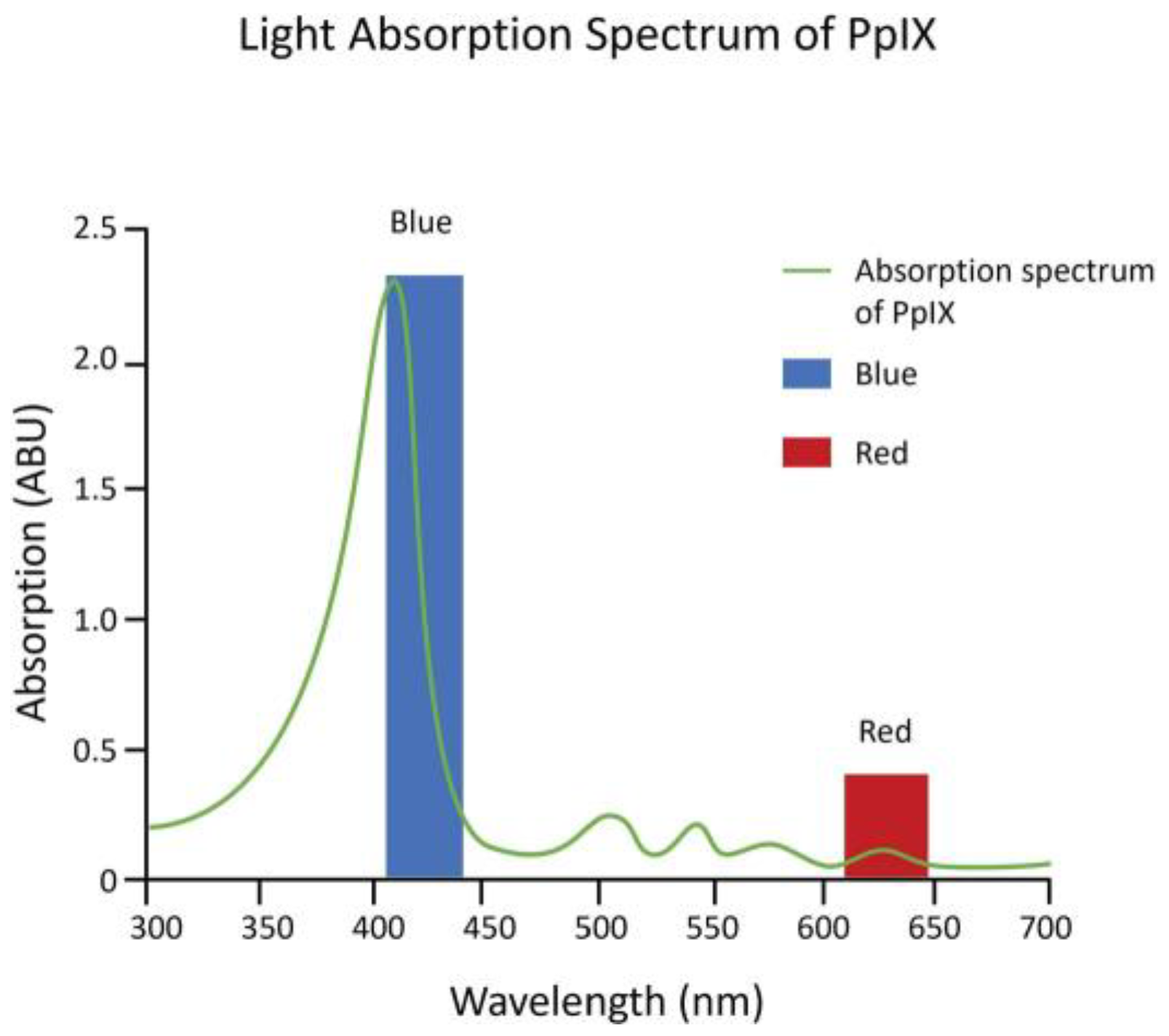
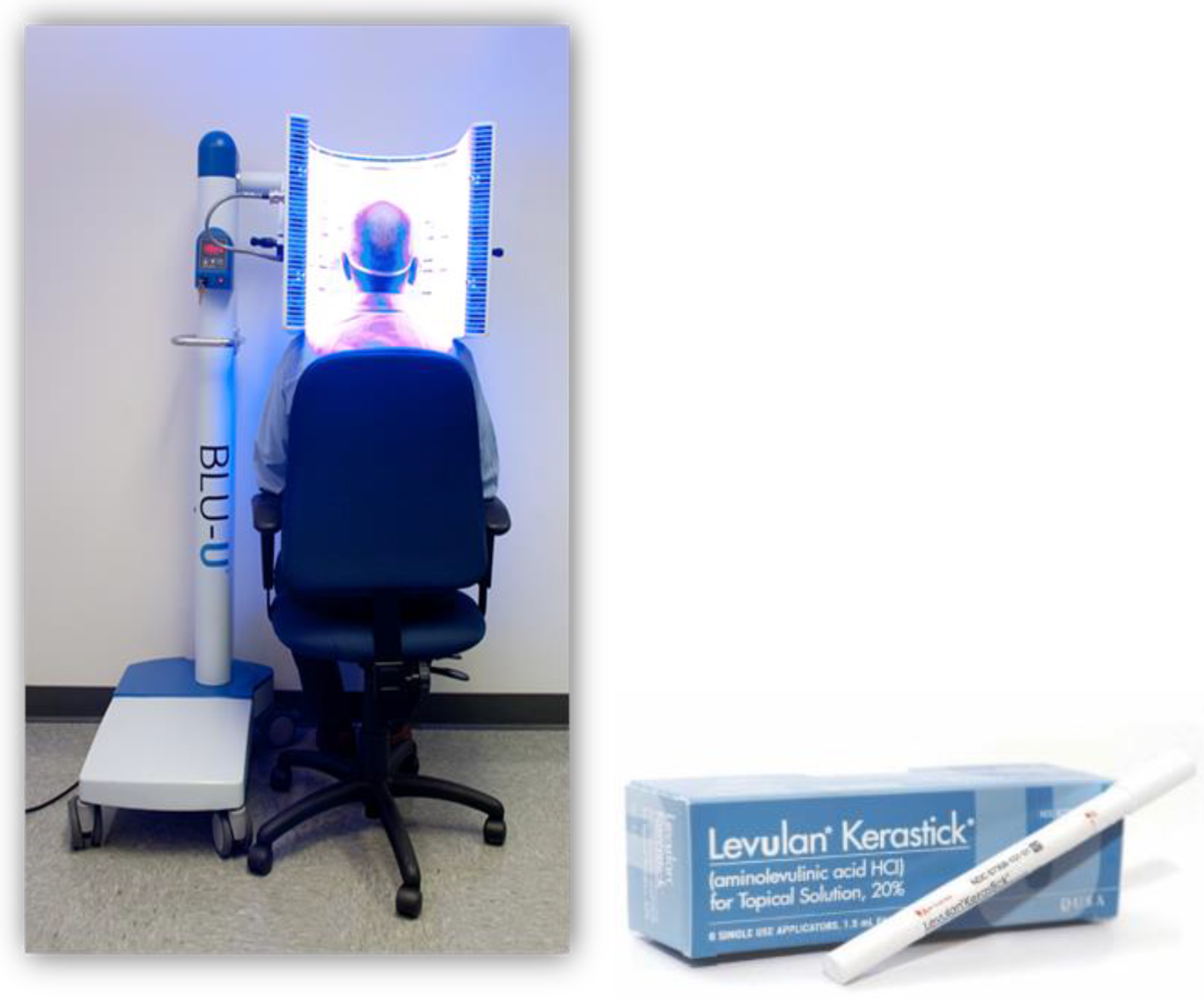
2. The Beginning of PpIX Therapeutics—Topical ALA PDT: From Red Light to Blue Light
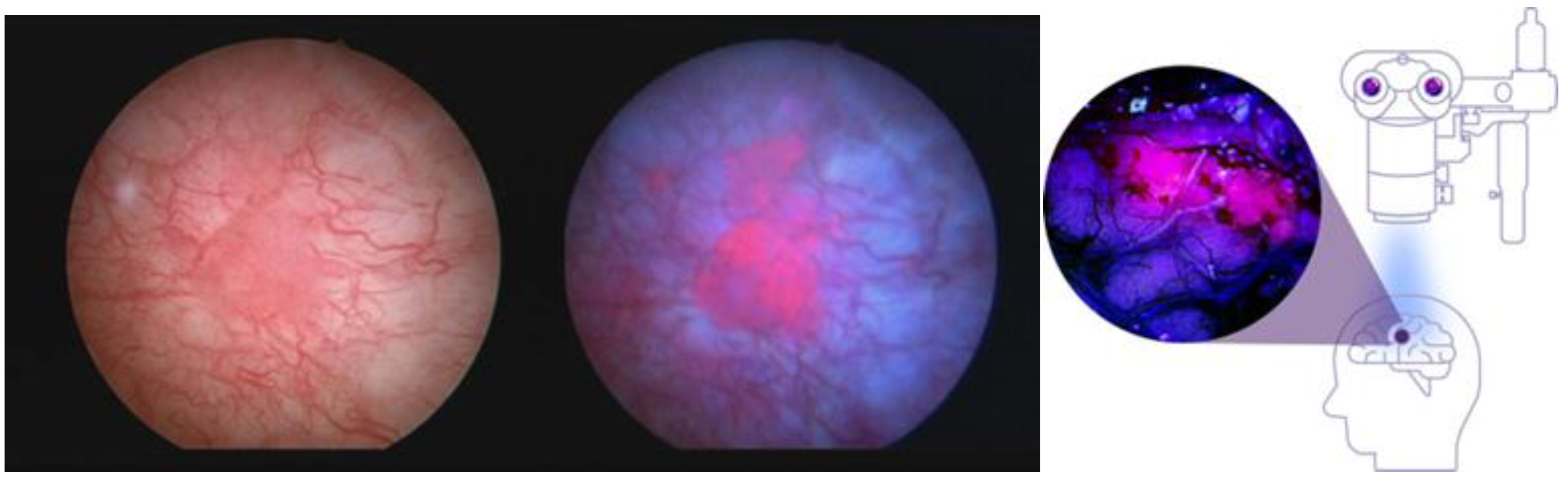
2.1. PpIX Photodynamic Diagnosis (PDD)—The Next Step in the Evolution toward Sonodynamic Therapy
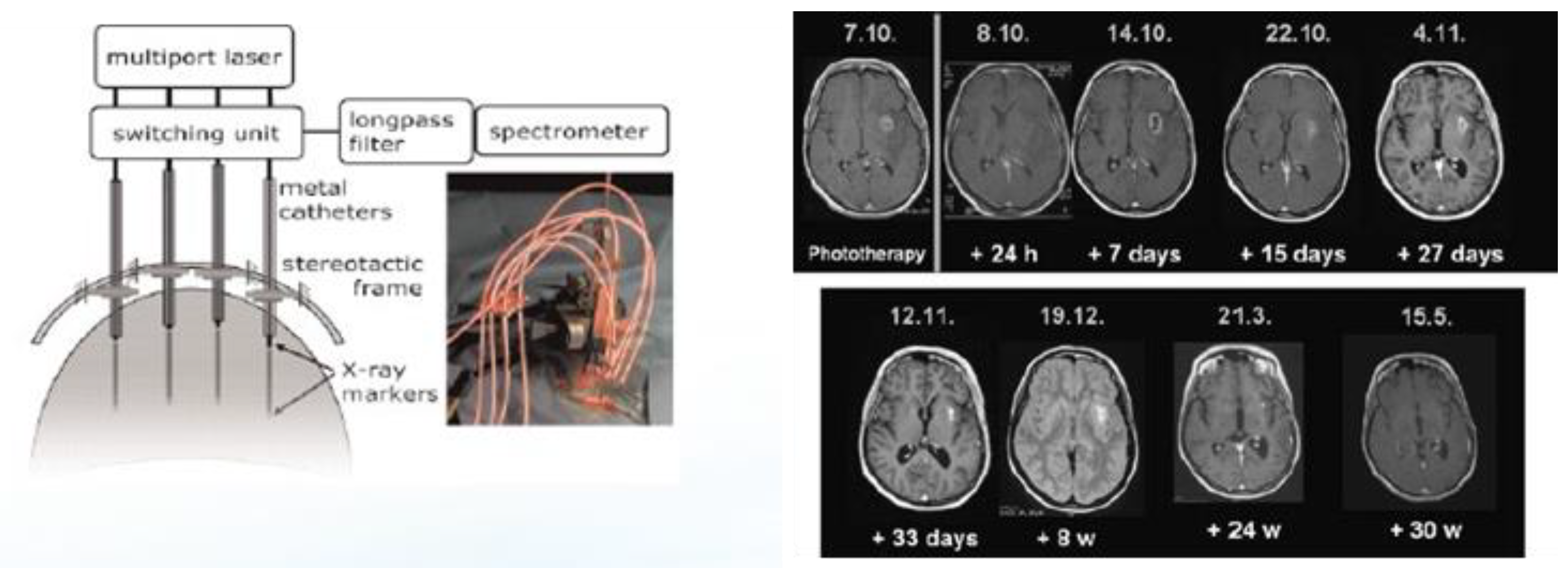
2.2. Interstitial ALA PDT (iPDT) for the Treatment of Recurrent Glioblastomas (rGBMs)
3. From PDT to SDT—Preclinical Studies in Rodent Models on SDT for Malignant Gliomas
3.1. Optimization of Single-Treatment ALA SDT in a C6 Rat Glioma Has Positive Effects on Survival
3.2. DIPG (DMG) Tissue Culture Cells Accumulate PpIX When Exposed to Exogenous ALA
4. Current SDT Clinical Trials
4.1. First-in-Man Phase 0/1 Clinical Trial of SDT
4.2. Ongoing Phase 1/2 Clinical Trials of ALA SDT for Malignant Glioma
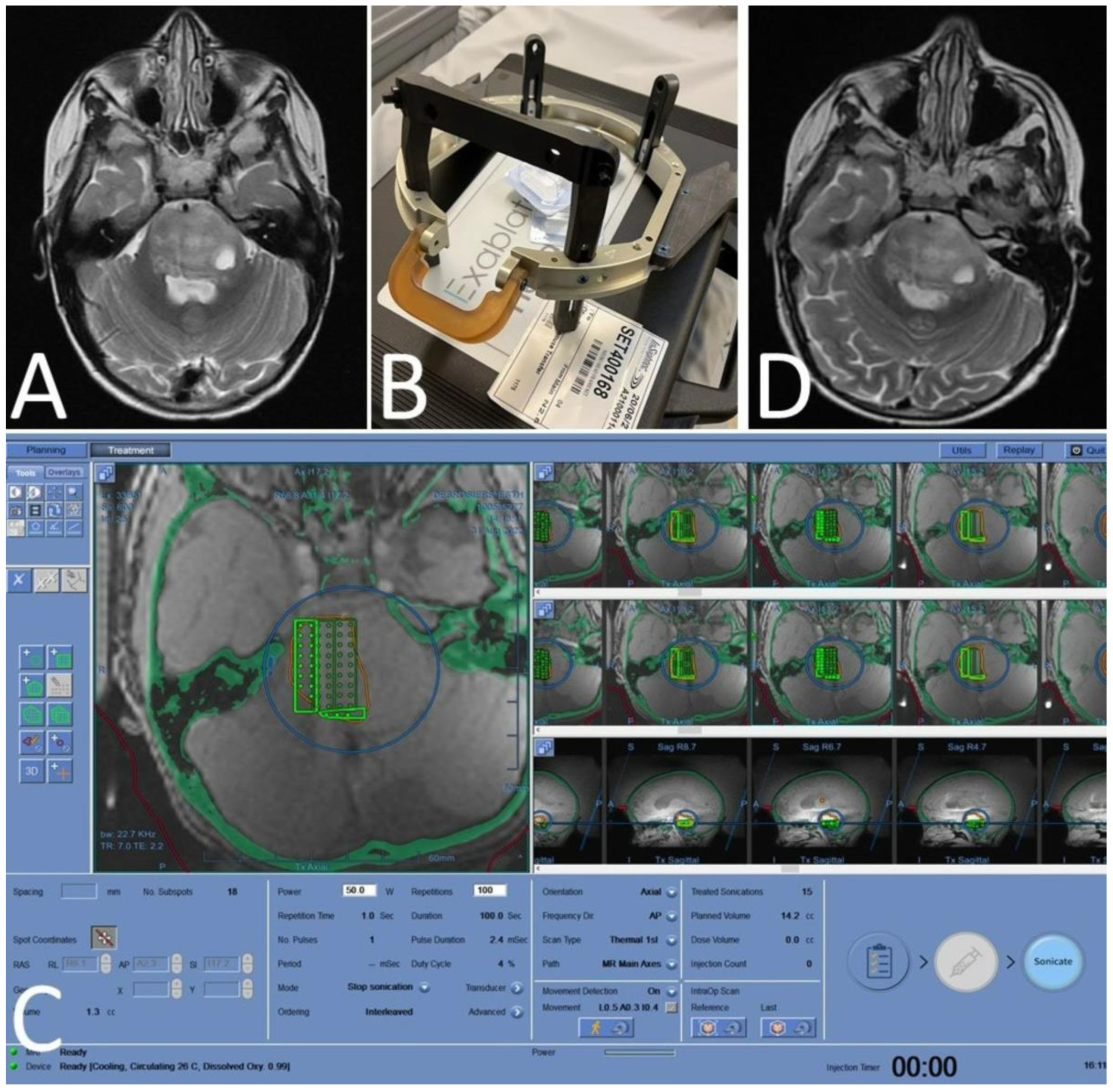
4.3. First-in-Child Pediatric SDT Trial for Diffuse Intrinsic Pontine Glioma (DIPG)
5. Likely Regulatory Path for US Commercial Clinical Development of ALA iPDT and ALA SDT in Neuro-Oncology: Potential Barriers
5.1. SDT: Suggestions for Commercial Clinical Development in the Treatment of Gliomas
- In the US, the drug label will likely carry sonication treatment methods.
- Clearly define device frequency, power, the nature of and frequency of pulses, and some acoustic parameter(s) as well as total energy delivered.
- Determine, with the FDA, the type of sonication parameters required for inclusion in the label and standardize those parameters across SDT clinical trials, regardless of the type of device used to produce ultrasound energies.
5.2. Future Research—Elucidating the Short-Term and Long-Term Cellular Effects of ALA iPDT and ALA SDT on Malignant Gliomas: How Many Ways Can You Kill a Cell?
6. Conclusions and Future Directions in the Evolution of and Research into Clinical SDT
6.1. Future Directions in the Evolution of Clinical SDT—How Clinical Indications and Use Will Be Determined by the Type of Device Used to Produce PpIX-Activating Ultrasound
6.1.1. Diffusely Targeting Large Brain Areas for SDT (Alpheus Medical)
6.1.2. SDT Using a Neuronavigation-Guided Device (Navifus Device)
6.1.3. MRgFUS—Using ALA SDT as a Neurosurgical Tool
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sunder, S.S.; Sharma, U.C.; Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody–drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Marcus, S.L.; Pottier, R.H. Photodynamic Therapy (PDT) and Photodiagnosis (PD) Using Endogenous Photosensitization Induced by 5-Aminolevulinic Acid (ALA): Mechanisms and Clinical Results. J. Clin. Laser Med. Surg. 1996, 14, 289–304. [Google Scholar] [CrossRef]
- Balwani, M. Erythropoietic Protoporphyria and X-Linked Protoporphyria: Pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019, 128, 298–303. [Google Scholar] [CrossRef]
- Scenesse (Afamelanotide) Prescribing Information. Available online: www.scenesse.com (accessed on 1 September 2023).
- Kennedy, J.; Pottier, R.; Pross, D. Photodynamic therapy with endogenous protoporphyrin: IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B Biol. 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Marcus, S.L.; McIntyre, W.R. Photodynamic therapy systems and applications. Expert Opin. Emerg. Drugs 2002, 7, 321–334. [Google Scholar] [CrossRef]
- Ameluz Prescribing Information. Available online: https://us.ameluz.com/ (accessed on 1 October 2023).
- Metvix SmPC. Available online: https://www.medicines.org.uk/emc/product/6777/smpc/print (accessed on 1 October 2023).
- Levulan Kerastick Prescribing Information. Available online: https://www.levulan.com/ (accessed on 8 October 2023).
- Jichlinski, P.; Forrer, M.; Mizeret, J.; Glanzmann, T.; Braichotte, D.; Wagnières, G.; Zimmer, G.; Guillou, L.; Schmidlin, F.; Graber, P.; et al. Clinical evaluation of a method for detecting superficial surgical transitional cell carcinoma of the bladder by light-induced flu-orescence of protoporphyrin IX following the topical application of 5-aminolevulinic acid: Preliminary results. Lasers Surg. Med. 1997, 20, 402–408. [Google Scholar] [CrossRef]
- Cysview Prescribing Information. Available online: https://www.cysview.com/ (accessed on 15 October 2023).
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef]
- Gliolan® SmPC. Available online: https://www.ema.europa.eu/en/documents/product-information/gliolan-epar-product-information_en.pdf (accessed on 1 October 2023).
- Gleolan Prescribing Information. Available online: https://gleolan.com/hcp (accessed on 1 October 2023).
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F. Protoporphyrin IX fluorescence and photo-bleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Lietke, S.; Schmutzer, M.; Schwartz, C.; Weller, J.; Siller, S.; Aumiller, M.; Heckl, C.; Forbrig, R.; Niyazi, M.; Egensperger, R.; et al. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 2021, 13, 1767. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Yumita, N.; Nishigaki, R.; Umemura, K. Mechanism of Cell Damage by Ultrasound in Combination with Hematoporphyrin. Jpn. J. Cancer Res. 1990, 81, 962–966. [Google Scholar] [CrossRef]
- Jeong, E.-J.; Seo, S.-J.; Ahn, Y.-J.; Choi, K.-H.; Kim, K.-H.; Kim, J.-K. Sonodynamically induced antitumor effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic rat glioma model. Ultrasound Med. Biol. 2012, 38, 2143–2150. [Google Scholar] [CrossRef]
- Suehiro, S.; Ohnishi, T.; Yamashita, D.; Kohno, S.; Inoue, A.; Nishikawa, M.; Ohue, S.; Tanaka, J.; Kunieda, T. Enhancement of antitumor activity by using 5-ALA–mediated sonodynamic therapy to induce apoptosis in malignant gliomas: Significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J. Neurosurg. 2018, 129, 1416–1428. [Google Scholar] [CrossRef]
- Yoshida, M.; Kobayashi, H.; Terasaka, S.; Endo, S.; Yamaguchi, S.; Motegi, H.; Itay, R.; Suzuki, S.; Brokman, O.; Shapira, Y.; et al. Sonodynamic Therapy for Malignant Glioma Using 220-kHz Transcranial Magnetic Resonance Imaging-Guided Focused Ultrasound and 5-Aminolevulinic acid. Ultrasound Med. Biol. 2019, 45, 526–538. [Google Scholar] [CrossRef]
- Wu, S.-K.; Santos, M.A.; Marcus, S.L.; Hynynen, K. MR-guided Focused Ultrasound Facilitates Sonodynamic Therapy with 5-Aminolevulinic Acid in a Rat Glioma Model. Sci. Rep. 2019, 9, 10465. [Google Scholar] [CrossRef]
- Schaab, L.; Ferry, Y.; Ozdas, M.; Kritzer, B.; Mourabit, S.; Marcus, S.; Nazarian, J. EXTH-49. Focused Ultrasound and 5-Ala Mediated Elimination of Diffuse Midline Glioma. Neuro-Oncology 2021, 23, vi174. [Google Scholar] [CrossRef]
- Sanai, N.; Tien, A.C.; Tovmasyan, A.; Chang, Y.W.; Margaryan, T.; Hendrickson, K.; Eschbacher, J.; Yoo, W.; Harmon, J.; Hong, A.; et al. A first-in-human phase 0/1 trial of 5-Aminolevulinic Acid Sonodynamic Therapy (5-ALA SDT) in recurrent glioblastoma. In Proceedings of the Society for Neuro-Oncology Conference, Tampa Bay, FL, USA, 16–20 November 2022. [Google Scholar]
- Hoffman, L.M.; van Zanten, S.E.V.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report from the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Syed, H.R.; Kilburn, L.; Fonseca, A.; Nazarian, J.; Oluigbo, C.; Myseros, J.S.; Packer, R.J.; Keating, R.F. First-in-human sonodynamic therapy with ALA for pediatric diffuse intrinsic pontine glioma: A phase 1/2 study using low-intensity focused ultrasound: Technical communication. J. Neuro-Oncol. 2023, 162, 449–451. [Google Scholar] [CrossRef]
- Photofrin Important Safety Information. Available online: https://photofrin.com/ (accessed on 1 November 2023).
- Marcus, S.L.; Dugan, M.H. Global status of clinical photodynamic therapy: The registration process for a new therapy. Lasers Surg. Med. 1992, 12, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mela, A.; Argenziano, M.G.; Banu, M.A.; Furnari, J.; Kotidis, C.; Sperring, C.P.; Humala, N.; Mahajan, A.; Bruce, J.N.; et al. Single-cell analysis of 5-aminolevulinic acid intraoperative labeling specificity for glioblastoma. J. Neurosurg. 2023, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, K.; Fujiwara, T.; Ito, T.; Suzuki, C.; Sasaki, K.; Ono, K.; Saito, K.; Fukuhara, N.; Onishi, Y.; Yokoyama, H.; et al. Flow Cytometry-Based Photodynamic Diagnosis with 5-Aminolevulinic Acid for the Detection of Minimal Residual Disease in Multiple Myeloma. Tohoku J. Exp. Med. 2019, 249, 19–28. [Google Scholar] [CrossRef]
- Darvekar, S.; Juzenas, P.; Oksvold, M.; Kleinauskas, A.; Holien, T.; Christensen, E.; Stokke, T.; Sioud, M.; Peng, Q. Selective Killing of Activated T Cells by 5-Aminolevulinic Acid Mediated Photodynamic Effect: Potential Improvement of Extracorporeal Photopheresis. Cancers 2020, 12, 377. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neuro-Oncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]



| NCT Number | Study Title | Condition | Description | Treatment | Endpoints |
|---|---|---|---|---|---|
| NCT06039709 | Sonodynamic Therapy in Patients with Recurrent GBM | rGBM | Phase 1, single center | Single treatment of oral 5-ALA with Neuronavigation-guided LIFU (NaviFUS), 1–3 weeks before surgery | Safety, biomarker analysis |
| NCT04845919 | Sonodynamic Therapy with ExAblate System in GBM Patients (Sonic ALA) | GBM | Phase 2, single center | Single treatment of oral 5-ALA with MRgFUS (Exablate), followed by surgery 15–21 days post SDT | Safety, biomarker analysis |
| NCT04559685 | Study of Sonodynamic Therapy in Participants with Recurrent HGG | HGG | Phase 0, single center | Single treatment of IV ALA (SONALA-001) with MRgFUS (Exablate), followed by surgery 4–6 days post SDT | Safety, biomarker analysis, immune profiling |
| NCT05362409 | Study to Evaluate 5-ALA Combined with CV01 Delivery of Ultrasound in Recurrent HGG | HGG | Phase 1, multicenter | Monthly treatment with oral ALA with CV01 ultrasound | Safety, determination of Maximum Tolerable Duration of Sonication (MTDu) |
| NCT05370508 | A Study of Sonodynamic Therapy Using SONALA-001 and Exablate 4000 Type 2.0 in Subjects with Recurrent GBM | rGBM | Phase 1/2, multicenter | Monthly treatments of IV ALA (SONALA-001) with MRgFUS (Exablate) device | Safety and tolerability, determination of MTD and RP2D |
| NCT05123534 | A Phase 2 Study of Sonodynamic Therapy Using SONALA-001 and Exablate 4000 Type 2.0 in Patients With DIPG | DIPG | Phase 1/2, multicenter | Monthly treatments of IV ALA (SONALA-001) with MRgFUS (Exablate) device | Safety and tolerability, determination of MTD and RP2D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcus, S.L.; de Souza, M.P. Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT). Cancers 2024, 16, 740. https://doi.org/10.3390/cancers16040740
Marcus SL, de Souza MP. Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT). Cancers. 2024; 16(4):740. https://doi.org/10.3390/cancers16040740
Chicago/Turabian StyleMarcus, Stuart L., and Mark P. de Souza. 2024. "Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT)" Cancers 16, no. 4: 740. https://doi.org/10.3390/cancers16040740
APA StyleMarcus, S. L., & de Souza, M. P. (2024). Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT). Cancers, 16(4), 740. https://doi.org/10.3390/cancers16040740





