ATOH1, TFAP2B, and CEACAM6 as Immunohistochemical Markers to Distinguish Merkel Cell Carcinoma and Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Differential Gene Expression Analysis
2.3. SCLC Subtype Analysis
2.4. Immunohistochemistry
2.5. Analysis of Expression
3. Results
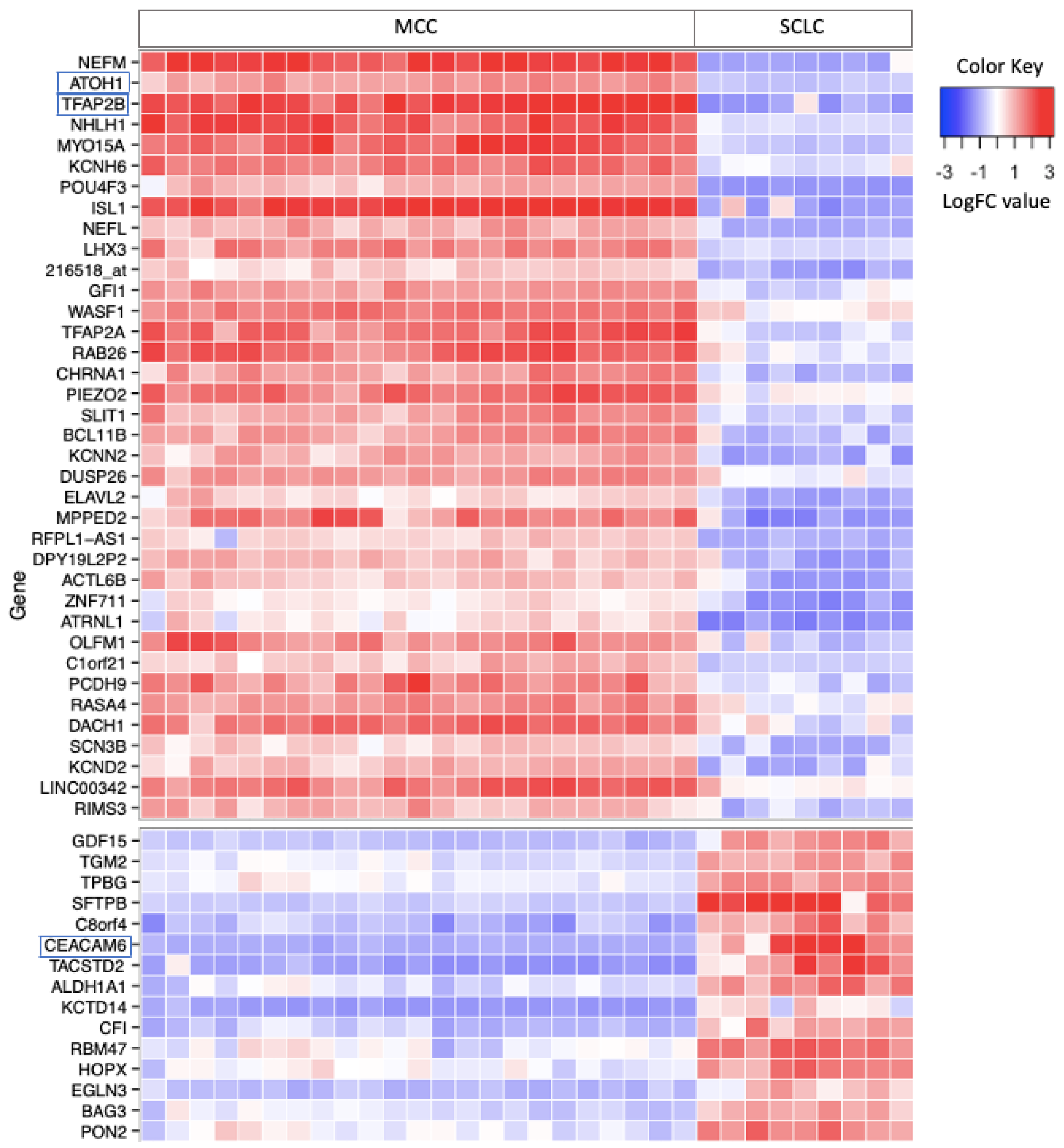
3.1. Differential Gene Expression in MCC and SCLC
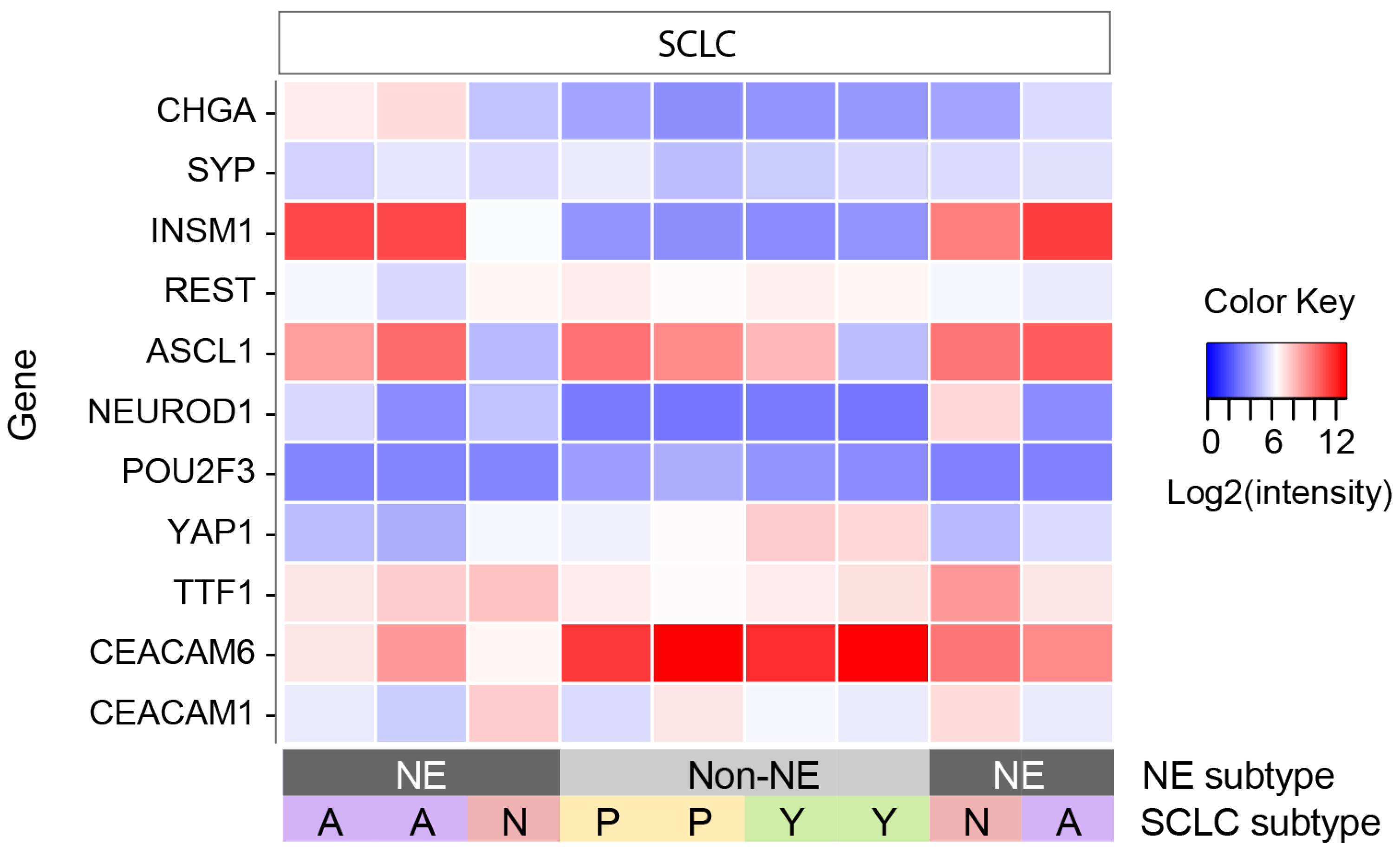
3.2. SCLC Subtype Analysis
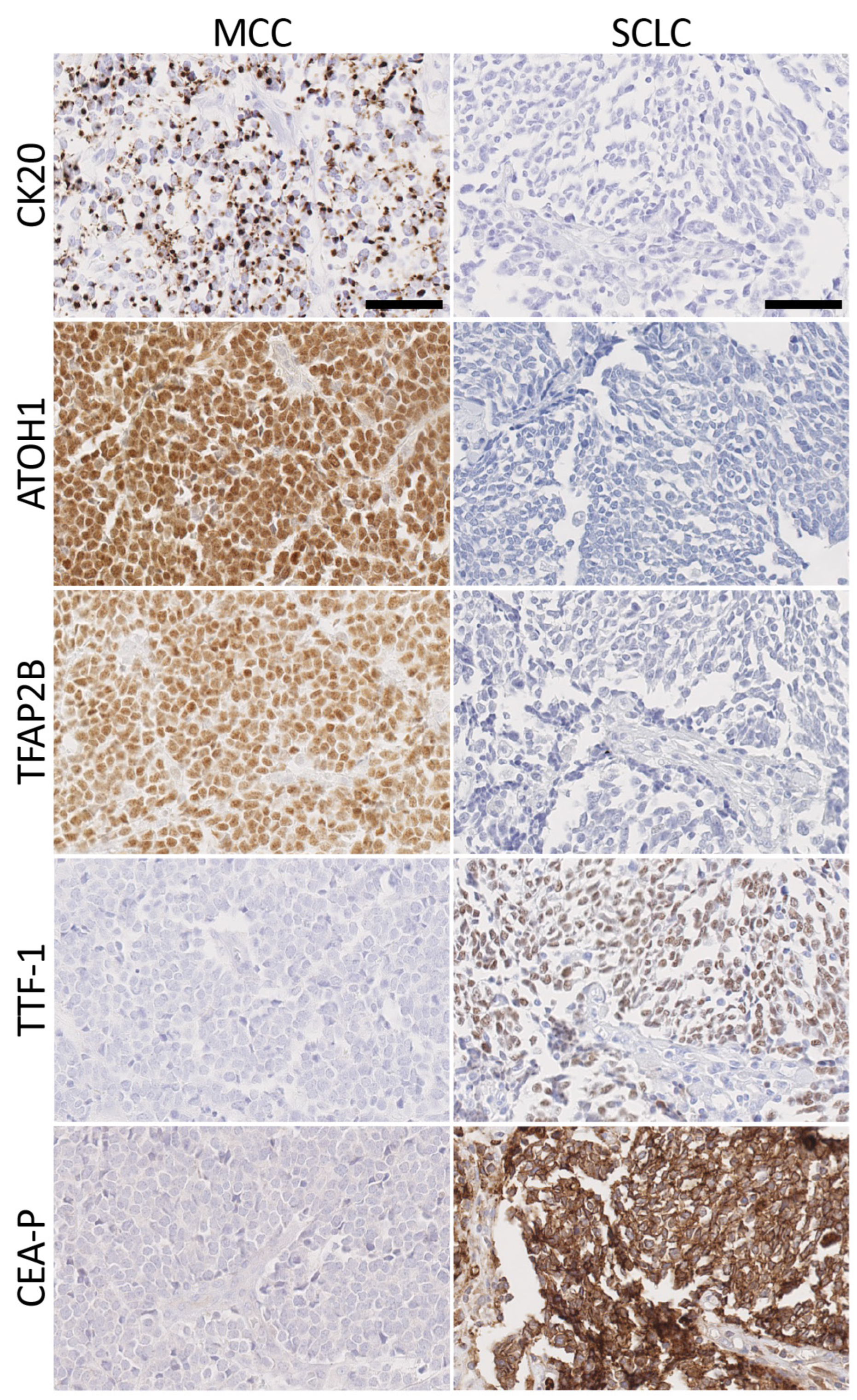
3.3. Immunohistochemical Staining
3.4. Merkel Cell Carcinoma Markers
3.5. Small Cell Lung Cancer Markers
3.6. Comparison of Antibody Panels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harms, P.W.; on behalf of the International Workshop on Merkel Cell Carcinoma Research (IWMCC) Working Group; Harms, K.L.; Moore, P.S.; DeCaprio, J.A.; Nghiem, P.; Wong, M.K.K.; Brownell, I. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018, 15, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, T.; Ring, J.; Andres, C. Histological, Immunohistological, and Clinical Features of Merkel Cell Carcinoma in Correlation to Merkel Cell Polyomavirus Status. J. Ski. Cancer 2012, 2012, 983421. [Google Scholar] [CrossRef] [PubMed]
- Mollet, T.W.; Garcia, C.A.; Koester, G. Skin metastases from lung cancer. Dermatol. Online J. 2009, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Byrd-Gloster, A.L.; Khoor, A.; Glass, L.F.; Messina, J.L.; Whitsett, J.A.; Livingston, S.K.; Cagle, P.T. Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and merkel cell tumor. Hum. Pathol. 2000, 31, 58–62. [Google Scholar] [CrossRef]
- Cheuk, W.; Kwan, M.Y.; Suster, S.; Chan, J.K. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch. Pathol. Lab. Med. 2001, 125, 228–231. [Google Scholar] [CrossRef]
- Hanly, A.J.; Elgart, G.W.; Jorda, M.; Smith, J.; Nadji, M. Analysis of thyroid transcription factor-1 and cytokeratin 20 separates merkel cell carcinoma from small cell carcinoma of lung. J. Cutan. Pathol. 2000, 27, 118–120. [Google Scholar] [CrossRef]
- Leech, S.N. Merkel Cell Carcinoma Can Be Distinguished from Metastatic Small Cell Carcinoma Using Anti-bodies to Cytokeratin 20 and Thyroid Transcription Factor 1. J. Clin. Pathol. 2001, 54, 727–729. [Google Scholar] [CrossRef]
- Metz, K.A.; Jacob, M.; Schmidt, U.; Steuhl, K.-P.; Leder, L.-D. Merkel cell carcinoma of the eyelid: Histological and immunohistochemical features with special respect to differential diagnosis. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 236, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, N.G. Value of Thyroid Transcription Factor-1 Immunostaining in Distinguishing Small Cell Lung Carcinomas From Other Small Cell Carcinomas. Am. J. Surg. Pathol. 2000, 24, 1217–1223. [Google Scholar] [CrossRef]
- Schmidt, U.; Müller, U.; Metz, K.A.; Leder, L.-D. Cytokeratin and Neurofilament Protein Staining in Merkel Cell Carcinoma of the Small Cell Type and Small Cell Carcinoma of the Lung. Am. J. Dermatopathol. 1998, 20, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.T. CD117, CK20, TTF-1, and DNA topoisomerase II-alpha antigen expression in small cell tumors. J. Cutan. Pathol. 2004, 31, 254–261. [Google Scholar] [CrossRef]
- Bobos, M.; Hytiroglou, P.; Kostopoulos, I.; Karkavelas, G.; Papadimitriou, C.S. Immunohistochemical Distinction Between Merkel Cell Carcinoma and Small Cell Carcinoma of the Lung. Am. J. Dermatopathol. 2006, 28, 99–104. [Google Scholar] [CrossRef]
- Fukuhara, M.; Agnarsdóttir, M.; Edqvist, P.-H.; Coter, A.; Ponten, F. SATB2 is expressed in Merkel cell carcinoma. Arch. Dermatol. Res. 2016, 308, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, M.; Hanna, W.; Raphael, S.J.; Ghorab, Z. Expression of TdT in Merkel Cell Carcinoma and Small Cell Lung Carcinoma. Am. J. Clin. Pathol. 2011, 135, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Kolhe, R.; Reid, M.D.; Lee, J.R.; Cohen, C.; Ramalingam, P. Immunohistochemical expression of PAX5 and TdT by Merkel cell carcinoma and pulmonary small cell carcinoma: A potential diagnostic pitfall but useful discriminatory marker. Int. J. Clin. Exp. Pathol. 2013, 6, 142–147. [Google Scholar] [PubMed]
- Ralston, J. MASH1: A Useful Marker in Differentiating Pulmonary Small Cell Carcinoma from Merkel Cell Car-cinoma. Mod. Pathol. 2008, 21, 1357–1362. [Google Scholar] [CrossRef]
- Moll, R. Cytokeratin 20 in Human Carcinomas. A New Histodiagnostic Marker Detected by Monoclonal Anti-bodies. Am. J. Pathol. 1992, 140, 427–447. [Google Scholar] [PubMed]
- Chan, J.K. Cytokeratin 20 Immunoreactivity Distinguishes Merkel Cell (Primary Cutaneous Neuroendocrine) Carcinomas and Salivary Gland Small Cell Carcinomas from Small Cell Carcinomas of Various Sites. Am. J. Surg. Pathol. 1997, 21, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Chu, P. Cytokeratin 7 and Cytokeratin 20 Expression in Epithelial Neoplasms: A Survey of 435 Cases. Mod. Pathol. 2000, 13, 962–972. [Google Scholar] [CrossRef]
- Nicholson, S.A.; McDermott, M.B.; Swanson, P.E.; Wick, M.R. CD99 and Cytokeratin-20 in Small-Cell and Basaloid Tumors of the Skin. Appl. Immunohistochem. Mol. Morphol. 2000, 8, 37–41. [Google Scholar] [CrossRef]
- Kaufmann, O.; Dietel, M. Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology 2000, 36, 415–420. [Google Scholar] [CrossRef]
- Kervarrec, T. Differentiating Merkel Cell Carcinoma of Lymph Nodes without a Detectable Primary Skin Tu-mor from Other Metastatic Neuroendocrine Carcinomas: The ELECTHIP Criteria. J. Am. Acad. Dermatol. 2018, 78, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, A.; Browning, D.; Salama, S. CD99 expression in Merkel cell carcinoma: A case series with an unusual paranuclear dot-like staining pattern. J. Cutan. Pathol. 2012, 40, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Stanoszek, L.M.; Chan, M.P.; Palanisamy, N.; Carskadon, S.; Siddiqui, J.; Patel, R.M.; Harms, K.L.; Lowe, L.; Fullen, D.R.; Harms, P.W. Neurofilament is superior to cytokeratin 20 in supporting cutaneous origin for neuroendocrine carcinoma. Histopathology 2018, 74, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Filtenborg-Barnkob, B.E.; Bzorek, M. Expression of anaplastic lymphoma kinase in Merkel cell carcinomas. Hum. Pathol. 2013, 44, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.S. SMARCA4 (BRG1) and SMARCB1 (INI1) Expression in TTF-1 Negative Neuroendocrine Carcino-mas Including Merkel Cell Carcinoma. Pathol. Res. Pract. 2021, 219, 153341. [Google Scholar] [CrossRef] [PubMed]
- Busam, K.J.; Jungbluth, A.A.; Rekthman, N.; Coit, D.; Pulitzer, M.; Bini, J.B.; Arora, R.B.; Hanson, N.C.B.; Tassello, J.A.B.; Frosina, D.B.; et al. Merkel Cell Polyomavirus Expression in Merkel Cell Carcinomas and Its Absence in Combined Tumors and Pulmonary Neuroendocrine Carcinomas. Am. J. Surg. Pathol. 2009, 33, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Vanderbeck, K.; Nagarajan, P.; Milton, D.R.; Gill, P.; Wang, W.-L.; Curry, J.L.; Torres-Cabala, C.A.; Ivan, D.; Prieto, V.G.; et al. SOX11 Is an Effective Discriminatory Marker, When Used in Conjunction with CK20 and TTF1, for Merkel Cell Carcinoma: Comparative Analysis of SOX11, CK20, PAX5, and TTF1 Expression in Merkel Cell Carcinoma and Pulmonary Small Cell Carcinoma. Arch. Pathol. Lab. Med. 2023, 147, 758–766. [Google Scholar] [CrossRef]
- Daily, K.; Coxon, A.; Williams, J.S.; Lee, C.-C.R.; Coit, D.G.; Busam, K.J.; Brownell, I. Assessment of Cancer Cell Line Representativeness Using Microarrays for Merkel Cell Carcinoma. J. Investig. Dermatol. 2015, 135, 1138–1146. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Schwendenwein, A.; Megyesfalvi, Z.; Barany, N.; Valko, Z.; Bugyik, E.; Lang, C.; Ferencz, B.; Paku, S.; Lantos, A.; Fillinger, J.; et al. Molecular profiles of small cell lung cancer subtypes: Therapeutic implications. Mol. Ther. Oncolytics 2021, 20, 470–483. [Google Scholar] [CrossRef]
- Paulson, K.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463.e2. [Google Scholar] [CrossRef]
- Ostrowski, S.M. Ectopic Atoh1 Expression Drives Merkel Cell Production in Embryonic, Postnatal and Adult Mouse Epidermis. Development 2015, 142, 2533–2544. [Google Scholar]
- Gambichler, T.; Mohtezebsade, S.; Wieland, U.; Silling, S.; Höh, A.-K.; Dreißigacker, M.; Schaller, J.; Schulze, H.-J.; Oellig, F.; Kreuter, A.; et al. Prognostic relevance of high atonal homolog-1 expression in Merkel cell carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hilger-Eversheim, K.; Moser, M.; Schorle, H.; Buettner, R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene 2000, 260, 1–12. [Google Scholar] [CrossRef]
- Jin, C.; Luo, Y.; Liang, Z.; Li, X.; Kołat, D.; Zhao, L.; Xiong, W. Crucial role of the transcription factors family activator protein 2 in cancer: Current clue and views. J. Transl. Med. 2023, 21, 371. [Google Scholar] [CrossRef]
- Dankner, M.; Gray-Owen, S.D.; Huang, Y.-H.; Blumberg, R.S.; Beauchemin, N. CEACAM1 as a Multi-Purpose Target for Cancer Immunotherapy. OncoImmunology 2017, 6, e1328336. [Google Scholar] [CrossRef] [PubMed]
- Ilantzis, C.; Demarte, L.; Screaton, R.A.; Stanners, C.P. Deregulated Expression of the Human Tumor Marker CEA and CEA Family Member CEACAM6 Disrupts Tissue Architecture and Blocks Colonocyte Differentiation. Neoplasia 2002, 4, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Iliadis, A.; Koletsa, T.; Kostopoulos, I.; Tzioufa, V. Letter to the Editor Diffuse TTF-1 expression in a case of Merkel cell carcinoma. Pol. J. Pathol. 2015, 2, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Koba, S.; Inoue, T.; Okawa, T.; Tara, A.; Yada, N.; Misago, N.; Narisawa, Y. Merkel cell carcinoma with cytokeratin 20-negative and thyroid transcription factor-1-positive immunostaining admixed with squamous cell carcinoma. J. Dermatol. Sci. 2011, 64, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Salvador, J.M. Primary Cutaneous Neuroendocrine Carcinoma with Diffuse Expression of Thyroid Tran-scription Factor-1: Report of Two Cases. Indian J Dermatol 2019, 64, 251. [Google Scholar] [CrossRef] [PubMed]
- Reddi, D.M.; Puri, P.K. Expression of Focal TTF-1 Expression in a Case of CK7/CK20-Positive Merkel Cell Car-cinoma. J Cutan Pathol 2013, 40, 431–433. [Google Scholar] [CrossRef]
- Shalin, S.C.; Cifarelli, C.P.; Suen, J.Y.; Gao, L. Loss of Cytokeratin 20 and Acquisition of Thyroid Transcription Factor-1 Expression in a Merkel Cell Carcinoma Metastasis to the Brain. Am. J. Dermatopathol. 2014, 36, 904–906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sierakowski, A.; Al-Janabi, K.; van Dam, H.; Sood, M. Metastatic Merkel Cell Carcinoma with Positive Expression of Thyroid Transcription Factor-1-A Case Report. Am. J. Dermatopathol. 2009, 31, 384–386. [Google Scholar] [CrossRef]
- Czapiewski, P.; Majewska, H.; Kutzner, H.; Kazakov, D.; Renkielska, A.; Biernat, W. TTF-1 and PAX5 Are Frequently Expressed in Combined Merkel Cell Carcinoma. Am. J. Dermatopathol. 2016, 38, 513–516. [Google Scholar] [CrossRef]
- Hughes, A.J.; French, M.; Ah-Weng, A.; Singh, M. Diffuse and Strong TTF-1 Positivity in a Combined Merkel Cell Carcinoma. Am. J. Dermatopathol. 2020, 42, 229–231. [Google Scholar] [CrossRef]
- Martin, B.; Poblet, E.; Rios, J.J.; Kazakov, D.; Kutzner, H.; Brenn, T.; Calonje, E. Merkel cell carcinoma with divergent differentiation: Histopathological and immunohistochemical study of 15 cases with PCR analysis for Merkel cell polyomavirus. Histopathology 2013, 62, 711–722. [Google Scholar] [CrossRef]
- Pulitzer, M.P.; Brannon, A.R.; Berger, M.F.; Louis, P.; Scott, S.N.; A Jungbluth, A.; Coit, D.G.; Brownell, I.; Busam, K.J. Cutaneous squamous and neuroendocrine carcinoma: Genetically and immunohistochemically different from Merkel cell carcinoma. Mod. Pathol. 2015, 28, 1023–1032. [Google Scholar] [CrossRef]
- Czapiewski, P.; Biernat, W. Comment on “Diffuse TTF-1 expression in a case of Merkel cell carcinoma”. Pol. J. Pathol. 2016, 67, 300. [Google Scholar] [CrossRef]
| MCC, % (+/n) | SCLC, % (+/n) | |||
|---|---|---|---|---|
| Source | CK20+ | TTF-1+ | CK20+ | TTF-1+ |
| Byrd-Gloster et al. [4] | 76% (16/21) | 0% (0/21) | 3% (1/36) | 97% (35/36) |
| Cheuk et al. [5] | 100% (23/23) | 0% (0/23) | 0% (0/52) | 83% (43/52) |
| Hanly et al. [6] | 95% (20/21) | 0% (0/21) | 33% (11/33) | 85% (28/33) |
| Leech et al. [7] | 91% (10/11) | 0% (0/11) | 0% (0/10) | 100% (10/10) |
| Metz et al. [8] | 100% (6/6) | 0% (0/22) | ||
| Ordonez [9] | 76% (16/21) | 0% (0/18) | 4% (1/28) | 96% (27/28) |
| Schmidt et al. [8], [10] | 77% (43/56) | 0% (0/18) | ||
| Yang et al. [11] | 86% (19/22) | 0% (0/22) | 0% (0/9) | 100% (9/9) |
| Bobos et al. [12] | 100% (13/13) | 0% (0/13) | 8% (1/13) | 85% (11/13) |
| Fukuhara et al. [13] | 75% (15/20) | 0% (0/4) | ||
| Sidiropoulos et al. [14] | 88% (35/40) | 3% (1/40) | 0% (0/30) | 77% (23/30) |
| Kolhe et al. [15] | 94% (15/16) | 0% (0/10) | 100% (2/2) | |
| Ralston et al. [16] | 3% (1/30) | 73% (43/59) | ||
| Moll et al. [17] | 100% (15/15) | 20% (3/15) | ||
| Chan et al. [18] | 97% (32/33) | 3% (1/37) | ||
| Chu et al. [19] | 78% (7/9) | 0% (0/7) | ||
| Nicholson et al. [20] | 67% (18/27) | 0% (0/5) | ||
| Kaufmann et al. [21] | 86% (24/28) | 0% (0/16) | 0% (0/5) | 81% (30/37) |
| Kervarrec et al. [22] | 86% (12/14) | 4% (1/28) | 0% (0/7) | 100% (5/5) |
| Rajagopalan et al. [23] | 95% (37/39) | 14% (4/28) | ||
| Stanoszek et al. [24] | 76% (37/49) | 41% (25/61) | ||
| Filtenborg-Barnkob et al. [25] | 88% 28/32 | 22% (7/32) | 33% (4/12) | 100% (12/12) |
| Gandhi et al. [26] | 0% (0/23) | 56% (9/16) | ||
| Busam et al. [27] | 89% (32/36) | 0% (0/16) | ||
| Cho et al. [28] | 89% (42/47) | 4% (2/47) | 3% (1/29) | 93% (27/29) |
| Cumulative Totals | 86% (515/599) | 3% (12/355) | 11% (52/477) | 85% (314/371) |
| MCC Markers, +/n | SCLC markers, +/n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Individual | Combination | Individual | Combination | ||||||
| CK20 | ATOH1 | TFAP2B | CK20, ATOH1 | CK20, TFAP2B | CK20, ATOH1, TFAP2B | TTF-1 | CEA-P | TTF-1, CEA-P | |
| MCC (n = 43) | 43/43 | 43/43 | 40/43 | 43/43 | 43/43 | 43/43 | 1/43 | 0/43 | 1/43 |
| SCLC (n = 59) | 0/59 | 0/59 | 0/59 | 0/59 | 0/59 | 0/59 | 47/59 | 49/59 | 55/59 |
| Sensitivity, % | 100 | 100 | 93 | 100 | 100 | 100 | 80 | 83 | 93 * |
| Specificity, % | 100 | 100 | 100 | 100 | 100 | 100 | 98 | 100 | 98 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilasi, S.M.; Nguyen, J.; Wang, C.J.; Miao, L.; Daily, K.; Eid, M.; Song, J.S.; Jiang, H.; Ylaya, K.; Busam, K.J.; et al. ATOH1, TFAP2B, and CEACAM6 as Immunohistochemical Markers to Distinguish Merkel Cell Carcinoma and Small Cell Lung Cancer. Cancers 2024, 16, 788. https://doi.org/10.3390/cancers16040788
Vilasi SM, Nguyen J, Wang CJ, Miao L, Daily K, Eid M, Song JS, Jiang H, Ylaya K, Busam KJ, et al. ATOH1, TFAP2B, and CEACAM6 as Immunohistochemical Markers to Distinguish Merkel Cell Carcinoma and Small Cell Lung Cancer. Cancers. 2024; 16(4):788. https://doi.org/10.3390/cancers16040788
Chicago/Turabian StyleVilasi, Serena M., Jannett Nguyen, Catherine J. Wang, Lingling Miao, Kenneth Daily, Mary Eid, Joon Seon Song, Hong Jiang, Kris Ylaya, Klaus J. Busam, and et al. 2024. "ATOH1, TFAP2B, and CEACAM6 as Immunohistochemical Markers to Distinguish Merkel Cell Carcinoma and Small Cell Lung Cancer" Cancers 16, no. 4: 788. https://doi.org/10.3390/cancers16040788
APA StyleVilasi, S. M., Nguyen, J., Wang, C. J., Miao, L., Daily, K., Eid, M., Song, J. S., Jiang, H., Ylaya, K., Busam, K. J., Gaiser, M. R., Hewitt, S. M., & Brownell, I. (2024). ATOH1, TFAP2B, and CEACAM6 as Immunohistochemical Markers to Distinguish Merkel Cell Carcinoma and Small Cell Lung Cancer. Cancers, 16(4), 788. https://doi.org/10.3390/cancers16040788






