Noncoding RNAs in Hepatocellular Carcinoma: Potential Applications in Combined Therapeutic Strategies and Promising Candidates of Treatment Response
Abstract
Simple Summary
Abstract
1. Introduction
2. Role of Noncoding RNAs in Hepatocarcinogenesis
3. Combination of Noncoding RNA-Based Strategies with TKIs in HCC
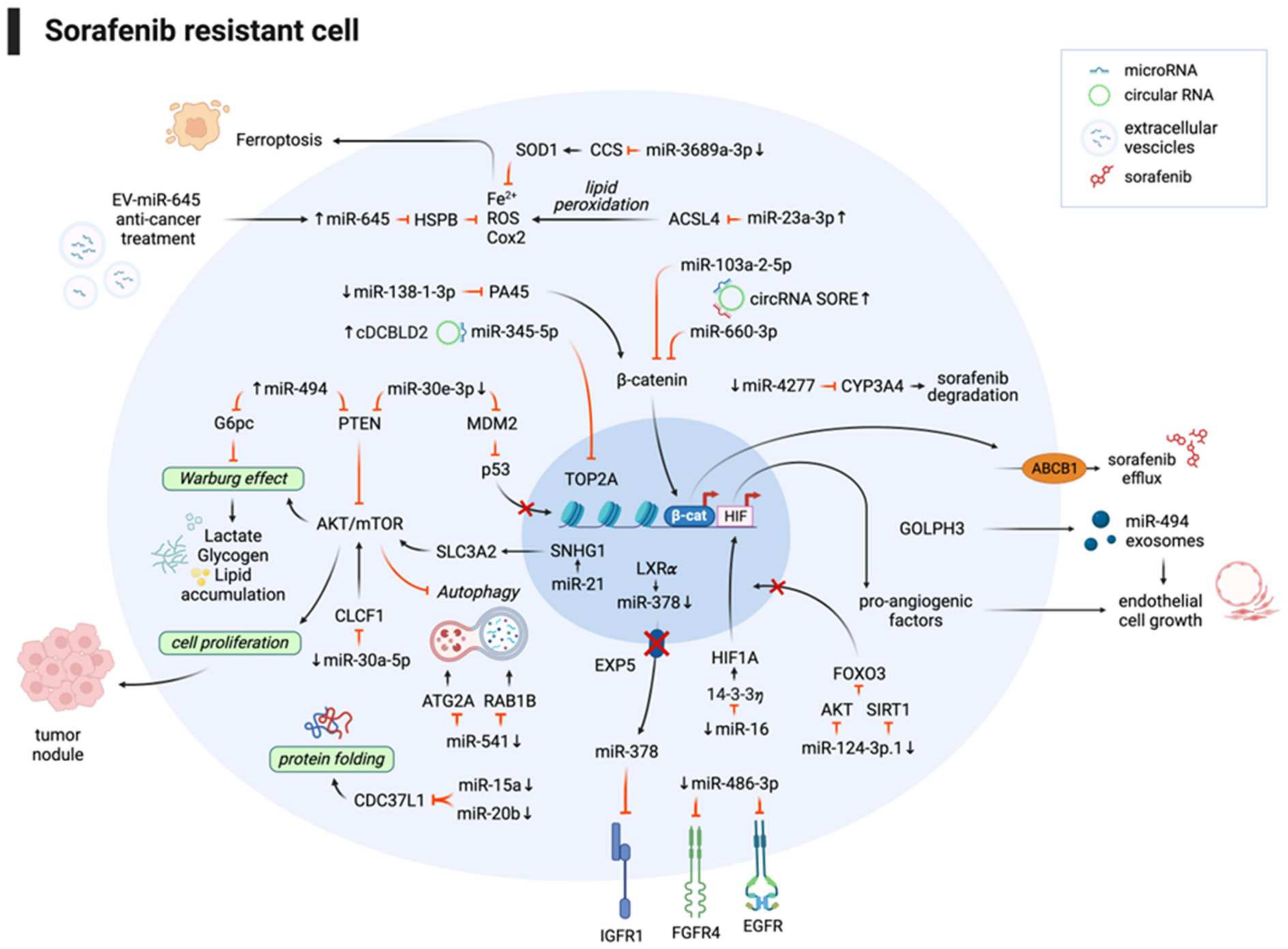
3.1. Noncoding RNAs and Sorafenib Combination Improves the Therapeutic Response
3.1.1. CRISPR/Cas9 High-Throughput Screening Identifies miRNAs with a Role in Sorafenib Sensitization
3.1.2. Noncoding RNAs Affect Sorafenib Response by Modulating Hypoxia-Related Signaling and Angiogenesis
3.1.3. Noncoding RNAs Affect Sorafenib Response by Interfering with Tumor Cell Metabolism
3.1.4. Noncoding RNAs Affect Sorafenib Response by Interfering with Ferroptosis
3.1.5. Noncoding RNAs Affect Sorafenib Response by Activating Oncogenic Pathways
3.1.6. Noncoding RNAs Affect Sorafenib Response by Modulating Autophagy
3.1.7. Noncoding RNAs Affect Sorafenib Response by Modulating Its Metabolism and Extrusion
3.2. Noncoding RNAs and lenvatinib Combination Improves the Therapeutic Response
3.2.1. MicroRNAs Affect Lenvatinib Response by Modulating Oncogenic Pathways
3.2.2. Circular RNAs and Long Noncoding RNAs Affect Lenvatinib Response by Modulating Oncogenic Pathways
3.2.3. MicroRNAs Exert Antitumor Effects Comparable to Lenvatinib Treatment
4. Combination of ncRNA-Based Strategies and ICIs Improves Therapeutic Efficacy in HCC Preclinical Models
4.1. MiRNAs Modulate Gene Expression in Immune System Cells, Preventing Tumor Development
4.2. Noncoding RNAs Affect Immunotherapy Response by Interfering with Tumor Cell Metabolism
4.3. Noncoding RNAs Affect Immunotherapy Response by Interfering with CD8+ T Cells Recruitment
4.4. Noncoding RNAs Affect Immunotherapy Response by Mediating Cell–Cell Interactions
5. Noncoding RNAs As Biomarkers of Treatment Response in HCC
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | hepatocellular carcinoma |
| TKIs | tyrosine kinase inhibitors |
| ICIs | immune checkpoint inhibitors |
| OS | overall survival |
| AFP | alpha fetoprotein |
| NASH | nonalcoholic steatohepatitis |
| ncRNAs | noncoding RNAs |
| miRNAs | microRNAs |
| lncRNAs | long noncoding RNAs |
| circRNAs | circular RNAs |
| ceRNA | competing endogenous RNA |
| TS | tumor suppressor |
| PDX | patient-derived xenograft |
| PDO | patient-derived organoid |
| EMT | epithelial–mesenchymal transition |
| DFS | disease-free survival |
| HCV | hepatitis C virus |
| Atezo/Beva | atezolizumab/bevacizumab |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| Cas9 | CRISPR-associated protein 9 |
| ROS | reactive oxygen species |
| DEN | diethylnitrosamine |
| SR | sorafenib-resistant |
| MVI | microvascular invasion |
| EV | extracellular vesicle |
| shRNA | short hairpin RNA |
| LR | lenvatinib-resistant |
| T-ICs | tumor-initiating cells |
| Treg | T regulatory lymphocytes |
| NAFLD | non-alcoholic fatty liver disease |
| TME | tumor microenvironment |
| NK | natural killer |
| DCs | dendritic cells |
| KCs | Kupffer cells |
| CTLs | cytotoxic T lymphocytes |
| PD1 | programmed cell death protein 1 |
| FA | fatty acids |
| TIDE | tumor immune dysfunction and exclusion |
| PD-L1 | programmed death-ligand 1 |
| HBV | hepatitis B virus |
| OE | overexpressing |
| HuNSG | humanized NOD/SCID gamma |
| IFN-γ | interferon gamma |
| TNF-α | tumor necrosis factor alpha |
| LPCs | liver progenitor cells |
| BCLC-C | Barcelona Clinic Liver Cancer staging system—stage C |
| ELISA | enzyme-linked immunosorbent assay |
| qPCR | quantitative polymerase chain reaction |
| ddPCR | digital droplet polymerase chain reaction |
| RNAseq | RNA sequencing |
| C19MC | chromosome 19 miRNA cluster |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after Sorafenib in Patients with Advanced Hepatocellular Carcinoma and Increased α-Fetoprotein Concentrations (REACH-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 Randomized, Open-Label, Multicenter Study of Tremelimumab (T) and Durvalumab (D) as First-Line Therapy in Patients (Pts) with Unresectable Hepatocellular Carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022, 40, 379. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH Limits Anti-Tumour Surveillance in Immunotherapy-Treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef]
- Afra, F.; Mahboobipour, A.A.; Salehi Farid, A.; Ala, M. Recent Progress in the Immunotherapy of Hepatocellular Carcinoma: Non-Coding RNA-Based Immunotherapy May Improve the Outcome. Biomed. Pharmacother. 2023, 165, 115104. [Google Scholar] [CrossRef] [PubMed]
- Fornari, F.; Giovannini, C.; Piscaglia, F.; Gramantieri, L. Elucidating the Molecular Basis of Sorafenib Resistance in HCC: Current Findings and Future Directions. J. Hepatocell. Carcinoma 2021, 8, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Makunin, I.V. Non-Coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Non-Coding RNAs in Human Disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-Coding RNAs in Disease: From Mechanisms to Therapeutics. Nat. Rev. Genet. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wei, T.; Yan, L.; Zhu, S.; Jin, W.; Bai, Y.; Zeng, Y.; Zhang, X.; Yin, Z.; Yang, J.; et al. Hypoxia-Responsive lncRNA AC115619 Encodes a Micropeptide That Suppresses m6A Modifications and Hepatocellular Carcinoma Progression. Cancer Res. 2023, 83, 2496–2512. [Google Scholar] [CrossRef]
- Gramantieri, L.; Baglioni, M.; Fornari, F.; Laginestra, M.A.; Ferracin, M.; Indio, V.; Ravaioli, M.; Cescon, M.; De Pace, V.; Leoni, S.; et al. LncRNAs as Novel Players in Hepatocellular Carcinoma Recurrence. Oncotarget 2018, 9, 35085–35099. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, J.-K.; Peng, Y.; He, W.; Huang, C. The Role of Long Noncoding RNAs in Hepatocellular Carcinoma. Mol. Cancer 2020, 19, 77. [Google Scholar] [CrossRef]
- Cai, Y.; Lyu, T.; Li, H.; Liu, C.; Xie, K.; Xu, L.; Li, W.; Liu, H.; Zhu, J.; Lyu, Y.; et al. LncRNA CEBPA-DT Promotes Liver Cancer Metastasis through DDR2/β-Catenin Activation via Interacting with hnRNPC. J. Exp. Clin. Cancer Res. 2022, 41, 335. [Google Scholar] [CrossRef]
- Wang, F.; Hu, Y.; Wang, H.; Hu, P.; Xiong, H.; Zeng, Z.; Han, S.; Wang, D.; Wang, J.; Zhao, Y.; et al. LncRNA FTO-IT1 Promotes Glycolysis and Progression of Hepatocellular Carcinoma through Modulating FTO-Mediated N6-Methyladenosine Modification on GLUT1 and PKM2. J. Exp. Clin. Cancer Res. 2023, 42, 267. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Tu, X.; Li, H.; Cao, P.; Chen, X.; Song, J.; Han, H.; Li, Y.; Guo, B.; Yang, L.; et al. Long Noncoding RNA P53-Stabilizing and Activating RNA Promotes P53 Signaling by Inhibiting Heterogeneous Nuclear Ribonucleoprotein K deSUMOylation and Suppresses Hepatocellular Carcinoma. Hepatology 2020, 71, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, J.; Yang, F.; Tao, Q.; Zhang, J.; Wang, L.; Yang, Y.; Liu, H.; Wang, Z.; Xu, Q.; et al. Long Noncoding RNA DANCR Increases Stemness Features of Hepatocellular Carcinoma by Derepression of CTNNB1. Hepatology 2016, 63, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-Y.; Cai, Z.-R.; Liu, J.; Wang, D.-S.; Ju, H.-Q.; Xu, R.-H. Circular RNA: Metabolism, Functions and Interactions with Proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Ma, S.; Xu, J.; Ren, X.; Guo, P.; Liu, H.; Li, P.; Yin, F.; Liu, M.; Wang, Q.; et al. A Novel Polypeptide Encoded by the Circular RNA ZKSCAN1 Suppresses HCC via Degradation of mTOR. Mol. Cancer 2023, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Li, S.; Zhou, Y.; Disoma, C.; Liao, Y.; Zhang, Y.; Chen, Z.; Yang, Q.; Liu, P.; Liu, S.; et al. M6A-Mediated Upregulation of circMDK Promotes Tumorigenesis and Acts as a Nanotherapeutic Target in Hepatocellular Carcinoma. Mol. Cancer 2022, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liang, M.; Liu, H.; Huang, J.; Li, P.; Wang, C.; Zhang, Y.; Lin, Y.; Jiang, X. CircRNA hsa_circRNA_104348 Promotes Hepatocellular Carcinoma Progression through Modulating miR-187-3p/RTKN2 Axis and Activating Wnt/β-Catenin Pathway. Cell Death Dis. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Z.-Q.; Yu, S.-Y.; Mao, L.; Zhou, Z.-J.; Wang, P.-C.; Gong, Y.; Su, S.; Zhou, J.; Fan, J.; et al. CircRPN2 Inhibits Aerobic Glycolysis and Metastasis in Hepatocellular Carcinoma. Cancer Res. 2022, 82, 1055–1069. [Google Scholar] [CrossRef]
- Wang, L.; Long, H.; Zheng, Q.; Bo, X.; Xiao, X.; Li, B. Circular RNA circRHOT1 Promotes Hepatocellular Carcinoma Progression by Initiation of NR2F6 Expression. Mol. Cancer 2019, 18, 119. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; He, L.; Zhang, J.; Zhu, X.; Liu, N.; Wang, J.; Lu, T.; He, L.; Tian, Y.; et al. Circular RNA circIPO11 Drives Self-Renewal of Liver Cancer Initiating Cells via Hedgehog Signaling. Mol. Cancer 2021, 20, 132. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Huang, Z.-L.; Huang, J.; Xu, B.; Huang, X.-Y.; Xu, Y.-H.; Zhou, J.; Tang, Z.-Y. Exosomal circRNA-100338 Promotes Hepatocellular Carcinoma Metastasis via Enhancing Invasiveness and Angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 20. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many Roads to Maturity: microRNA Biogenesis Pathways and Their Regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, C.; Bi, Z.-Y.; Zhou, Q.; Zhang, H.; Li, L.-L.; Zhang, J.; Zhu, W.; Song, Y.-Y.-Y.; Zhang, F.; et al. Comprehensive Landscape of Extracellular Vesicle-Derived RNAs in Cancer Initiation, Progression, Metastasis and Cancer Immunology. Mol. Cancer 2020, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, H.; Dai, B.; Li, J.; Shang, L.; Huang, J.; Shi, X. Hepatocellular Carcinoma-Derived Exosomal miRNA-21 Contributes to Tumor Progression by Converting Hepatocyte Stellate Cells to Cancer-Associated Fibroblasts. J. Exp. Clin. Cancer Res. 2018, 37, 324. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, S.K.; Alahari, S.K. MicroRNA Function in Cancer: Oncogene or a Tumor Suppressor? Cancer Metastasis Rev. 2009, 28, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Fornari, F.; Milazzo, M.; Galassi, M.; Callegari, E.; Veronese, A.; Miyaaki, H.; Sabbioni, S.; Mantovani, V.; Marasco, E.; Chieco, P.; et al. P53/Mdm2 Feedback Loop Sustains miR-221 Expression and Dictates the Response to Anticancer Treatments in Hepatocellular Carcinoma. Mol. Cancer Res. 2014, 12, 203–216. [Google Scholar] [CrossRef]
- Gramantieri, L.; Pollutri, D.; Gagliardi, M.; Giovannini, C.; Quarta, S.; Ferracin, M.; Casadei-Gardini, A.; Callegari, E.; De Carolis, S.; Marinelli, S.; et al. MiR-30e-3p Influences Tumor Phenotype through MDM2/TP53 Axis and Predicts Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Res. 2020, 80, 1720–1734. [Google Scholar] [CrossRef]
- Salvi, A.; Abeni, E.; Portolani, N.; Barlati, S.; De Petro, G. Human Hepatocellular Carcinoma Cell-Specific miRNAs Reveal the Differential Expression of miR-24 and miR-27a in Cirrhotic/Non-Cirrhotic HCC. Int. J. Oncol. 2013, 42, 391–402. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA Signatures in Human Cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Gramantieri, L.; Ferracin, M.; Fornari, F.; Veronese, A.; Sabbioni, S.; Liu, C.-G.; Calin, G.A.; Giovannini, C.; Ferrazzi, E.; Grazi, G.L.; et al. Cyclin G1 Is a Target of miR-122a, a microRNA Frequently down-Regulated in Human Hepatocellular Carcinoma. Cancer Res. 2007, 67, 6092–6099. [Google Scholar] [CrossRef]
- Ladeiro, Y.; Couchy, G.; Balabaud, C.; Bioulac-Sage, P.; Pelletier, L.; Rebouissou, S.; Zucman-Rossi, J. MicroRNA Profiling in Hepatocellular Tumors Is Associated with Clinical Features and Oncogene/Tumor Suppressor Gene Mutations. Hepatology 2008, 47, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Budhu, A.; Jia, H.-L.; Forgues, M.; Liu, C.-G.; Goldstein, D.; Lam, A.; Zanetti, K.A.; Ye, Q.-H.; Qin, L.-X.; Croce, C.M.; et al. Identification of Metastasis-Related microRNAs in Hepatocellular Carcinoma. Hepatology 2008, 47, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of Hepatitis C Virus RNA Abundance by a Liver-Specific MicroRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.F.; Berger, U.; et al. LNA-Mediated microRNA Silencing in Non-Human Primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in Vivo with “Antagomirs”. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Komoll, R.-M.; Hu, Q.; Olarewaju, O.; von Döhlen, L.; Yuan, Q.; Xie, Y.; Tsay, H.-C.; Daon, J.; Qin, R.; Manns, M.P.; et al. MicroRNA-342-3p Is a Potent Tumour Suppressor in Hepatocellular Carcinoma. J. Hepatol. 2021, 74, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Kota, J.; Chivukula, R.R.; O’Donnell, K.A.; Wentzel, E.A.; Montgomery, C.L.; Hwang, H.-W.; Chang, T.-C.; Vivekanandan, P.; Torbenson, M.; Clark, K.R.; et al. Therapeutic microRNA Delivery Suppresses Tumorigenesis in a Murine Liver Cancer Model. Cell 2009, 137, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Callegari, E.; Domenicali, M.; Shankaraiah, R.C.; D’Abundo, L.; Guerriero, P.; Giannone, F.; Baldassarre, M.; Bassi, C.; Elamin, B.K.; Zagatti, B.; et al. MicroRNA-Based Prophylaxis in a Mouse Model of Cirrhosis and Liver Cancer. Mol. Ther. Nucleic Acids 2019, 14, 239–250. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 Study of MRX34, a Liposomal miR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Kim, T.; Croce, C.M. MicroRNA: Trends in Clinical Trials of Cancer Diagnosis and Therapy Strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef]
- Zanuso, V.; Rimassa, L.; Braconi, C. The Rapidly Evolving Landscape of HCC: Selecting the Optimal Systemic Therapy. Hepatology 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, S.; Chen, J.; Quan, M.; Gao, Y.; Li, Y. miR-15a and miR-20b Sensitize Hepatocellular Carcinoma Cells to Sorafenib through Repressing CDC37L1 and Consequent PPIA Downregulation. Cell Death Discov. 2022, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chan, Y.-T.; Wu, J.; Feng, Z.; Yuan, H.; Li, Q.; Xing, T.; Xu, L.; Zhang, C.; Tan, H.-Y.; et al. CRISPR/Cas9 Screens Unravel miR-3689a-3p Regulating Sorafenib Resistance in Hepatocellular Carcinoma via Suppressing CCS/SOD1-Dependent Mitochondrial Oxidative Stress. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemoter. 2023, 71, 101015. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lee, D.; Law, C.-T.; Zhang, M.S.; Shen, J.; Chin, D.W.-C.; Zhang, A.; Tsang, F.H.-C.; Wong, C.L.-S.; Ng, I.O.-L.; et al. Genome-Wide CRISPR/Cas9 Library Screening Identified PHGDH as a Critical Driver for Sorafenib Resistance in HCC. Nat. Commun. 2019, 10, 4681. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Chevalier, N.; Calderoni, M.; Dubuis, G.; Dormond, O.; Ziros, P.G.; Sykiotis, G.P.; Widmann, C. CRISPR/Cas9 Genome-Wide Screening Identifies KEAP1 as a Sorafenib, Lenvatinib, and Regorafenib Sensitivity Gene in Hepatocellular Carcinoma. Oncotarget 2019, 10, 7058–7070. [Google Scholar] [CrossRef] [PubMed]
- Luk, J.M.; Burchard, J.; Zhang, C.; Liu, A.M.; Wong, K.F.; Shek, F.H.; Lee, N.P.; Fan, S.T.; Poon, R.T.; Ivanovska, I.; et al. DLK1-DIO3 Genomic Imprinted microRNA Cluster at 14q32.2 Defines a Stemlike Subtype of Hepatocellular Carcinoma Associated with Poor Survival. J. Biol. Chem. 2011, 286, 30706–30713. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Balakrishnan, A.; Huskey, N.; Jones, K.D.; Jodari, M.; Ng, R.; Song, G.; Riordan, J.; Anderton, B.; Cheung, S.-T.; et al. MicroRNA-494 within an Oncogenic microRNA Megacluster Regulates G1/S Transition in Liver Tumorigenesis through Suppression of Mutated in Colorectal Cancer. Hepatology 2014, 59, 202–215. [Google Scholar] [CrossRef]
- Pollutri, D.; Patrizi, C.; Marinelli, S.; Giovannini, C.; Trombetta, E.; Giannone, F.A.; Baldassarre, M.; Quarta, S.; Vandewynckel, Y.P.; Vandierendonck, A.; et al. The Epigenetically Regulated miR-494 Associates with Stem-Cell Phenotype and Induces Sorafenib Resistance in Hepatocellular Carcinoma. Cell Death Dis. 2018, 9, 4. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, Z.; Qi, Y.; Peng, H.; Ma, W.; Wang, R.; Li, W. Golgi Phosphoprotein 3 Promotes Angiogenesis and Sorafenib Resistance in Hepatocellular Carcinoma via Upregulating Exosomal miR-494-3p. Cancer Cell Int. 2022, 22, 35. [Google Scholar] [CrossRef]
- Mao, G.; Liu, Y.; Fang, X.; Liu, Y.; Fang, L.; Lin, L.; Liu, X.; Wang, N. Tumor-Derived microRNA-494 Promotes Angiogenesis in Non-Small Cell Lung Cancer. Angiogenesis 2015, 18, 373–382. [Google Scholar] [CrossRef]
- Shen, J.; Jiang, F.; Yang, Y.; Huang, G.; Pu, F.; Liu, Q.; Chen, L.; Ju, L.; Lu, M.; Zhou, F.; et al. 14-3-3η Is a Novel Growth-Promoting and Angiogenic Factor in Hepatocellular Carcinoma. J. Hepatol. 2016, 65, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Shan, W.; Yang, Y.; Jin, M.; Dai, Y.; Yang, H.; Jiao, R.; Xia, Y.; Liu, Q.; Ju, L.; et al. Reversal of Sorafenib Resistance in Hepatocellular Carcinoma: Epigenetically Regulated Disruption of 14-3-3η/Hypoxia-Inducible Factor-1α. Cell Death Discov. 2019, 5, 120. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zuo, X.; Zhang, Y.; Han, G.; Zhang, L.; Wu, J.; Wang, X. MiR-3662 Suppresses Hepatocellular Carcinoma Growth through Inhibition of HIF-1α-Mediated Warburg Effect. Cell Death Dis. 2018, 9, 549. [Google Scholar] [CrossRef]
- Ye, J.; Xiao, X.; Han, Y.; Fan, D.; Zhu, Y.; Yang, L. MiR-3662 Suppresses Cell Growth, Invasion and Glucose Metabolism by Targeting HK2 in Hepatocellular Carcinoma Cells. Neoplasma 2020, 67, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, C.; Leoni, I.; Rizzardi, N.; Melli, M.; Galvani, G.; Coada, C.A.; Giovannini, C.; Monti, E.; Liparulo, I.; Valenti, F.; et al. MiR-494 Induces Metabolic Changes through G6pc Targeting and Modulates Sorafenib Response in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2023, 42, 145. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, X.; Luo, J.; Yao, H.; Si, Z.; Tong, J.-S. The miR-30a-5p/CLCF1 Axis Regulates Sorafenib Resistance and Aerobic Glycolysis in Hepatocellular Carcinoma. Cell Death Dis. 2020, 11, 902. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Dong, P.-Y.; Yang, G.-M.; Gurunathan, S. A Comprehensive Review on the Composition, Biogenesis, Purification, and Multifunctional Role of Exosome as Delivery Vehicles for Cancer Therapy. Biomed. Pharmacother. 2023, 165, 115087. [Google Scholar] [CrossRef]
- Nicodemou, A.; Bernátová, S.; Čeháková, M.; Danišovič, Ľ. Emerging Roles of Mesenchymal Stem/Stromal-Cell-Derived Extracellular Vesicles in Cancer Therapy. Pharmaceutics 2023, 15, 1453. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Q.; Jiang, Y.; Cai, Z.; Liu, H.; Zuo, H. Engineered Small Extracellular Vesicles Loaded with miR-654-5p Promote Ferroptosis by Targeting HSPB1 to Alleviate Sorafenib Resistance in Hepatocellular Carcinoma. Cell Death Discov. 2023, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chan, Y.-T.; Tan, H.-Y.; Zhang, C.; Guo, W.; Xu, Y.; Sharma, R.; Chen, Z.-S.; Zheng, Y.-C.; Wang, N.; et al. Epigenetic Regulation of Ferroptosis via ETS1/miR-23a-3p/ACSL4 Axis Mediates Sorafenib Resistance in Human Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 3. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.W.; Yoon, J.H.; Ahn, H.R.; Kim, S.; Kim, Y.B.; Lim, S.B.; Park, W.; Kang, T.W.; Baek, G.O.; Yoon, M.G.; et al. Cancer-Associated Fibroblast-Derived Secreted Phosphoprotein 1 Contributes to Resistance of Hepatocellular Carcinoma to Sorafenib and Lenvatinib. Cancer Commun. 2023, 43, 455–479. [Google Scholar] [CrossRef]
- Fornari, F.; Gramantieri, L.; Callegari, E.; Shankaraiah, R.C.; Piscaglia, F.; Negrini, M.; Giovannini, C. MicroRNAs in Animal Models of HCC. Cancers 2019, 11, 1906. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-B.; Wu, H.-M.; He, Y.-C.; Huang, Z.-T.; Weng, Y.-H.; Li, H.; Liang, C.; Yu, W.-M.; Chen, W. MiRNA-124-3p.1 Sensitizes Hepatocellular Carcinoma Cells to Sorafenib by Regulating FOXO3a by Targeting AKT2 and SIRT1. Cell Death Dis. 2022, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Tovar, V.; Cornella, H.; Moeini, A.; Vidal, S.; Hoshida, Y.; Sia, D.; Peix, J.; Cabellos, L.; Alsinet, C.; Torrecilla, S.; et al. Tumour Initiating Cells and IGF/FGF Signalling Contribute to Sorafenib Resistance in Hepatocellular Carcinoma. Gut 2017, 66, 530–540. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, J.; Ma, L.; Shan, J.; Shen, J.; Yang, Z.; Liu, L.; Luo, Y.; Yao, C.; Qian, C. MicroRNA-122 Confers Sorafenib Resistance to Hepatocellular Carcinoma Cells by Targeting IGF-1R to Regulate RAS/RAF/ERK Signaling Pathways. Cancer Lett. 2016, 371, 171–181. [Google Scholar] [CrossRef]
- Lin, Z.; Xia, S.; Liang, Y.; Ji, L.; Pan, Y.; Jiang, S.; Wan, Z.; Tao, L.; Chen, J.; Lin, C.; et al. LXR Activation Potentiates Sorafenib Sensitivity in HCC by Activating microRNA-378a Transcription. Theranostics 2020, 10, 8834–8850. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Wan, Z.; Xia, S.; Jiang, S.; Cen, D.; Cai, L.; Xu, J.; Cai, X. miR-486-3p Mediates Hepatocellular Carcinoma Sorafenib Resistance by Targeting FGFR4 and EGFR. Cell Death Dis. 2020, 11, 250. [Google Scholar] [CrossRef]
- Xu, J.; Wan, Z.; Tang, M.; Lin, Z.; Jiang, S.; Ji, L.; Gorshkov, K.; Mao, Q.; Xia, S.; Cen, D.; et al. N6-Methyladenosine-Modified CircRNA-SORE Sustains Sorafenib Resistance in Hepatocellular Carcinoma by Regulating β-Catenin Signaling. Mol. Cancer 2020, 19, 163. [Google Scholar] [CrossRef]
- Ruan, Y.; Chen, T.; Zheng, L.; Cai, J.; Zhao, H.; Wang, Y.; Tao, L.; Xu, J.; Ji, L.; Cai, X. cDCBLD2 Mediates Sorafenib Resistance in Hepatocellular Carcinoma by Sponging miR-345-5p Binding to the TOP2A Coding Sequence. Int. J. Biol. Sci. 2023, 19, 4608–4626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dong, X.; He, C.; Tan, G.; Li, Z.; Zhai, B.; Feng, J.; Jiang, X.; Liu, C.; Jiang, H.; et al. LncRNA SNHG1 Contributes to Sorafenib Resistance by Activating the Akt Pathway and Is Positively Regulated by miR-21 in Hepatocellular Carcinoma Cells. J. Exp. Clin. Cancer Res. 2019, 38, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Hu, F.; Jiang, X.; Xu, J.; Zhao, D.; Liu, B.; Pan, S.; Dong, X.; Tan, G.; Wei, Z.; et al. Inhibition of Akt Reverses the Acquired Resistance to Sorafenib by Switching Protective Autophagy to Autophagic Cell Death in Hepatocellular Carcinoma. Mol. Cancer Ther. 2014, 13, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-P.; Liu, J.-P.; Feng, J.-F.; Zhu, C.-P.; Yang, Y.; Zhou, W.-P.; Ding, J.; Huang, C.-K.; Cui, Y.-L.; Ding, C.-H.; et al. miR-541 Potentiates the Response of Human Hepatocellular Carcinoma to Sorafenib Treatment by Inhibiting Autophagy. Gut 2020, 69, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, H.; Jiang, Q.; Chai, Y.; Li, X.; Wang, Z.; Zhu, B.; You, S.; Li, B.; Hao, J.; et al. Hsa-miR-4277 Decelerates the Metabolism or Clearance of Sorafenib in HCC Cells and Enhances the Sensitivity of HCC Cells to Sorafenib by Targeting Cyp3a4. Front. Oncol. 2021, 11, 735447. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Mou, J.; Pan, Y.-J.; Huo, F.-C.; Du, W.-Q.; Liang, J.; Wang, Y.; Zhang, L.-S.; Pei, D.-S. MicroRNA-138-1-3p Sensitizes Sorafenib to Hepatocellular Carcinoma by Targeting PAK5 Mediated β-Catenin/ABCB1 Signaling Pathway. J. Biomed. Sci. 2021, 28, 56. [Google Scholar] [CrossRef] [PubMed]
- Spallanzani, A.; Orsi, G.; Andrikou, K.; Gelsomino, F.; Rimini, M.; Riggi, L.; Cascinu, S. Lenvatinib as a Therapy for Unresectable Hepatocellular Carcinoma. Expert Rev. Anticancer Ther. 2018, 18, 1069–1076. [Google Scholar] [CrossRef]
- Ladd, A.D.; Duarte, S.; Sahin, I.; Zarrinpar, A. Mechanisms of Drug Resistance in HCC. Hepatology 2023. online ahead of print. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, L.; Han, T.; Ding, S. miR-3154 Promotes Hepatocellular Carcinoma Progression via Suppressing HNF4α. Carcinogenesis 2022, 43, 1002–1014. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Chen, L.; Chen, H.-Y.; Hu, L.; Li, L.; Sun, H.-Y.; Jiang, F.; Zhao, J.; Liu, G.-M.-Y.; Tang, J.; et al. MUC15 Inhibits Dimerization of EGFR and PI3K-AKT Signaling and Is Associated with Aggressive Hepatocellular Carcinomas in Patients. Gastroenterology 2013, 145, 1436–1448.e1-12. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zheng, H.; Zhang, J.; Yang, P.; Li, H.; Cheng, Z.; Xiang, D.; Wang, R. Downregulation of MUC15 by miR-183-5p.1 Promotes Liver Tumor-Initiating Cells Properties and Tumorigenesis via Regulating c-MET/PI3K/AKT/SOX2 Axis. Cell Death Dis. 2022, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, W.; Han, P.; Zhang, J.; Tong, L.; Sun, X. MicroRNA-128-3p Mediates Lenvatinib Resistance of Hepatocellular Carcinoma Cells by Downregulating c-Met. J. Hepatocell. Carcinoma 2022, 9, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, W.; Chen, X.; Yin, J.; Ma, L.; Liu, M.; Zhou, X.; Xian, L.; Li, P.; Tan, X.; et al. circKCNN2 Suppresses the Recurrence of Hepatocellular Carcinoma at Least Partially via Regulating miR-520c-3p/Methyl-DNA-Binding Domain Protein 2 Axis. Clin. Transl. Med. 2022, 12, e662. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yu, J.; Lu, L.; Zhang, Y.; Zhou, Y.; Zhou, Y.; Huang, F.; Sun, L.; Guo, Z.; Hou, G.; et al. MT1JP-Mediated miR-24-3p/BCL2L2 Axis Promotes Lenvatinib Resistance in Hepatocellular Carcinoma Cells by Inhibiting Apoptosis. Cell. Oncol. 2021, 44, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Tao, H.; Zhu, J.; Lu, Y.; Cheng, F.; Xiong, Y.; Liu, J.; Cai, G.; Zhang, Z.; et al. Targeting LINC01607 Sensitizes Hepatocellular Carcinoma to Lenvatinib via Suppressing Mitophagy. Cancer Lett. 2023, 576, 216405. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Setayesh, T.; Vaziri, F.; Wu, X.; Hwang, S.T.; Chen, X.; Yvonne Wan, Y.-J. miR-22 Gene Therapy Treats HCC by Promoting Anti-Tumor Immunity and Enhancing Metabolism. Mol. Ther. 2023, 31, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Iwama, H.; Fujita, K.; Kobara, H.; Nishiyama, N.; Fujihara, S.; Goda, Y.; Yoneyama, H.; Morishita, A.; Tani, J.; et al. Evaluating the Effect of Lenvatinib on Sorafenib-Resistant Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 13071. [Google Scholar] [CrossRef]
- Liu, N.; Wang, X.; Steer, C.J.; Song, G. MicroRNA-206 Promotes the Recruitment of CD8+ T Cells by Driving M1 Polarisation of Kupffer Cells. Gut 2022, 71, 1642–1655. [Google Scholar] [CrossRef]
- Liu, N.; Chang, C.W.; Steer, C.J.; Wang, X.W.; Song, G. MicroRNA-15a/16-1 Prevents Hepatocellular Carcinoma by Disrupting the Communication Between Kupffer Cells and Regulatory T Cells. Gastroenterology 2022, 162, 575–589. [Google Scholar] [CrossRef]
- Li, X.; Wenes, M.; Romero, P.; Huang, S.C.-C.; Fendt, S.-M.; Ho, P.-C. Navigating Metabolic Pathways to Enhance Antitumour Immunity and Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 425–441. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Z.; Zhang, Y.; Wang, J.; Zhang, Z.; Wu, J.; Mao, J.; Zuo, X. CircRHBDD1 Augments Metabolic Rewiring and Restricts Immunotherapy Efficacy via m6A Modification in Hepatocellular Carcinoma. Mol. Ther. Oncolytics 2022, 24, 755–771. [Google Scholar] [CrossRef]
- Xu, K.; Xia, P.; Chen, X.; Ma, W.; Yuan, Y. ncRNA-Mediated Fatty Acid Metabolism Reprogramming in HCC. Trends Endocrinol. Metab. 2023, 34, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Yi, J.; Jiang, J.; Zou, Z.; Mo, Y.; Ren, Q.; Lin, Z.; Lu, Y.; Zhang, J.; Liu, J. Identification and Validation of a Fatty Acid Metabolism-Related lncRNA Signature as a Predictor for Prognosis and Immunotherapy in Patients with Liver Cancer. BMC Cancer 2022, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Q.; Cheng, J.; Shen, X.; Li, J.; Chen, M.; Zhou, C.; Zhou, J. LncRNA SNHG1 Upregulates FANCD2 and G6PD to Suppress Ferroptosis by Sponging miR-199a-5p/3p in Hepatocellular Carcinoma. Drug Discov. Ther. 2023, 17, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Zhang, P.-F.; Wei, C.-Y.; Peng, R.; Lu, J.-C.; Gao, C.; Cai, J.-B.; Yang, X.; Fan, J.; Ke, A.-W.; et al. Circular RNA circMET Drives Immunosuppression and Anti-PD1 Therapy Resistance in Hepatocellular Carcinoma via the miR-30-5p/Snail/DPP4 Axis. Mol. Cancer 2020, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the Dipeptidyl Peptidase DPP4 (CD26) Reveals IL-33-Dependent Eosinophil-Mediated Control of Tumor Growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pan, T.; Zhou, W.; Zhang, Y.; Xu, G.; Xu, Q.; Li, S.; Gao, Y.; Wang, Z.; Xu, J.; et al. Long Noncoding RNA LINC01132 Enhances Immunosuppression and Therapy Resistance via NRF1/DPP4 Axis in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 270. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Mackowiak, B.; Feng, D.; Lu, H.; Guan, Y.; Lehner, T.; Pan, H.; Wang, X.W.; He, Y.; Gao, B. MicroRNA-223 Attenuates Hepatocarcinogenesis by Blocking Hypoxia-Driven Angiogenesis and Immunosuppression. Gut 2023, 72, 1942–1958. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, X.; Ren, Y.; Yang, Y.; Song, F.; Fu, J.; Liu, S.; Yu, M.; Chen, J.; Wang, S.; et al. An RNA-RNA Crosstalk Network Involving HMGB1 and RICTOR Facilitates Hepatocellular Carcinoma Tumorigenesis by Promoting Glutamine Metabolism and Impedes Immunotherapy by PD-L1+ Exosomes Activity. Signal Transduct. Target. Ther. 2021, 6, 421. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, G.; Zhao, Y.; Gao, H.; Li, L.; Yin, Y.; Jiang, J.; Wang, L.; Mang, Y.; Gao, Y.; et al. Exosome-Derived circCCAR1 Promotes CD8 + T-Cell Dysfunction and Anti-PD1 Resistance in Hepatocellular Carcinoma. Mol. Cancer 2023, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-F.; Gao, C.; Huang, X.-Y.; Lu, J.-C.; Guo, X.-J.; Shi, G.-M.; Cai, J.-B.; Ke, A.-W. Cancer Cell-Derived Exosomal circUHRF1 Induces Natural Killer Cell Exhaustion and May Cause Resistance to Anti-PD1 Therapy in Hepatocellular Carcinoma. Mol. Cancer 2020, 19, 110. [Google Scholar] [CrossRef]
- Dong, Z.-R.; Cai, J.-B.; Shi, G.-M.; Yang, Y.-F.; Huang, X.-Y.; Zhang, C.; Dong, R.-Z.; Wei, C.-Y.; Li, T.; Ke, A.-W.; et al. Oncogenic miR-93-5p/Gal-9 Axis Drives CD8 (+) T-Cell Inactivation and Is a Therapeutic Target for Hepatocellular Carcinoma Immunotherapy. Cancer Lett. 2023, 564, 216186. [Google Scholar] [CrossRef]
- Wei, L.; Wang, X.; Lv, L.; Liu, J.; Xing, H.; Song, Y.; Xie, M.; Lei, T.; Zhang, N.; Yang, M. The Emerging Role of microRNAs and Long Noncoding RNAs in Drug Resistance of Hepatocellular Carcinoma. Mol. Cancer 2019, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Miotto, E.; Saccenti, E.; Lupini, L.; Callegari, E.; Negrini, M.; Ferracin, M. Quantification of Circulating miRNAs by Droplet Digital PCR: Comparison of EvaGreen- and TaqMan-Based Chemistries. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Villanueva, A.; Korangy, F.; Ruf, B.; Yarchoan, M.; Ma, L.; Ruppin, E.; Wang, X.W. Biomarkers for Immunotherapy of Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2023, 20, 780–798. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The Mechanisms of Sorafenib Resistance in Hepatocellular Carcinoma: Theoretical Basis and Therapeutic Aspects. Signal Transduct. Target. Ther. 2020, 5, 87. [Google Scholar] [CrossRef]
- Fornari, F.; Pollutri, D.; Patrizi, C.; La Bella, T.; Marinelli, S.; Casadei Gardini, A.; Marisi, G.; Baron Toaldo, M.; Baglioni, M.; Salvatore, V.; et al. In Hepatocellular Carcinoma miR-221 Modulates Sorafenib Resistance through Inhibition of Caspase-3-Mediated Apoptosis. Clin. Cancer Res. 2017, 23, 3953–3965. [Google Scholar] [CrossRef]
- De la Cruz-Ojeda, P.; Schmid, T.; Boix, L.; Moreno, M.; Sapena, V.; Praena-Fernández, J.M.; Castell, F.J.; Falcón-Pérez, J.M.; Reig, M.; Brüne, B.; et al. miR-200c-3p, miR-222-5p, and miR-512-3p Constitute a Biomarker Signature of Sorafenib Effectiveness in Advanced Hepatocellular Carcinoma. Cells 2022, 11, 2673. [Google Scholar] [CrossRef]
- Fernández-Tussy, P.; Rodríguez-Agudo, R.; Fernández-Ramos, D.; Barbier-Torres, L.; Zubiete-Franco, I.; Davalillo, S.L.d.; Herraez, E.; Goikoetxea-Usandizaga, N.; Lachiondo-Ortega, S.; Simón, J.; et al. Anti-miR-518d-5p Overcomes Liver Tumor Cell Death Resistance through Mitochondrial Activity. Cell Death Dis. 2021, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Arizumi, T.; Hagiwara, S.; Ida, H.; Sakurai, T.; Kudo, M. MicroRNAs for the Prediction of Early Response to Sorafenib Treatment in Human Hepatocellular Carcinoma. Liver Cancer 2017, 6, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-Y.; Chen, P.-S.; Lin, L.-I.; Lee, B.-S.; Ling, A.; Cheng, A.-L.; Hsu, C.; Ou, D.-L. Low miR-10b-3p Associated with Sorafenib Resistance in Hepatocellular Carcinoma. Br. J. Cancer 2022, 126, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.-P.; Wang, C.-Y.; Jin, X.-H.; Li, M.; Wang, F.-W.; Huang, W.-J.; Yun, J.-P.; Xu, R.-H.; Cai, Q.-Q.; Xie, D. Acidic Microenvironment Up-Regulates Exosomal miR-21 and miR-10b in Early-Stage Hepatocellular Carcinoma to Promote Cancer Cell Proliferation and Metastasis. Theranostics 2019, 9, 1965–1979. [Google Scholar] [CrossRef]
- Finn, R.S.; Kudo, M.; Cheng, A.-L.; Wyrwicz, L.; Ngan, R.K.C.; Blanc, J.-F.; Baron, A.D.; Vogel, A.; Ikeda, M.; Piscaglia, F.; et al. Pharmacodynamic Biomarkers Predictive of Survival Benefit with Lenvatinib in Unresectable Hepatocellular Carcinoma: From the Phase III REFLECT Study. Clin. Cancer Res. 2021, 27, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Teufel, M.; Seidel, H.; Köchert, K.; Meinhardt, G.; Finn, R.S.; Llovet, J.M.; Bruix, J. Biomarkers Associated with Response to Regorafenib in Patients with Hepatocellular Carcinoma. Gastroenterology 2019, 156, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Toffanin, S.; Lachenmayer, A.; Villanueva, A.; Minguez, B.; Llovet, J.M. Molecular Classification and Novel Targets in Hepatocellular Carcinoma: Recent Advancements. Semin. Liver Dis. 2010, 30, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kondo, S.; Matsuzaki, J.; Esaki, M.; Okusaka, T.; Shimada, K.; Murakami, Y.; Enomoto, M.; Tamori, A.; Kato, K.; et al. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients with Liver Disease. Hepatol. Commun. 2020, 4, 284–297. [Google Scholar] [CrossRef]
- Moshiri, F.; Salvi, A.; Gramantieri, L.; Sangiovanni, A.; Guerriero, P.; De Petro, G.; Bassi, C.; Lupini, L.; Sattari, A.; Cheung, D.; et al. Circulating miR-106-b-3p, miR-101-3p and miR-1246 as Diagnostic Biomarkers of Hepatocellular Carcinoma. Oncotarget 2018, 9, 15350–15364. [Google Scholar] [CrossRef]

| Noncoding RNA | Target Gene/Sponged miRNA/Other Targets | Experimental In Vivo Models | Therapeutic/Experimental Strategy | Effect on Immune Cells | Treatment Combination | Ref. No. |
|---|---|---|---|---|---|---|
| miR-206 | Klf4/CCL2 | AKT/Ras and Sleeping Beauty transposon hydrodynamic injection in FVB/NJ mice | Minicircle and Sleeping Beauty hydrodynamic injection for miR-206 overexpression | Decreased Treg recruitment | None | [99] |
| miR-15a/16-1 | Nf-kB/CCL22 | AKT/Ras, Myc hydrodynamic injection in FVB/NJ mice | Hydrodynamic injection for miRNA overexpression | M1 macrophage polarization | None | [100] |
| circRHBDD1 | YTHDF1/PIK3R1 | PDX NOD/SCID, BALBc mice; Hepa1-6 cells in xenograft C57BL/6 mice | circRHBDD1 interference vector | N/A | Anti-PD1 | [102] |
| circMET | miR-30-5p/SNAI1/DPP4/CXCL10 | Hepa1-6 cells in xenograft C57BL/6 mice | Sitagliptin (DPP4 inhibitor) | Increased CD8+ T cells recruitment | Anti-PD1 | [106] |
| LINC01132 | NRF1/DPP4 | PDX nude mice; Hepa1-6 cells in C57BL/6 xenograft mice | LINC01132 adenovirus interference vector | Increased CD8+ T cells recruitment | Anti-PD-L1 | [108] |
| miR-223 | HIF1/CD39/CD73 | miR-223 KO mice + DEN or CCL4; C57BL/4J mice + DEN+CCl4 | miR-223 adenovirus vector | Decreased PD1/PD-L1 expression | None | [109] |
| CircCCAR1 | miR-127-5p/WTAP | HCCLM3 cells in BALBc, HuNSG xenograft mice | circCCAR1 overexpression vector | CD8+ T cells dysfunction | Anti-PD1 | [111] |
| circUHRF1 | miR-449c-5p/TIM3 | HCCLM3 cells in NOD/SCID xenograft mice | circUHRF1 interference vector | Increased NK activity | Anti-PD1 | [112] |
| miR-93-5p | GAL-9 | LPC cells in xenograft and orthotopic nude mice | Anti-GAL-9 | Increased CD8+ T cells recruitment | Anti-PD1 | [113] |
| miRNA Name | Blood Specimen | Timepoint of Analysis | Circulating Levels in Responders | Treatment | Ref. No. |
|---|---|---|---|---|---|
| miR-221 | Serum | Basal On treatment (2 m) | Low High | Sorafenib | [119] |
| miR-200c-3p miR-222-5p miR-512-3p | Plasma | Basal On treatment (1 m) On treatment (1 m) | High Low Low | Sorafenib | [120] |
| miR-30e-3p | Serum | On treatment (2 m) | Low | Sorafenib | [37] |
| miR-518d-5p | Serum | Basal | Low | Sorafenib | [121] |
| miR-181a-5p | Serum | Basal | High | Sorafenib | [122] |
| miR-10b-3p | Serum | Basal | High | Sorafenib | [123] |
| miR-494 | Serum | Basal | Low | Sorafenib | [68] |
| miR-30a, miR-122, miR-125b, miR-200a, miR-347b; miR-15b, miR-107, miR-320; miR-645 | Plasma | Basal | High Low Absent | Regorafenib | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vianello, C.; Monti, E.; Leoni, I.; Galvani, G.; Giovannini, C.; Piscaglia, F.; Stefanelli, C.; Gramantieri, L.; Fornari, F. Noncoding RNAs in Hepatocellular Carcinoma: Potential Applications in Combined Therapeutic Strategies and Promising Candidates of Treatment Response. Cancers 2024, 16, 766. https://doi.org/10.3390/cancers16040766
Vianello C, Monti E, Leoni I, Galvani G, Giovannini C, Piscaglia F, Stefanelli C, Gramantieri L, Fornari F. Noncoding RNAs in Hepatocellular Carcinoma: Potential Applications in Combined Therapeutic Strategies and Promising Candidates of Treatment Response. Cancers. 2024; 16(4):766. https://doi.org/10.3390/cancers16040766
Chicago/Turabian StyleVianello, Clara, Elisa Monti, Ilaria Leoni, Giuseppe Galvani, Catia Giovannini, Fabio Piscaglia, Claudio Stefanelli, Laura Gramantieri, and Francesca Fornari. 2024. "Noncoding RNAs in Hepatocellular Carcinoma: Potential Applications in Combined Therapeutic Strategies and Promising Candidates of Treatment Response" Cancers 16, no. 4: 766. https://doi.org/10.3390/cancers16040766
APA StyleVianello, C., Monti, E., Leoni, I., Galvani, G., Giovannini, C., Piscaglia, F., Stefanelli, C., Gramantieri, L., & Fornari, F. (2024). Noncoding RNAs in Hepatocellular Carcinoma: Potential Applications in Combined Therapeutic Strategies and Promising Candidates of Treatment Response. Cancers, 16(4), 766. https://doi.org/10.3390/cancers16040766





