Male Fertility and Fatherhood in Chronic Myeloid Leukemia: Current Understanding and Future Perspectives
Abstract
Simple Summary
Abstract
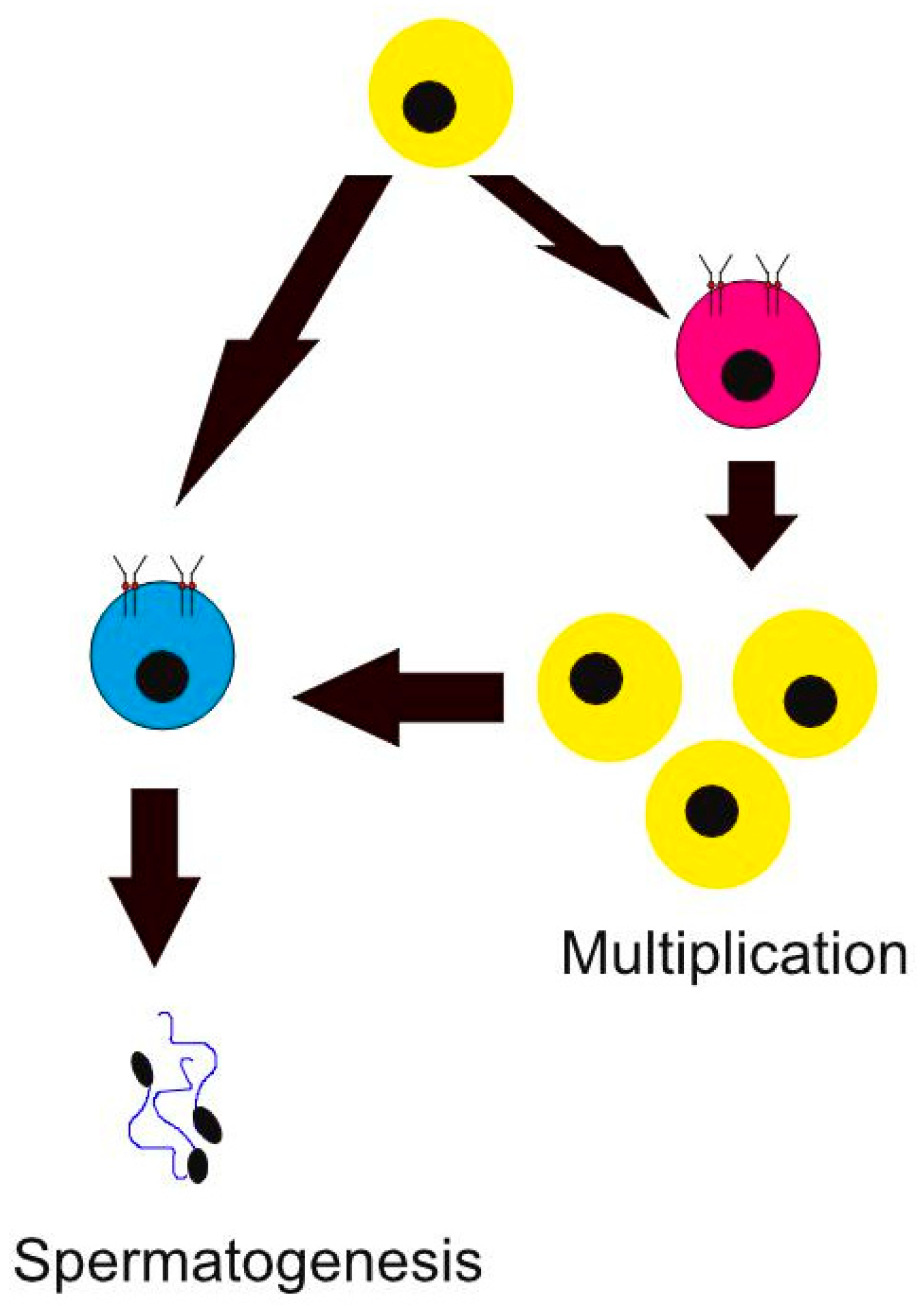
1. Introduction
CML Treatments
2. Findings
2.1. Hydroxyurea
2.2. Interferon
2.3. TKIs
2.3.1. Imatinib
2.3.2. Nilotinib
2.3.3. Dasatinib
2.3.4. Other TKIs
2.4. Transplantation
3. Limitations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minciacchi, V.R.; Kumar, R.; Krause, D.S. Chronic Myeloid Leukemia: A Model Disease of the Past, Present and Future. Cells 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Abruzzese, E.; Trawinska, M.M.; de Fabritiis, P.; Baccarani, M. Management of pregnant chronic myeloid leukemia patients. Expert Rev. Hematol. 2016, 9, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Saglio, G.; Kantarjian, H.M.; Baccarani, M.; Mayer, J.; Boqué, C.; Shah, N.P.; Chuah, C.; Casanova, L.; Bradley-Garelik, B.; et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J. Clin. Oncol. 2016, 34, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.; Radich, J.; Garcia-Gonzalez, P. Meeting the needs of CML patients in resource-poor countries. Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.A.-J.; Aldapt, M.B.; Chandra, P.; Akiki, S.J.; Fernyhough, L.; Sardar, S.; Chapra, A.; Nashwan, A.J.; Yassin, M.A. Chronic Myeloid Leukemia in Adolescents and Youngadults: Clinicopathological Variables and Outcomes. Blood 2022, 140, 3911–3912. [Google Scholar] [CrossRef]
- Elhadary, M.; Elsabagh, A.A.; Ferih, K.; Elsayed, B.; Elshoeibi, A.M.; Kaddoura, R.; Akiki, S.; Ahmed, K.; Yassin, M. Applications of Machine Learning in Chronic Myeloid Leukemia. Diagnostics 2023, 13, 1330. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Eide, C.A.; Druker, B.J. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell 2020, 37, 530–542. [Google Scholar] [CrossRef]
- Yun, S.; Vincelette, N.D.; Segar, J.M.; Dong, Y.; Shen, Y.; Kim, D.W.; Abraham, I. Comparative Effectiveness of Newer Tyrosine Kinase Inhibitors Versus Imatinib in the First-Line Treatment of Chronic-Phase Chronic Myeloid Leukemia Across Risk Groups: A Systematic Review and Meta-Analysis of Eight Randomized Trials. Clin. Lymphoma Myeloma Leuk. 2016, 16, e85–e94. [Google Scholar] [CrossRef]
- Pfirrmann, M.; Baccarani, M.; Saussele, S.; Guilhot, J.; Cervantes, F.; Ossenkoppele, G.; Hoffmann, V.S.; Castagnetti, F.; Hasford, J.; Hehlmann, R.; et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016, 30, 48–56. [Google Scholar] [CrossRef]
- Ahmed, K.; Kaddoura, R.; Yassin, M.A. A practical guide to managing hypertension, hyperlipidemia, and hyperglycemia in patients with chronic myeloid leukemia. Front. Med. 2022, 9, 1025392. [Google Scholar] [CrossRef]
- Kaddoura, R.; Dabdoob, W.A.; Ahmed, K.; Yassin, M.A. A practical guide to managing cardiopulmonary toxicities of tyrosine kinase inhibitors in chronic myeloid leukemia. Front. Med. 2023, 10, 1163137. [Google Scholar] [CrossRef]
- Ciftciler, R.; Haznedaroglu, I.C. Tailored tyrosine kinase inhibitor (TKI) treatment of chronic myeloid leukemia (CML) based on current evidence. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7787–7798. [Google Scholar] [CrossRef]
- Narlı Özdemir, Z.; Kılıçaslan, N.A.; Yılmaz, M.; Eşkazan, A.E. Guidelines for the treatment of chronic myeloid leukemia from the NCCN and ELN: Differences and similarities. Int. J. Hematol. 2023, 117, 3–15. [Google Scholar] [CrossRef]
- Ramstein, J.J.; Tsai, K.K.; Smith, J.F. Tyrosine Kinase Inhibitors and Male Reproductive Health. Clin. Pharmacol. Ther. 2017, 102, 754–756. [Google Scholar] [CrossRef]
- Haznedaroğlu, İ.; Kuzu, I.; İlhan, O. WHO 2016 Definition of Chronic Myeloid Leukemia and Tyrosine Kinase Inhibitors. Turk. J. Haematol. 2020, 37, 42–47. [Google Scholar] [CrossRef]
- Al-Dewik, N.I.; Morsi, H.M.; Samara, M.M.; Ghasoub, R.S.; Gnanam, C.C.; Bhaskaran, S.K.; Nashwan, A.J.; Al-Jurf, R.M.; Ismail, M.A.; AlSharshani, M.M.; et al. Is Adherence to Imatinib Mesylate Treatment Among Patients with Chronic Myeloid Leukemia Associated with Better Clinical Outcomes in Qatar? Clin. Med. Insights Oncol. 2016, 10, 95–104. [Google Scholar] [CrossRef]
- Ali, E.; Soliman, A.; De Sanctis, V.; Nussbaumer, D.; Yassin, M. Priapism in Patients with Chronic Myeloid Leukemia (CML): A Systematic Review. Acta Biomed. 2021, 92, e2021193. [Google Scholar] [CrossRef]
- Hehlmann, R.; Kister, P.; Willer, A.; Simon, M.; Schenk, M.; Seifarth, W.; Papakonstantinou, G.; Saussele, S.; Kolb, H.J.; Ansari, H. Therapeutic progress and comparative aspects in chronic myelogenous leukemia (CML): Interferon alpha vs. hydroxyurea vs. busulfan and expression of MMTV-related endogenous retroviral sequences in CML. German CML Study Group. Leukemia 1994, 8 (Suppl. 1), S127–S132. [Google Scholar]
- Program, N.T. NTP-CERHR monograph on the potential human reproductive and developmental effects of hydroxyurea. NTP Cerhr. Mon. 2008, vii–viii, v, ix–III1. [Google Scholar]
- Jones, K.M.; Niaz, M.S.; Brooks, C.M.; Roberson, S.I.; Aguinaga, M.P.; Hills, E.R.; Rice, V.M.; Bourne, P.; Bruce, D.; Archibong, A.E. Adverse effects of a clinically relevant dose of hydroxyurea used for the treatment of sickle cell disease on male fertility endpoints. Int. J. Environ. Res. Public Health 2009, 6, 1124–1144. [Google Scholar] [CrossRef]
- Grigg, A. Effect of hydroxyurea on sperm count, motility and morphology in adult men with sickle cell or myeloproliferative disease. Intern. Med. J. 2007, 37, 190–192. [Google Scholar] [CrossRef]
- Garozzo, G.; Disca, S.; Fidone, C.; Bonomo, P. Azoospermia in a patient with sickle cell disease treated with hydroxyurea. Haematologica 2000, 85, 1216–1218. [Google Scholar]
- Soliman, A.T.; Alaaraj, N.; Yassin, M. The Effects of Treatment with Blood Transfusion, Iron Chelation and Hydroxyurea on Puberty, Growth and Spermatogenesis in Sickle Cell Disease (SCD): A short update. Acta Biomed. 2021, 92, e2021386. [Google Scholar] [CrossRef]
- Simonsson, B.; Gedde-Dahl, T.; Markevärn, B.; Remes, K.; Stentoft, J.; Almqvist, A.; Björeman, M.; Flogegård, M.; Koskenvesa, P.; Lindblom, A.; et al. Combination of pegylated IFN-α2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood 2011, 118, 3228–3235. [Google Scholar] [CrossRef]
- Palandri, F.; Castagnetti, F.; Iacobucci, I.; Martinelli, G.; Amabile, M.; Gugliotta, G.; Poerio, A.; Testoni, N.; Breccia, M.; Bocchia, M.; et al. The response to imatinib and interferon-alpha is more rapid than the response to imatinib alone: A retrospective analysis of 495 Philadelphia-positive chronic myeloid leukemia patients in early chronic phase. Haematologica 2010, 95, 1415–1419. [Google Scholar] [CrossRef]
- Burchert, A.; Müller, M.C.; Kostrewa, P.; Erben, P.; Bostel, T.; Liebler, S.; Hehlmann, R.; Neubauer, A.; Hochhaus, A. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J. Clin. Oncol. 2010, 28, 1429–1435. [Google Scholar] [CrossRef]
- Hehlmann, R.; Lauseker, M.; Saußele, S.; Pfirrmann, M.; Krause, S.; Kolb, H.J.; Neubauer, A.; Hossfeld, D.K.; Nerl, C.; Gratwohl, A.; et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 2017, 31, 2398–2406. [Google Scholar] [CrossRef]
- Talpaz, M.; Mercer, J.; Hehlmann, R. The interferon-alpha revival in CML. Ann. Hematol. 2015, 94 (Suppl. 2), S195–S207. [Google Scholar] [CrossRef]
- Law, A.D.; Dong Hwan Kim, D.; Lipton, J.H. Pregnancy: Part of life in chronic myelogenous leukemia. Leuk. Lymphoma 2017, 58, 280–287. [Google Scholar] [CrossRef]
- Gazdaru, S.; Perey, L.; Rosselet, A.; Mathevet, P.; Chalandon, Y.; Vulliemoz, N. Successful Ovarian Stimulation for Fertility Preservation in a Patient with Chronic Myeloid Leukemia: Switch from Nilotinib to Interferon-α. Oncologist 2018, 23, 719–721. [Google Scholar] [CrossRef]
- De Leo, S.; Trevisan, M.; Moneta, C.; Colombo, C. Endocrine-related adverse conditions induced by tyrosine kinase inhibitors. Ann. Endocrinol. 2023, 84, 374–381. [Google Scholar] [CrossRef]
- Nurmio, M.; Toppari, J.; Zaman, F.; Andersson, A.M.; Paranko, J.; Söder, O.; Jahnukainen, K. Inhibition of tyrosine kinases PDGFR and C-Kit by imatinib mesylate interferes with postnatal testicular development in the rat. Int. J. Androl. 2007, 30, 366–376, discussion 376. [Google Scholar] [CrossRef]
- Seshadri, T.; Seymour, J.F.; McArthur, G.A. Oligospermia in a patient receiving imatinib therapy for the hypereosinophilic syndrome. N. Engl. J. Med. 2004, 351, 2134–2135. [Google Scholar] [CrossRef]
- Prasad, A.M.; Ramnarayan, K.; Nalini, K.; Bairy, K.L. Effect of imatinib on the biochemical parameters of the reproductive function in male Swiss albino mice. Indian J. Pharmacol. 2011, 43, 389–392. [Google Scholar] [CrossRef]
- Garcia, D.N.; Hense, J.D.; Zanini, B.M.; Isola, J.V.V.; Pradiee, J.; Prosczek, J.B.; Alvarado-Rincón, J.A.; Mondadori, R.G.; Mason, J.B.; Brieño-Enríquez, M.A.; et al. Dasatinib and quercetin increase testosterone and sperm concentration in mice. Physiol. Int. 2023, 110, 121–134. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, C.D.; Liu, X.M.; Bao, H.C.; Qu, Q.L.; Hao, C.F. Effect of PDGF-Rb antagonist imatinib on endometrial injury repairing in mouse model. Asian Pac. J. Trop. Med. 2015, 8, 555–559. [Google Scholar] [CrossRef]
- Ozkavukcu, S.; Kuscu, N.; Adiguzel, D.; Cengiz-Seval, G.; Celik-Ozenci, C. Deleterious reproductive effects of nilotinib in mouse model. Reproduction 2021, 161, 295–306. [Google Scholar] [CrossRef]
- Cortes, J.E.; Gambacorti-Passerini, C.; Deininger, M.; Abruzzese, E.; DeAnnuntis, L.; Brümmendorf, T.H. Pregnancy outcomes in patients treated with bosutinib. Int. J. Hematol. Oncol. 2020, 9, IJH26. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, L.; Chen, X.; Xu, B.; Cheng, Y.; Sun, S.; Fang, M.; Xiang, Y. Impact of Imatinib on the Fertility of Male Patients with Chronic Myelogenous Leukaemia in the Chronic Phase. Target Oncol. 2017, 12, 827–832. [Google Scholar] [CrossRef]
- Mariani, S.; Basciani, S.; Fabbri, A.; Agati, L.; Ulisse, S.; Lubrano, C.; Spera, G.; Gnessi, L. Severe oligozoospermia in a young man with chronic myeloid leukemia on long-term treatment with imatinib started before puberty. Fertil. Steril. 2011, 95, 1120.e15–1120.e17. [Google Scholar] [CrossRef]
- Gentile, M.; Guido, M.; Lucia, E.; Vigna, E.; Mazzone, C.; Recchia, A.G.; Morabito, F. Favorable conception and pregnancy involving a male patient affected by chronic myeloid leukemia while taking dasatinib. Leuk. Lymphoma 2014, 55, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Oweini, H.; Otrock, Z.K.; Mahfouz, R.A.; Bazarbachi, A. Successful pregnancy involving a man with chronic myeloid leukemia on dasatinib. Arch. Gynecol. Obstet. 2011, 283, 133–134. [Google Scholar] [CrossRef]
- Chethan, R.; Malik, P.S.; Sahoo, R.K.; Sharawat, S.; Singh, M.; Garg, V.; Bhatia, K.; Kantak, A.; Kumar, S.; Kumar, L. Fertility and pregnancy in chronic myeloid leukemia: Real-world experience from an Indian tertiary care institution. Ann. Hematol. 2023, 102, 2087–2096. [Google Scholar] [CrossRef]
- Gnessi, L.; Basciani, S.; Mariani, S.; Arizzi, M.; Spera, G.; Wang, C.; Bondjers, C.; Karlsson, L.; Betsholtz, C. Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A-deficient mice. J. Cell Biol. 2000, 149, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Basciani, S.; Mariani, S.; Spera, G.; Gnessi, L. Role of platelet-derived growth factors in the testis. Endocr. Rev. 2010, 31, 916–939. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Soliman, A.T.; Elawa, A.S.; El-Ayoubi, H.R.; Desanctis, V. Effects of Tyrosine Kinase Inhibitors On Spermatogenesis and Pituitary Gonadal Axis in Males with Chronic Myeloid Leukemia. Blood 2012, 120, 1688. [Google Scholar] [CrossRef]
- Berman, E.; Druker, B.J.; Burwick, R. Chronic Myelogenous Leukemia: Pregnancy in the Era of Stopping Tyrosine Kinase Inhibitor Therapy. J. Clin. Oncol. 2018, 36, 1250–1256. [Google Scholar] [CrossRef]
- Abu-Tineh, M.; Ali, E.A.; Alshurafa, A.; Nashwan, A.J.; Albsheer, K.; Ahmed, A.; Hailan, Y.; Rozi, W.; Aljaloudi, E.; Yassin, M.A. The Impact of Tyrosine Kinase Inhibitors on Fatherhood in Patients with Chronic Myeloid Leukemia: A Mixed-Method Study. Cureus 2023, 15, e33407. [Google Scholar] [CrossRef]
- Dou, X.L.; Qin, Y.Z.; Shi, H.X.; Lai, Y.Y.; Hou, Y.; Huang, X.J.; Jiang, Q. Fertility and disease outcomes in patients with chronic myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi 2019, 40, 980–985. [Google Scholar]
- Millot, F.; Guilhot, J.; Baruchel, A.; Petit, A.; Leblanc, T.; Bertrand, Y.; Mazingue, F.; Lutz, P.; Vérité, C.; Berthou, C.; et al. Growth deceleration in children treated with imatinib for chronic myeloid leukaemia. Eur. J. Cancer 2014, 50, 3206–3211. [Google Scholar] [CrossRef]
- Shima, H.; Tokuyama, M.; Tanizawa, A.; Tono, C.; Hamamoto, K.; Muramatsu, H.; Watanabe, A.; Hotta, N.; Ito, M.; Kurosawa, H.; et al. Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia. J. Pediatr. 2011, 159, 676–681. [Google Scholar] [CrossRef]
- Ault, P.; Kantarjian, H.; O’Brien, S.; Faderl, S.; Beran, M.; Rios, M.B.; Koller, C.; Giles, F.; Keating, M.; Talpaz, M.; et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J. Clin. Oncol. 2006, 24, 1204–1208. [Google Scholar] [CrossRef]
- Breccia, M.; Cannella, L.; Montefusco, E.; Frustaci, A.; Pacilli, M.; Alimena, G. Male patients with chronic myeloid leukemia treated with imatinib involved in healthy pregnancies: Report of five cases. Leuk. Res. 2008, 32, 519–520. [Google Scholar] [CrossRef]
- Ramasamy, K.; Hayden, J.; Lim, Z.; Mufti, G.J.; Ho, A.Y. Successful pregnancies involving men with chronic myeloid leukaemia on imatinib therapy. Br. J. Haematol. 2007, 137, 374–375. [Google Scholar] [CrossRef]
- Abruzzese, E.; Mauro, M.; Apperley, J.; Chelysheva, E. Tyrosine kinase inhibitors and pregnancy in chronic myeloid leukemia: Opinion, evidence, and recommendations. Ther. Adv. Hematol. 2020, 11, 2040620720966120. [Google Scholar] [CrossRef]
- Salooja, N.; Szydlo, R.; Socie, G.; Rio, B.; Chatterjee, R.; Ljungman, P.; Van Lint, M.; Powles, R.; Jackson, G.; Hinterberger-Fischer, M.; et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: A retrospective survey. Lancet 2001, 358, 271–276. [Google Scholar] [CrossRef]
- Apperley, J. CML in pregnancy and childhood. Best Prac. Res. Clin. Haematol. 2009, 22, 455–474. [Google Scholar] [CrossRef]
- Schleicher, O.; Horndasch, A.; Krumbholz, M.; Sembill, S.; Bremensdorfer, C.; Grabow, D.; Erdmann, F.; Karow, A.; Metzler, M.; Suttorp, M. Patient-reported long-term outcome following allogeneic hematopoietic stem cell transplantation in pediatric chronic myeloid leukemia. Front. Oncol. 2022, 12, 963223. [Google Scholar] [CrossRef]
| Medication | Imatinib | Dasatinib | Nilotinib | Ponatinib | Bosutinib | Asciminib |
|---|---|---|---|---|---|---|
| FDA information on fertility | Not highlighted—males who are worried about their fertility while on imatinib should consult their doctor. | Not highlighted in males. | Not highlighted in males. | May impair male and female fertility. | Not highlighted. | Not highlighted. |
| Animal studies | Available. Studied on mice and rats: studies show impaired fertility and negative effect on germ-line stem cell formation [32,33]. Was found to reduce intratesticular testosterone and increase LDH levels significantly, but was reversible [34]. | Available. A study on mice showed no effect on fertility, but a reduction in seminal vesicle weight [35]. | Available. Study on mice found lower conception rates in nilotinib-treated male mice. Offspring had lower chances of conception [36,37]. | Available. Studies on rats and monkeys showed degeneration of epithelium of the testes [2]. | Available. Bosutinib resulted in reduced fertility in males as demonstrated by 16% reduction in number of pregnancies. It was not mutagenic in a battery of testes [38]. | Not available. |
| Human studies | Available. Several case studies show no effect on male fertility. Studies also show no effect on sperm morphology and motility. Other reports show effects like reduction in sperm density, count, survival, and overall activity [39,40]. | Available. A 2016 study showed no effect on conception; however, the study had a small sample size as a significant limitation. Several case studies of normal conception while taking medication [3,37,41,42]. | Available. A study with 38 males showed it can be used safely in both men and women [43]. | Not available. | Not available. | Not available. |
| Paper | Type of Study | Conclusions | Drug Used | Limitations |
|---|---|---|---|---|
| [20] | Animal study—transgenic sickle cell mice | Hydroxyurea use was associated with reduction in testosterone levels and sperm motility (negatively affects fertility). Leydig cell hyperplasia was observed. | Hydroxyurea | Animal study with sickle cell models, which may not be an indicator of the effects seen in CML patients. High doses of nitric oxide could be the main cause of the adverse effects. |
| [21] | Human study—retrospective review including male adult patients with sickle cell disease | Azoospermia observed. Effects are reversible after discontinuation. | Hydroxyurea | A case-series. External validity compromised. Study conducted retrospectively. No baseline data recorded before hydroxyurea usage. |
| [32] | Animal study—rats | Imatininb was associated with impaired fertility in rats as it interferes with maturation processes in rat testes. This includes gonocyte migration, testis growth, formation of spermatogonial stem cell and Leydig cell pools, and proliferation of type A spermatogonia. | Imatinib | Animal study on rats. Further human studies are needed to show the observed effect in human testis. |
| [34] | Animal study—Swiss albino mice were and their testes were studied after imatininb exposure | There is an effect on LDH and testosterone exerted by imatinib, but it is reversible. | Imatinib | Animal study on mice. Further human studies are needed to show the observed effect in human testis. |
| [39] | Human study—the effects of imatinib were observed on sperm parameters by a computer-assisted sperm assay and the male reproductive system by using ultrasound | Imatinib crosses the blood–testis barrier and has adverse effects on sperm production. | Imatinib | Limited sample size. |
| [35] | Animal study—mice | Dasatinib showed no adverse effect on fertility. | Dasatinib | Animal study. Human studies are needed to show the observed effect in human testis. This study also included quercitin with dasatinib as senolytic agents. A study examining the effect of the drug on CML subjects is needed. |
| [3] | Human study | No effect on conception in cases subjected to dasatinib. | Dasatinib | Small sample size was a significant limitation. |
| [38] | Human study | No evidence of adverse effects on babies born to a father who was exposed to bosutinib at the time of conception. | Bosutinib | Limited sample size. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsabagh, A.A.; Benkhadra, M.; Elmakaty, I.; Elsayed, A.; Elsayed, B.; Elmarasi, M.; Abutineh, M.; Qasem, N.M.; Ali, E.; Yassin, M. Male Fertility and Fatherhood in Chronic Myeloid Leukemia: Current Understanding and Future Perspectives. Cancers 2024, 16, 791. https://doi.org/10.3390/cancers16040791
Elsabagh AA, Benkhadra M, Elmakaty I, Elsayed A, Elsayed B, Elmarasi M, Abutineh M, Qasem NM, Ali E, Yassin M. Male Fertility and Fatherhood in Chronic Myeloid Leukemia: Current Understanding and Future Perspectives. Cancers. 2024; 16(4):791. https://doi.org/10.3390/cancers16040791
Chicago/Turabian StyleElsabagh, Ahmed Adel, Maria Benkhadra, Ibrahim Elmakaty, Abdelrahman Elsayed, Basant Elsayed, Mohamed Elmarasi, Mohammad Abutineh, Nabeel Mohammad Qasem, Elrazi Ali, and Mohamed Yassin. 2024. "Male Fertility and Fatherhood in Chronic Myeloid Leukemia: Current Understanding and Future Perspectives" Cancers 16, no. 4: 791. https://doi.org/10.3390/cancers16040791
APA StyleElsabagh, A. A., Benkhadra, M., Elmakaty, I., Elsayed, A., Elsayed, B., Elmarasi, M., Abutineh, M., Qasem, N. M., Ali, E., & Yassin, M. (2024). Male Fertility and Fatherhood in Chronic Myeloid Leukemia: Current Understanding and Future Perspectives. Cancers, 16(4), 791. https://doi.org/10.3390/cancers16040791






