The Microbiome Modulates the Immune System to Influence Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Poor Quality of Food Increases the Risk of Cancer
3. Gut Microbiome and Biomarkers for Unfavorable Microbiome
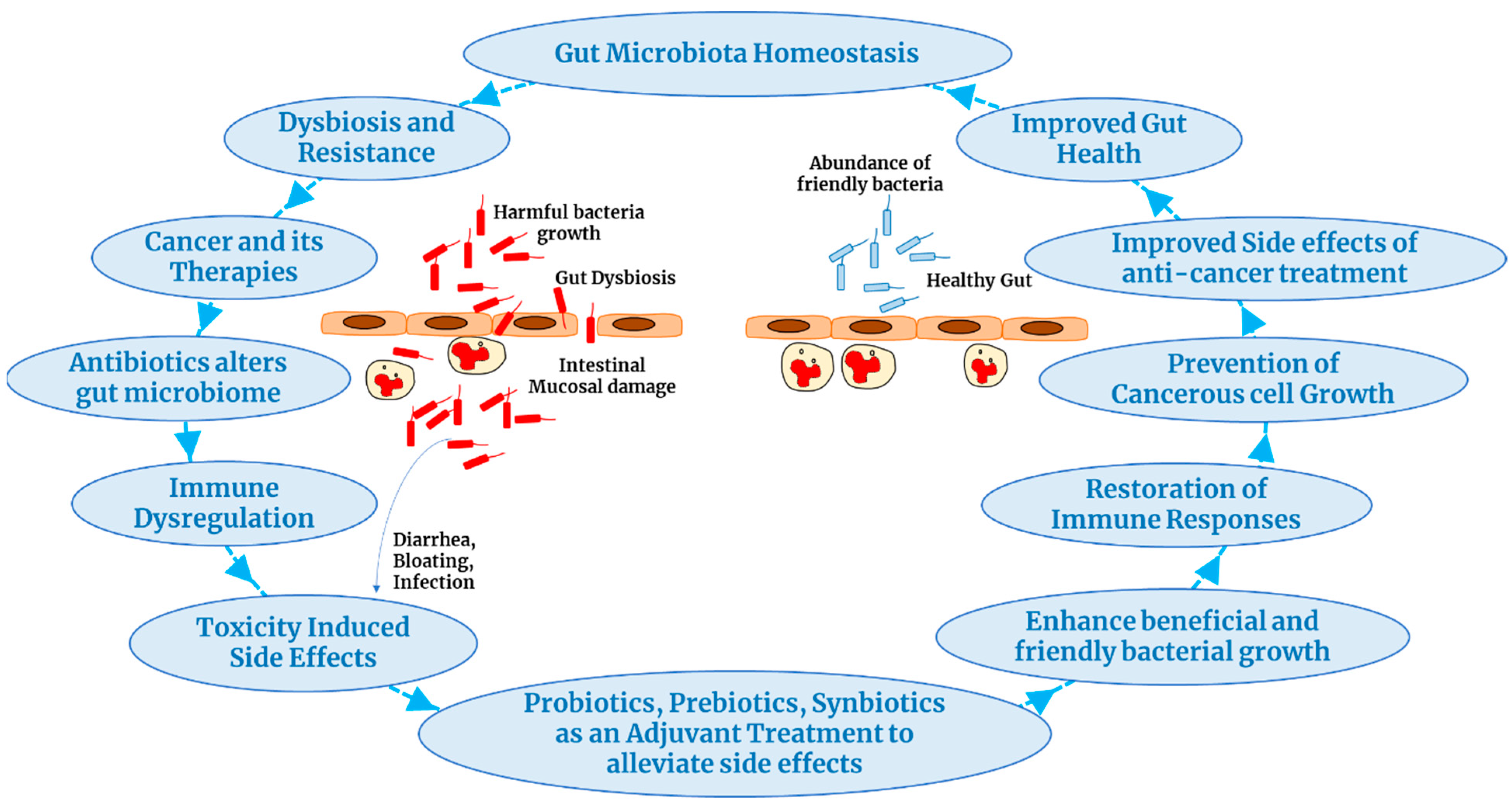
4. Probiotics Improve Cancer Treatment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Folch Ayora, A.; Ropero-Padilla, C. Probiotic Supplements on Oncology Patients’ Treatment-Related Side Effects: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 4265. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018; American Institute for Cancer Research: Arlington, VA, USA, 2018. [Google Scholar]
- Papadimitriou, N.; Markozannes, G.; Kanellopoulou, A.; Critselis, E.; Alhardan, S.; Karafousia, V.; Kasimis, J.C.; Katsaraki, C.; Papadopoulou, A.; Zografou, M.; et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun. 2021, 12, 4579. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. Bmj 2007, 335, 1134. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? Bmj 2020, 368, m511. [Google Scholar] [CrossRef] [PubMed]
- Link, W. Principles of Cancer Treatment and Anticancer Drug Development; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Loman, B.R.; Jordan, K.R.; Haynes, B.; Bailey, M.T.; Pyter, L.M. Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Sci. Rep. 2019, 9, 16490. [Google Scholar] [CrossRef]
- Appleby, P.N.; Key, T.J. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- WHO. Time to Deliver: Report of the WHO Independent High-Level Commission on Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Vergnaud, A.-C.; Romaguera, D.; Peeters, P.H.; Van Gils, C.H.; Chan, D.S.; Romieu, I.; Freisling, H.; Ferrari, P.; Clavel-Chapelon, F.; Fagherazzi, G. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: Results from the European Prospective Investigation into Nutrition and Cancer cohort study. Am. Clin. Nutr. 2013, 97, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer Red Meat and Processed Meat. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 114 IARC. 2018. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/Evaluations.pdf (accessed on 25 January 2024).
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Kim, Y.I. Folate: A magic bullet or a double edged sword for colorectal cancer prevention? Gut 2006, 55, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D.P. Editorial: Large-bowel cancer: An epidemiologic jigsaw puzzle. J. Natl. Cancer Inst. 1975, 54, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sawada, N.; Matsuda, T.; Iwasaki, M.; Sasazuki, S.; Shimazu, T.; Shibuya, K.; Tsugane, S. Attributable causes of cancer in Japan in 2005—Systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann. Oncol. 2012, 23, 1362–1369. [Google Scholar] [CrossRef]
- Petimar, J.; Smith-Warner, S.A.; Fung, T.T.; Rosner, B.; Chan, A.T.; Hu, F.B.; Giovannucci, E.L.; Tabung, F.K. Recommendation-based dietary indexes and risk of colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Am. J. Clin. Nutr. 2018, 108, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Wesselius, A.; Mehrkanoon, S.; Goosens, M.; Brinkman, M.; van den Brandt, P.; Grant, E.J.; White, E.; Weiderpass, E.; Le Calvez-Kelm, F.; et al. Vegetable intake and the risk of bladder cancer in the BLadder Cancer Epidemiology and Nutritional Determinants (BLEND) international study. BMC Med. 2021, 19, 56. [Google Scholar] [CrossRef]
- Pauwels, E.K.J.; Volterrani, D. Coffee Consumption and Cancer Risk: An Assessment of the Health Implications Based on Recent Knowledge. Med. Princ. Pract. 2021, 30, 401–411. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- World Cancer Research Fund; American Institute for Cancer Research. Resources and Toolkits. 2018. Available online: https://www.wcrf.org/diet-activity-and-cancer/global-cancer-update-programme/resources-and-toolkits/ (accessed on 25 January 2024).
- Figueiredo, J.C.; Grau, M.V.; Haile, R.W.; Sandler, R.S.; Summers, R.W.; Bresalier, R.S.; Burke, C.A.; McKeown-Eyssen, G.E.; Baron, J.A. Folic acid and risk of prostate cancer: Results from a randomized clinical trial. J. Natl. Cancer Inst. 2009, 101, 432–435. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, Y.; Huang, J.Y.; Zhang, A.Q.; Zhou, Y.H.; Wang, J.N. Folate intake, serum folate levels, and prostate cancer risk: A meta-analysis of prospective studies. BMC Public Health 2014, 14, 1326. [Google Scholar] [CrossRef]
- Liss, M.A.; White, J.R.; Goros, M.; Gelfond, J.; Leach, R.; Johnson-Pais, T.; Lai, Z.; Rourke, E.; Basler, J.; Ankerst, D.; et al. Metabolic Biosynthesis Pathways Identified from Fecal Microbiome Associated with Prostate Cancer. Eur. Urol. 2018, 74, 575–582. [Google Scholar] [CrossRef]
- Schmit, S.L.; Rennert, H.S.; Rennert, G.; Gruber, S.B. Coffee Consumption and the Risk of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Yiannakou, I.; Singer, M.R.; Jacques, P.F.; Xanthakis, V.; Ellison, R.C.; Moore, L.L. Adherence to a Mediterranean-Style Dietary Pattern and Cancer Risk in a Prospective Cohort Study. Nutrients 2021, 13, 4064. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International. Preservation and Processing of Foods and Cancer Risk. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Preservation-and-processing-of-foods.pdf (accessed on 25 January 2024).
- Wu, B.; Yang, D.; Yang, S.; Zhang, G. Dietary Salt Intake and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 801228. [Google Scholar] [CrossRef]
- Strumylaite, L.; Zickute, J.; Dudzevicius, J.; Dregval, L. Salt-preserved foods and risk of gastric cancer. Medicina 2006, 42, 164–170. [Google Scholar] [PubMed]
- Kimanya, M.E.; Routledge, M.N.; Mpolya, E.; Ezekiel, C.N.; Shirima, C.P.; Gong, Y.Y. Estimating the risk of aflatoxin-induced liver cancer in Tanzania based on biomarker data. PLoS ONE 2021, 16, e0247281. [Google Scholar] [CrossRef]
- Lin, M.H.; Li, C.Y.; Cheng, Y.Y.; Guo, H.R. Arsenic in Drinking Water and Incidences of Leukemia and Lymphoma: Implication for Its Dual Effects in Carcinogenicity. Front. Public Health 2022, 10, 863882. [Google Scholar] [CrossRef]
- Issanov, A.; Adewusi, B.; Dummer, T.J.B.; Saint-Jacques, N. Arsenic in Drinking Water and Urinary Tract Cancers: A Systematic Review Update. Water 2023, 15, 2185. [Google Scholar] [CrossRef]
- Brooks, A.W.; Priya, S.; Blekhman, R.; Bordenstein, S.R. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018, 16, e2006842. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Haro, C.; Vega-Rojas, A.; Alcala-Diaz, J.F.; Molina-Abril, H.; Leon-Acuña, A.; Lopez-Moreno, J.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; et al. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol. Nutr. Food Res. 2019, 63, e1800870. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Kochumon, S.; Al-Rashed, F.; Sindhu, S.; Thomas, R.; Miranda, L.; Al-Mulla, F.; Ahmad, R. Dectin-1 as a Potential Inflammatory Biomarker for Metabolic Inflammation in Adipose Tissue of Individuals with Obesity. Cells 2022, 11, 2879. [Google Scholar] [CrossRef]
- Nagy, K.; Sonkodi, I.; Szöke, I.; Nagy, E.; Newman, H. The microflora associated with human oral carcinomas. Oral Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; White, J.R.; Godoy-Vitorino, F.; Rodríguez-Hilario, A.; Navarro, K.; González, H.; Michailidi, C.; Jedlicka, A.; Canapp, S.; Bondy, J.; et al. High-resolution microbiome profiling uncovers Fusobacterium nucleatum, Lactobacillus gasseri/johnsonii, and Lactobacillus vaginalis associated to oral and oropharyngeal cancer in saliva from HPV positive and HPV negative patients treated with surgery and chemo-radiation. Oncotarget 2017, 8, 110931–110948. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Narikiyo, M.; Tanabe, C.; Yamada, Y.; Igaki, H.; Tachimori, Y.; Kato, H.; Muto, M.; Montesano, R.; Sakamoto, H.; Nakajima, Y.; et al. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 2004, 95, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Park, J.P.; Jeon, S.H.; Lee, Y.J.; Choi, H.J.; Jeong, K.M.; Lee, J.G.; Choi, S.P.; Lim, J.H.; Kim, Y.H.; et al. Purulent Pericarditis Caused by Group G Streptococcus as an Initial Presentation of Colon Cancer. J. Korean Med. Sci. 2002, 17, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- You, W.C.; Zhang, L.; Gail, M.H.; Chang, Y.S.; Liu, W.D.; Ma, J.L.; Li, J.Y.; Jin, M.L.; Hu, Y.R.; Yang, C.S.; et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J. Natl. Cancer Inst. 2000, 92, 1607–1612. [Google Scholar] [CrossRef]
- Golombos, D.M.; Ayangbesan, A.; O’Malley, P.; Lewicki, P.; Barlow, L.; Barbieri, C.E.; Chan, C.; DuLong, C.; Abu-Ali, G.; Huttenhower, C.; et al. The Role of Gut Microbiome in the Pathogenesis of Prostate Cancer: A Prospective, Pilot Study. Urology 2018, 111, 122–128. [Google Scholar] [CrossRef]
- Trend, S.; Leffler, J.; Jones, A.P.; Cha, L.; Gorman, S.; Brown, D.A.; Breit, S.N.; Kermode, A.G.; French, M.A.; Ward, N.C.; et al. Associations of serum short-chain fatty acids with circulating immune cells and serum biomarkers in patients with multiple sclerosis. Sci. Rep. 2021, 11, 5244. [Google Scholar] [CrossRef]
- Jan, G.; Belzacq, A.; Haouzi, D.; Rouault, A.; Metivier, D.; Kroemer, G.; Brenner, C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef]
- Amann, R.I.; Binder, B.J.; Olson, R.J.; Chisholm, S.W.; Devereux, R.; Stahl, D.A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 1990, 56, 1919–1925. [Google Scholar] [CrossRef]
- Frank, D.N.; Pace, N.R. Molecular-phylogenetic analyses of human gastrointestinal microbiota. Curr. Opin. Gastroenterol. 2001, 17, 52–57. [Google Scholar] [CrossRef]
- Half, E.; Keren, N.; Reshef, L.; Dorfman, T.; Lachter, I.; Kluger, Y.; Reshef, N.; Knobler, H.; Maor, Y.; Stein, A.; et al. Fecal microbiome signatures of pancreatic cancer patients. Sci. Rep. 2019, 9, 16801. [Google Scholar] [CrossRef]
- Shrestha, E.; White, J.R.; Yu, S.H.; Kulac, I.; Ertunc, O.; De Marzo, A.M.; Yegnasubramanian, S.; Mangold, L.A.; Partin, A.W.; Sfanos, K.S. Profiling the Urinary Microbiome in Men with Positive versus Negative Biopsies for Prostate Cancer. J. Urol. 2018, 199, 161–171. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Markowski, M.C.; Peiffer, L.B.; Ernst, S.E.; White, J.R.; Pienta, K.J.; Antonarakis, E.S.; Ross, A.E. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018, 21, 539–548. [Google Scholar] [CrossRef]
- Dou, Y.; Ma, C.; Wang, K.; Liu, S.; Sun, J.; Tan, W.; Neckenig, M.; Wang, Q.; Dong, Z.; Gao, W.; et al. Dysbiotic tumor microbiota associates with head and neck squamous cell carcinoma outcomes. Oral. Oncol. 2022, 124, 105657. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.-A.; Mani, S. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef]
- Mazzotti, E.; Antonini Cappellini, G.C.; Buconovo, S.; Morese, R.; Scoppola, A.; Sebastiani, C.; Marchetti, P. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer 2012, 20, 2553–2557. [Google Scholar] [CrossRef]
- Orlando, A.; Refolo, M.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2. 1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef]
- Rafter, J.J. The role of lactic acid bacteria in colon cancer prevention. Scand. J. Gastroenterol. 1995, 30, 497–502. [Google Scholar] [CrossRef]
- Blaut, M. Relationship of prebiotics and food to intestinal microflora. Eur. J. Nutr. 2002, 41, i11–i16. [Google Scholar] [CrossRef]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. 2019. Available online: https://www.jmb.or.kr/journal/view.html?uid=5262&vmd=Full (accessed on 25 January 2024).
- Lan, A.; Lagadic-Gossmann, D.; Lemaire, C.; Brenner, C.; Jan, G. Acidic extracellular pH shifts colorectal cancer cell death from apoptosis to necrosis upon exposure to propionate and acetate major end-products of the human probiotic propionibacteria. Apoptosis 2007, 12, 573591. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Boonprasom, P.; Niamsup, P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol. Lett. 2009, 31, 571–576. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kanmani, P.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Thirunavukkarasu, C.; Arul, V. Lactobacillus plantarum AS1 isolated from south Indian fermented food Kallappam suppress 1,2-dimethyl hydrazine (DMH)-induced colorectal cancer in male Wistar rats. Appl. Biochem. Biotechnol. 2012, 166, 620–631. [Google Scholar] [CrossRef]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef]
- Maroof, H.; Hassan, Z.M.; Mobarez, A.M.; Mohamadabadi, M.A. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J. Clin. Immunol. 2012, 32, 1353–1359. [Google Scholar] [CrossRef]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-control Study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kahouli, I.; Malhotra, M.; Tomaro-Duchesneau, C.; Rodes, L.S.; Alaoui-Jamali, M.A.; Prakash, S. Identification of lactobacillus fermentum strains with potential against colorectal cancer by characterizing short chain fatty acids production, anti-proliferative activity and survival in an intestinal fluid: In vitro analysis. J. Bioanal. Biomed. 2015, 7, 4. [Google Scholar]
- Lee, H.A.; Kim, H.; Lee, K.-W.; Park, K.-Y. Dead nano-sized Lactobacillus plantarum inhibits azoxymethane/dextran sulfate sodium-induced colon cancer in Balb/c mice. J. Med. Food 2015, 18, 1400–1405. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, N.-K.; Park, H.; Paik, H.-D. Anticancer and anti-inflammatory activity of probiotic Lactococcus lactis NK34. J. Microbiol. Biotechnol. 2015, 25, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Fujiya, M.; Tanaka, H.; Ueno, N.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Akutsu, H.; Tanabe, H.; Kohgo, Y. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 2016, 7, 12365. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef] [PubMed]
- Jacouton, E.; Chain, F.; Sokol, H.; Langella, P.; Bermudez-Humaran, L.G. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front. Immunol. 2017, 8, 1553. [Google Scholar] [CrossRef]
- Kahouli, I.; Malhotra, M.; Westfall, S.; Alaoui-Jamali, M.A.; Prakash, S. Design and validation of an orally administrated active L. fermentum-L. acidophilus probiotic formulation using colorectal cancer Apc Min/+ mouse model. Appl. Microbiol. Biotechnol. 2017, 101, 1999–2019. [Google Scholar] [CrossRef]
- An, B.C.; Hong, S.; Park, H.J.; Kim, B.-K.; Ahn, J.Y.; Ryu, Y.; An, J.H.; Chung, M.J. Anti-colorectal cancer effects of probiotic-derived p8 protein. Genes 2019, 10, 624. [Google Scholar] [CrossRef]
- Aghazadeh, Z.; Pouralibaba, F.; Khosroushahi, A.Y. The prophylactic effect of Acetobacter syzygii probiotic species against squamous cell carcinoma. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 208. [Google Scholar]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Yassin, A.M.; Al-Madboly, L.A.; El-Hawiet, A. A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-κB inflammatory pathways in human colon cancer. Microb. Cell Factories 2018, 17, 29. [Google Scholar] [CrossRef]
- Jacouton, E.; Torres Maravilla, E.; Boucard, A.-S.; Pouderous, N.; Pessoa Vilela, A.P.; Naas, I.; Chain, F.; Azevedo, V.; Langella, P.; Bermúdez-Humarán, L.G. Anti-tumoral Effects of Recombinant Lactococcus lactis Strain Secreting IL-17A Cytokine. Front. Microbiol. 2018, 9, 3355. [Google Scholar] [CrossRef]
- Tarrah, A.; Castilhos, J.d.; Rossi, R.C.; Duarte, V.d.S.; Ziegler, D.R.; Corich, V.; Giacomini, A. In vitro probiotic potential and anti-cancer activity of newly isolated folate-producing Streptococcus thermophilus strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Li, Q.; Tian, K.; Xu, L.; Liu, G.; Guo, C. Exopolysaccharide, isolated from a novel strain Bifidobacterium breve lw01 possess an anticancer effect on head and neck cancer–genetic and biochemical evidences. Front. Microbiol. 2019, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Chung, I.-C.; OuYang, C.-N.; Yuan, S.-N.; Lin, H.-C.; Huang, K.-Y.; Wu, P.-S.; Liu, C.-Y.; Tsai, K.-J.; Loi, L.-K.; Chen, Y.-J. Pretreatment with a heat-killed probiotic modulates the NLRP3 inflammasome and attenuates colitis-associated colorectal cancer in mice. Nutrients 2019, 11, 516. [Google Scholar] [CrossRef]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef]
- Ghanavati, R.; Asadollahi, P.; Shapourabadi, M.B.; Razavi, S.; Talebi, M.; Rohani, M. Inhibitory effects of Lactobacilli cocktail on HT-29 colon carcinoma cells growth and modulation of the Notch and Wnt/β-catenin signaling pathways. Microb. Pathog. 2020, 139, 103829. [Google Scholar] [CrossRef]
- Maghsood, F.; Johari, B.; Rohani, M.; Madanchi, H.; Saltanatpour, Z.; Kadivar, M. Anti-proliferative and anti-metastatic potential of high molecular weight secretory molecules from probiotic Lactobacillus reuteri cell-free supernatant against human colon cancer stem-like cells (HT29-ShE). Int. J. Pept. Res. Ther. 2020, 26, 2619–2631. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Movassaghpour, A.A.; Talebi, M.; Gargari, B.P. Modulatory role of exopolysaccharides of Kluyveromyces marxianus and Pichia kudriavzevii as probiotic yeasts from dairy products in human colon cancer cells. J. Funct. Foods 2020, 64, 103675. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Chen, H.; Hong, L.; Feng, J.; Yang, J.; Yang, Z.; Shi, C.; Wu, W.; Gao, R.; et al. The effect of perioperative probiotics treatment for colorectal cancer: Short-term outcomes of a randomized controlled trial. Oncotarget 2016, 7, 8432–8440. [Google Scholar] [CrossRef]
- Luo, M.; Hu, M.; Feng, X.; Xiao Li, W.; Dong, D.; Wang, W. Preventive effect of Lactobacillus reuteri on melanoma. Biomed. Pharmacother. 2020, 126, 109929. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, F.; Chen, S.; Cen, P.; Yang, Q.; Guan, J.; Cen, L.; Zhang, T.; Zhu, H.; Chen, Z. Lactobacillus reuteri improves function of the intestinal barrier in rats with acute liver failure through Nrf-2/HO-1 pathway. Nutrition 2022, 99–100, 111673. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, N.; Luo, Q.; Jiang, L.; He, B.; Yuan, X.; Shen, L. Probiotics Lactobacillus reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells. Front. Immunol. 2019, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Akaza, H.; Kotake, T.; Tsukamoto, T.; Imai, K.; Naito, S.; Group, B.S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. Eur. Urol. 1995, 27, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int. J. Mol. Sci. 2008, 9, 854–863. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Gao, Y.; Xue, Y.; Yan, Y.; Guo, X. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef]
- Xie, X.; He, Y.; Li, H.; Yu, D.; Na, L.; Sun, T.; Zhang, D.; Shi, X.; Xia, Y.; Jiang, T. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition 2019, 61, 132–142. [Google Scholar] [CrossRef]
- Krebs, B. Prebiotic and Synbiotic Treatment before Colorectal Surgery--Randomised Double Blind Trial. Coll. Antropol. 2016, 40, 35–40. [Google Scholar]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal Consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Silverman, M.S.; Davis, I.; Pillai, D.R. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2010, 8, 471–473. [Google Scholar] [CrossRef]
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: A population-based case-control pilot study. J. Natl. Cancer Inst. 2015, 107, djv147. [Google Scholar] [CrossRef]
- Molteni, A.; Brizio-Molteni, L.; Persky, V. In vitro hormonal effects of soybean isoflavones. J. Nutr. 1995, 125, 751S–756S. [Google Scholar] [PubMed]
- Sambrani, R.; Abdolalizadeh, J.; Kohan, L.; Jafari, B. Recent advances in the application of probiotic yeasts, particularly Saccharomyces, as an adjuvant therapy in the management of cancer with focus on colorectal cancer. Mol. Biol. Rep. 2021, 48, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Masuno, T.; Kishimoto, S.; Ogura, T.; Honma, T.; Niitani, H.; Fukuoka, M.; Ogawa, N. A comparative trial of LC9018 plus doxorubicin and doxorubicin alone for the treatment of malignant pleural effusion secondary to lung cancer. Cancer 1991, 68, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Österlund, P.; Ruotsalainen, T.; Peuhkuri, K.; Korpela, R.; Ollus, A.; Ikonen, M.; Joensuu, H.; Elomaa, I. Lactose intolerance associated with adjuvant 5-fluorouracil-based chemotherapy for colorectal cancer. Clin. Gastroenterol. Hepatol. 2004, 2, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Giralt, J.; Regadera, J.P.; Verges, R.; Romero, J.; de la Fuente, I.; Biete, A.; Villoria, J.; Cobo, J.M.; Guarner, F. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: Results from multicenter, randomized, placebo-controlled nutritional trial. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1213–1219. [Google Scholar] [CrossRef]
- Chitapanarux, I.; Chitapanarux, T.; Traisathit, P.; Kudumpee, S.; Tharavichitkul, E.; Lorvidhaya, V. Randomized controlled trial of live lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 2010, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Holma, R.; Korpela, R.; Sairanen, U.; Blom, M.; Rautio, M.; Poussa, T.; Saxelin, M.; Osterlund, P. Colonic methane production modifies gastrointestinal toxicity associated with adjuvant 5-fluorouracil chemotherapy for colorectal cancer. J. Clin. Gastroenterol. 2013, 47, 45–51. [Google Scholar] [CrossRef]
- Ki, Y.; Kim, W.; Nam, J.; Kim, D.; Lee, J.; Park, D.; Jeon, H.; Ha, H.; Kim, T.; Kim, D. Probiotics for rectal volume variation during radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 646–650. [Google Scholar] [CrossRef]
- Shao, F.; Xin, F.-Z.; Yang, C.-G.; Yang, D.-G.; Mi, Y.-T.; Yu, J.-X.; Li, G.-Y. The impact of microbial immune enteral nutrition on the patients with acute radiation enteritis in bowel function and immune status. Cell Biochem. Biophys. 2014, 69, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Dagnault, A.; Desjardins, J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 2014, 33, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Golkhalkhali, B.; Rajandram, R.; Paliany, A.S.; Ho, G.F.; Wan Ishak, W.Z.; Johari, C.S.; Chin, K.F. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: A randomized controlled trial. Asia-Pac. J. Clin. Oncol. 2018, 14, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Motoori, M.; Yano, M.; Miyata, H.; Sugimura, K.; Saito, T.; Omori, T.; Fujiwara, Y.; Miyoshi, N.; Akita, H.; Gotoh, K.; et al. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin. Nutr. 2017, 36, 93–99. [Google Scholar] [CrossRef]
- Linn, Y.H.; Thu, K.K.; Win, N.H.H. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: A randomized double-blind placebo-controlled study. Probiotics Antimicrob. Proteins 2019, 11, 638–647. [Google Scholar] [CrossRef]
- De Sanctis, V.; Belgioia, L.; Cante, D.; La Porta, M.R.; Caspiani, O.; Guarnaccia, R.; Argenone, A.; Muto, P.; Musio, D.; De Felice, F. Lactobacillus brevis CD2 for prevention of oral mucositis in patients with head and neck tumors: A multicentric randomized study. Anticancer. Res. 2019, 39, 1935–1942. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; Su, Y.; Xie, H.; Zeng, L.; Kuang, J. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef]
- Bajramagic, S.; Hodzic, E.; Mulabdic, A.; Holjan, S.; Smajlovic, S.V.; Rovcanin, A. Usage of Probiotics and its Clinical Significance at Surgically Treated Patients Sufferig from Colorectal Carcinoma. Med. Arch. 2019, 73, 316–320. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Boutell, S.; Taylor, M.W.; Douglas, R.G.; Biswas, K. Randomised, double-blind, placebo-controlled trial of oral probiotic Streptococcus salivarius M18 on head and neck cancer patients post-radiotherapy: A pilot study. Sci. Rep. 2020, 10, 13201. [Google Scholar] [CrossRef]
- Doppalapudi, R.; Vundavalli, S.; Prabhat, M. Effect of probiotic bacteria on oral Candida in head-and neck-radiotherapy patients: A randomized clinical trial. J. Cancer Res. Ther. 2020, 16, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, T.; Lu, J.; Wei, K.; Tian, H.; Liu, W.; Xu, T.; Wang, X.; Wang, S.; Yang, R.; et al. Adjuvant treatment and molecular mechanism of probiotic compounds in patients with gastric cancer after gastrectomy. Food Funct. 2021, 12, 6294–6308. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef]
- Lin, B.; Zhao, F.; Liu, Y.; Wu, X.; Feng, J.; Jin, X.; Yan, W.; Guo, X.; Shi, S.; Li, Z.; et al. Randomized Clinical Trial: Probiotics Alleviated Oral-Gut Microbiota Dysbiosis and Thyroid Hormone Withdrawal-Related Complications in Thyroid Cancer Patients Before Radioiodine Therapy Following Thyroidectomy. Front. Endocrinol. 2022, 13, 834674. [Google Scholar] [CrossRef] [PubMed]
- Motoori, M.; Sugimura, K.; Tanaka, K.; Shiraishi, O.; Kimura, Y.; Miyata, H.; Yamasaki, M.; Makino, T.; Miyazaki, Y.; Iwama, M.; et al. Comparison of synbiotics combined with enteral nutrition and prophylactic antibiotics as supportive care in patients with esophageal cancer undergoing neoadjuvant chemotherapy: A multicenter randomized study. Clin. Nutr. 2022, 41, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients. Nutrients 2023, 15, 356. [Google Scholar] [CrossRef] [PubMed]
| Biomarkers | Cancer Type | Abundance | Ref. |
|---|---|---|---|
| Veillonella, Fusobacterium, Prevotella, Porphyromonas, Actinomyces and Clostridium, Haemophilus, Enterobacteriaceae and Streptococcus spp. and Candida albicans | Oral carcinoma | Elevated in tumor sites | [39] |
| Capnocytophaga gingivalis, Prevotella melaninogenica and Streptococcus mitis | Oral squamous cell carcinoma (OSCC) | Elevated in the saliva of individuals with OSCC | [40] |
| Fusobacterium nucleatum, S salivarius: Streptococcus vestibularis, Prevotella oris, and Rothia mucilaginosa | Head and neck squamous cell carcinoma (HNSCC) | HNSCC patients had a significant loss in richness and diversity of microbiota species | [41] |
| Fusobacterium nucleatum | HNSCC | Elevated in the saliva | [42] |
| Corynebacterium and Kingella | HNSCC | Greater oral abundance of these commensals is associated with decreased risk of HNSCC | [43] |
| Capnocytophaga, Pseudomonas, and Atopobium | OSCC | Highly abundant in biopsy tissue | [44] |
| Lautropia, Staphylococcus, and Propionibacterium | Fibroepithelial polyp | Highly abundant in biopsy tissue | [44] |
| Treponema denticola, Streptococcus mitis, and Streptococcus anginosus | Esophageal cancer | Induction of inflammatory cytokines | [45] |
| Group G streptococci | Colon cancer | Large amount of pericardial effusion | [46] |
| Fusobacterium nucleatum | Colorectal carcinoma | Over-representation in tumor specimen | [47] |
| Neisseria elongate, Streptococcus mitis and Granulicatella adiacen | Pancreatic cancer and chronic pancreatitis | Salivary microbiota as an informative source for discovering noninvasive biomarkers of systemic diseases | [48] |
| Helicobacter pylori | Dysplasia or gastric cancer | Presence of H. pylori at baseline was associated with an increased risk of progression to dysplasia or gastric cancer | [49] |
| Streptococcus and Bacteroides species (Bacteroides massiliensis) | Prostate cancer | Higher relative abundance in prostate cancer cases compared to controls. | [26,50] |
| Probiotics | Cancer Type | Study Model | Effect | Ref. |
|---|---|---|---|---|
| Propionibacterium freuden reichii | Colorectal cancer | HT-29 cells | Induced cell cycle arrest in the G2/M phase | [70] |
| Enterococcus faecium RM11, Lactobacillus fermentum RM28 | Colon cancer | Caco-2 cells | Triggered antiproliferation of colon cancer cells | [71] |
| Yogurt probiotics | Colorectal cancer | Clinical trial | [72] | |
| Lactobacillus plantarum AS1 | Colon cancer | Rat | Antioxidant-dependent mechanism | [73] |
| Lactobacillus casei and Lactobacillus rhamnosus GG | Colorectal cancer | HCT-116 cells | Decreased metalloproteinase-9 activity and increased levels of tight junction protein zona occludens-1 | [74] |
| Lactobacillus plantarum AS1 | Colorectal cancer | Male Wistar rats | Antioxidant property reduced tumor volume diameter and total number of tumors | [73] |
| Lactobacillus acidophilus | Breast cancer | Balb/C inbred female mice | Induces production of IFNγ, IL-4, and TGF-β | [75] |
| L. casei Shirota | Breast cancer | Case –control study | NK cell activation and NK cell-mediated antitumor activity | [76] |
| Lactobacillus casei Shirota | Breast cancer | Population-based case –control study | Enhanced NK cell activity-mediated antitumor activity | [76] |
| Lactobacillus fermentum | Colorectal cancer | Caco-2 colon cancer cell | Antiproliferative activity | [77] |
| Dead nano-sized Lactobacillus plantarum | Colon cancer | Balb/c mice | Suppressed inflammation, induced cell cycle arrest and apoptosis, and enhanced IgA secretion | [78] |
| Lactobacillus lactis NK34 | Lung, colon, gastric adenocarcinoma, breast cancer | SK-MES-1, DLD-1, HT-29, LoVo, AGS, MCF-7, and RAW 264.7 cells | Reduced production of nitric oxide and proinflammatory cytokines (tumor necrosis factor-α, interleukin-18, and cyclooxygenase-2) | [79] |
| Lactobacillus casei ATCC334 | Colon cancer | Human colon cancer cell lines (Caco2bbe, SKCO-1, and SW620) and Xenografts (SW620 cells injected into male BALB/c nude mice) | Ferrichrome-induced apoptosis by activating C-jun N-terminal kinase and suppressed tumor growth | [80] |
| Lactobacillus casei ATCC 393 | Colon cancer | Murine (CT26) and human (HT29) colon carcinoma cell lines | Apoptotic cell death and upregulation of TRAIL in colon carcinoma cells | [81] |
| Lactobacillus casei BL23 | Colitis-associated colorectal cancer | C57BL6 mice | Immunomodulatory effect, mediated through the downregulation of the IL-22 cytokine, and an antiproliferative effect, mediated through the upregulation of caspase-7, caspase-9, and Bik | [82] |
| Lactobacillus acidophilus ATCC 314 and Lactobacillus fermentum NCIMB 5221 | Colorectal cancer | Apc Min/+ CRC mouse | Downregulated proliferation markers (Ki-67, E-cadherin, β-catenin) | [83] |
| Lactobacillus reuteri NCIMB 701,359 | Colorectal cancer | DLD-1 cell line | Probiotic-derived protein, p8, inhibits p53-p21-Cyclin B1/Cdk1 signal pathway | [84] |
| Acetobacter syzygii | Squamous cell carcinoma | Human oral cancer (KB) and human normal epithelial (KDR) cell lines | Induced apoptosis | [85] |
| Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium | Metastatic melanoma | Melanoma patients | Enhanced systemic and antitumor immune responses mediated by increased antigen presentation and improved effector T cell function | [86] |
| Akkermansia muciniphila | Non-small-cell lung cancer, renal cell cancer, and urothelial cancer | Mice | Increasing the recruitment of CCR9+CXCR3+CD4+ T lymphocytes | [87] |
| Lactobacillus acidophilus 20079 | Colon cancer | Colon cancer (CaCo-2) and human breast cancer (MCF7) cell lines | Increased apoptosis in sub-G0/G1 cell cycle phase, stimulated immune response, and inactivated NF-κB inflammatory pathway | [88] |
| Recombinant Lactococcus lactis | Mouse allograft model of human papilloma virus (HPV)-induced cancer and TC-1 cell line | Secreting IL-17 to stimulate the TH17 pathway | [89] | |
| Streptococcus thermophilus | Colorectal cancer | HT-29 human colorectal adenocarcinoma cells | High production of folic acid, tyramine, and histamine; high cytotoxic to cancer cells | [90] |
| Bifidobacterium breve lw01 | Head and neck cancer | SCC15, CAL 27, and WSU-HN6 cell lines | Increased expression of cell apoptosis protein caspase 3 and PARP and increased proportion of Cl-PARP/PARP | [91] |
| Lactobacillus and Bifidobacteria strain | CRC | Randomized double-blind placebo-controlled trial | Interfered with the signaling pathways to stimulate or suppress the level of cytokine production | [92] |
| Enterococcus faecalis | Colorectal cancer | C57BL/6 mice | Inhibited NLRP3 inflammasome activation in macrophages | [93] |
| Lactobacillus delbrueckii ssp. bulgaricus B3 | Colon cancer | HT-29 cells | Inhibited cell proliferation in HT-29 via apoptosis | [94] |
| Recombinant Lactococcus lactis | Colorectal cancer | Murine fibroblast 3T3 L1 cell lines and mouse allograft model of human papilloma virus-induced cancer | Efficiently secretes biologically active IL-17A cytokines | [89] |
| Lactobacilli cocktail | Colorectal cancer | HT-29 colon carcinoma cells | Antitumor effects on HT-29 cells by modulating the Notch and Wnt/β-catenin pathways | [95] |
| Lactobacillus reuteri | Colon Cancer | Colon cancer stem-like cells (HT29-ShE) | Antimetastatic and antiproliferative | [96] |
| Kluyveromyces marxianus and Pichia kudriavzevii | Colon cancer | Colon cancer cell lines (SW-480, HT-29, HCT-116) | Hindered AKT-1, mTOR, and JAK-1 pathways and induced apoptosis | [97] |
| Probiotics | Cancer Type (Year) | Effect and Results | Ref. |
|---|---|---|---|
| Lactobacillus casei LC9018 | Lung cancer (malignant pleural effusions secondary to lung cancer) (1991) |
| [113] |
| Lactobacillus rhamnosus GG ATCC 53103 | Colorectal cancer (lactose intolerance associated with adjuvant 5-fluorouracil-based chemotherapy) (2007) |
| [114] |
| Lactobacillus casei DN-114 001 | Endometrial adenocarcinoma patients (2008) |
| [115] |
| Lactobacillus acidophilus plus Bifidobacterium bifidum | Cervical cancer (2010) |
| [116] |
| Lactobacillus rhamnosus GG | Colorectal cancer (2013) |
| [117] |
| Lactobacillus acidophilus | Prostate cancer (2013) |
| [118] |
| Bifidobacterium, Lactobacillus and Streptococcus thermophilus | Acute radiation enteritis (2014) |
| [119] |
| Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei DN-114001 and Bifidobacterium bifidum | Abdominal or pelvic cancer (2014) |
| [120] |
| Lactobacillus acidophilus BMC12130, Lactobacillus casei BCMC12313, Lactobacillus lactis BCMC12451, Bifidobacterium bifidum BCMC02290, Bifidobacterium longum BCMC02120 and Bifidobacterium infantis BCMC02129 | Colorectal cancer (2017) |
| [121] |
| Bifidobacterium breve strain Yakult, Lactobacillus casei strain Shirota | Esophageal cancer (2017) |
| [122] |
| Lactobacillus acidophilus LA-5 plus Bifidobacterium animalis subsp. lactis BB-12 | Cervical cancer (2019) |
| [123] |
| Lactobacillus brevis CD2 | Head and neck cancer (2019) |
| [124] |
| Bifidobacterium longum, Lactobacillus lactis and Enterococcus faecium | Nasopharyngeal carcinoma (2019) |
| [125] |
| Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium lactis, Bifidobacterium bifidum, Bifidobacterium breve, Streptococcus thermophilus | Colorectal cancer (2019) |
| [126] |
| Streptococcus salivarius M18 | Head and neck cancer (2020) |
| [127] |
| Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum and Saccharomyces boulardii | Head and neck cancer (2020) |
| [128] |
| Lactobacillus plantarum MH-301 (CGMCC NO. 18618), L. rhamnosus LGG-18 (CGMCC NO. 14007), Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis LPL-RH (CGMCC NO. 4599) | Gastric cancer (2021) |
| [129] |
| Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis | Breast cancer (2022) |
| [130] |
| Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus | Thyroid cancer (2022) |
| [131] |
| Lacticaseibacillus paracasei strain Shirota (YIT9029), Bifidobacterium breve strain Yakult, | Esophageal cancer (2022) |
| [132] |
| Bifidobacterium infantis, L. acidophilus, Enterococcus faecalis, and Bacillus cereus | Colorectal cancer (2023) |
| [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, R.; Singh, S.K. The Microbiome Modulates the Immune System to Influence Cancer Therapy. Cancers 2024, 16, 779. https://doi.org/10.3390/cancers16040779
Roy R, Singh SK. The Microbiome Modulates the Immune System to Influence Cancer Therapy. Cancers. 2024; 16(4):779. https://doi.org/10.3390/cancers16040779
Chicago/Turabian StyleRoy, Ruchi, and Sunil Kumar Singh. 2024. "The Microbiome Modulates the Immune System to Influence Cancer Therapy" Cancers 16, no. 4: 779. https://doi.org/10.3390/cancers16040779
APA StyleRoy, R., & Singh, S. K. (2024). The Microbiome Modulates the Immune System to Influence Cancer Therapy. Cancers, 16(4), 779. https://doi.org/10.3390/cancers16040779





