LIONS PREY: A New Logistic Scoring System for the Prediction of Malignant Pulmonary Nodules
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Recruitment
2.2. Data Collection
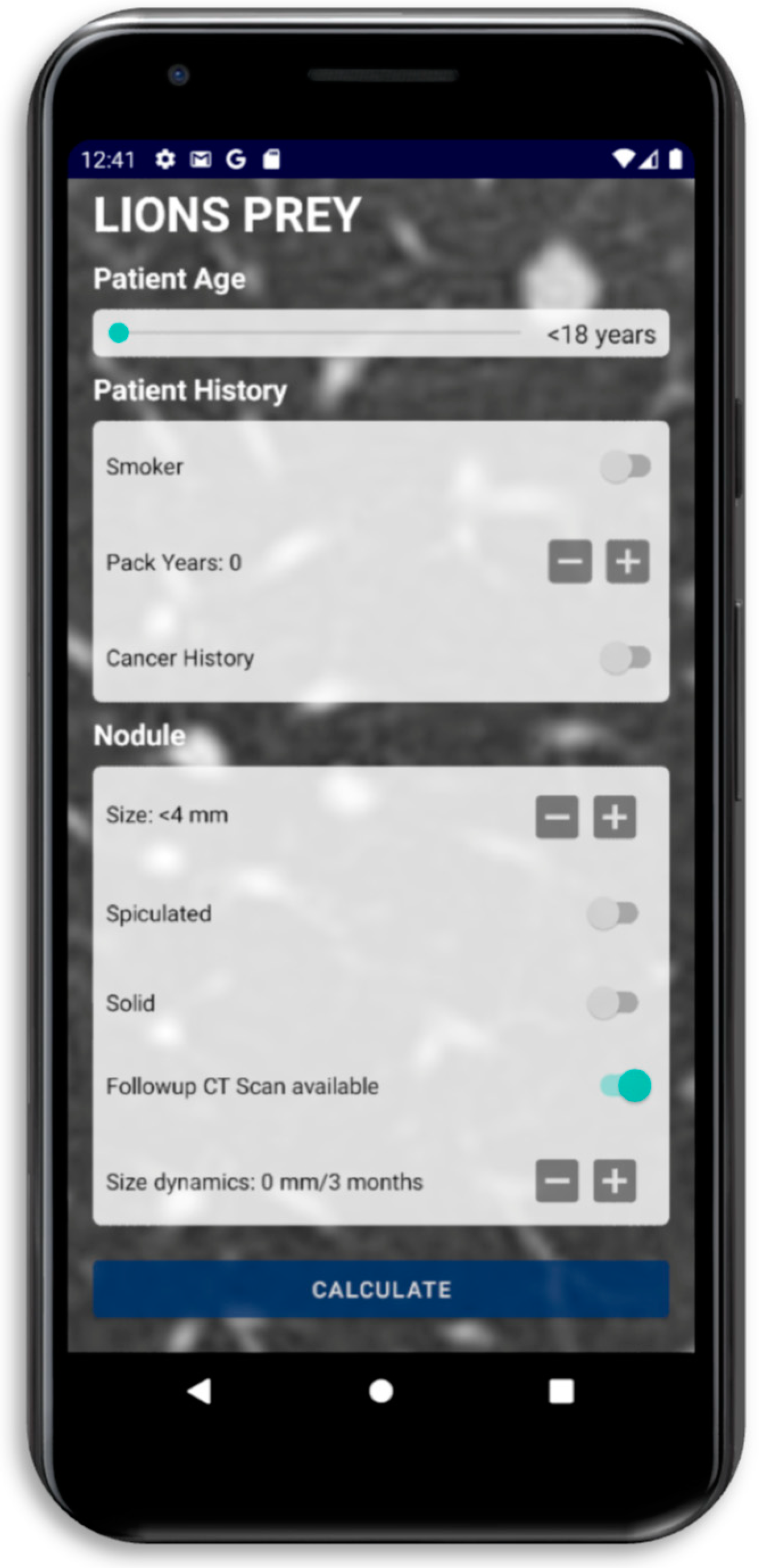
2.3. Score Development and Calculation
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
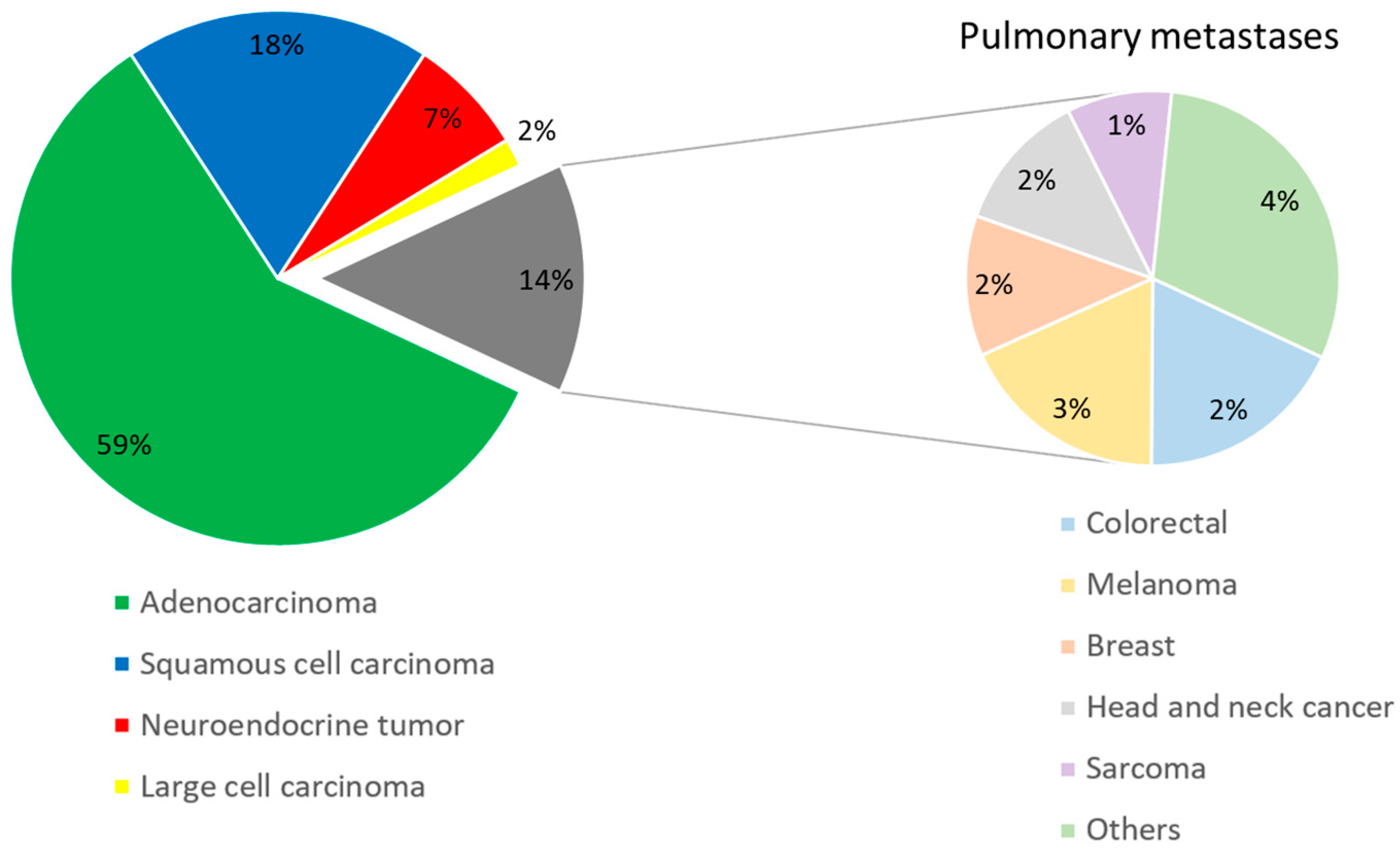
3.2. Radiologic Parameters
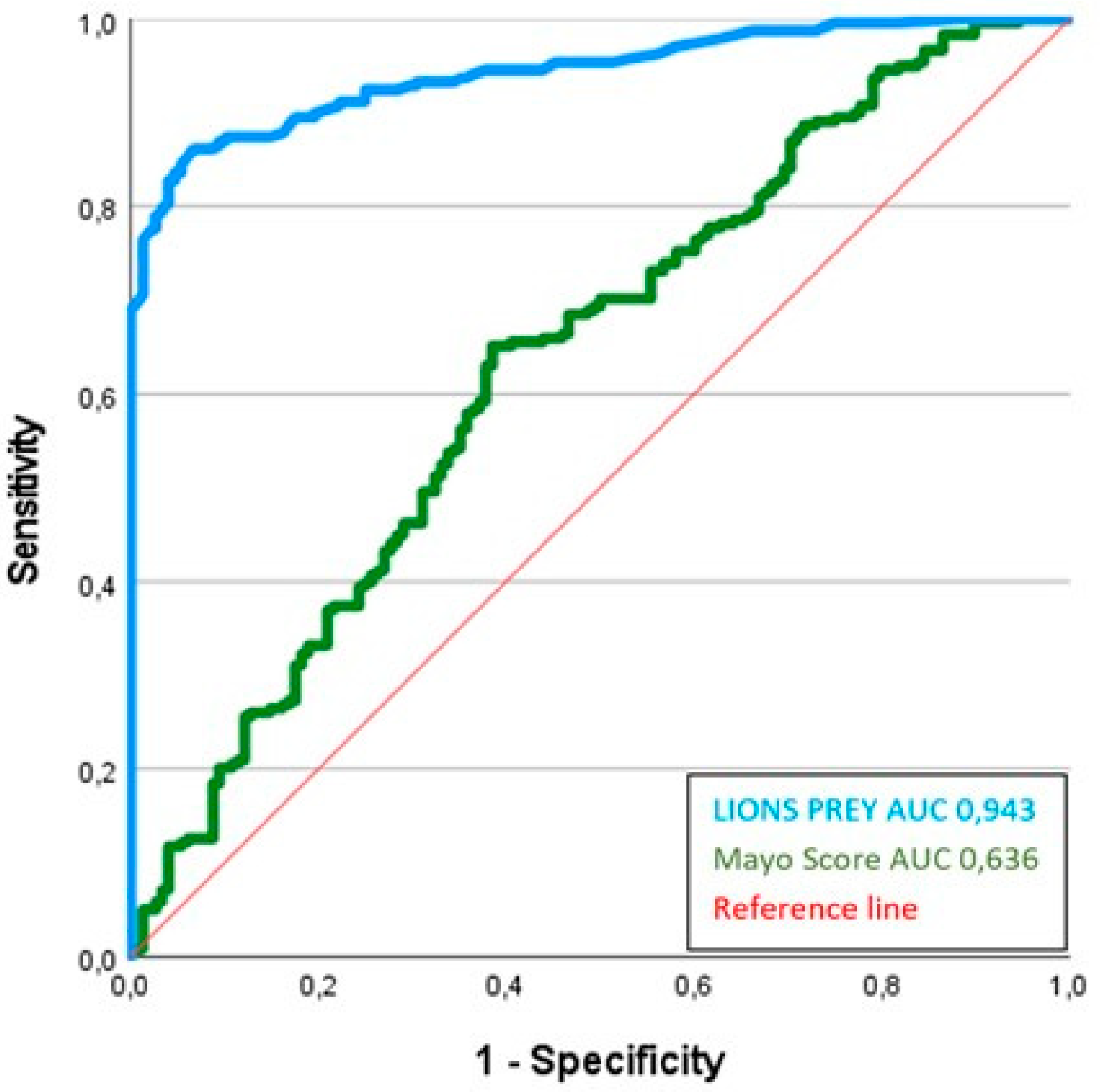
3.3. Performance Assessment
4. Discussion
4.1. Precision and Limits of a Scoring Model
4.2. The Clinical LIONS PREY Parameters
4.3. The Radiological LIONS PREY Parameters
4.4. Exclusion of Potentially Relevant Parameters
4.5. What Are the Strengths of LIONS PREY?
4.6. Validation of LIONS PREY by Comparison with the Mayo Score
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Vitzthum, K.; Thielke, L.; Deter, A.; Riemer, T.; Eggeling, S.; Pankow, W.; Mache, S. Smoking Lung Cancer Patients and Tobacco Cessation—Is the Current Treatment in Germany Sufficient? Pneumologie 2015, 69, 667–672. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.B.; Truitt, S.G.; Scheunemann, L.P.; Shah, S.A.; Rivera, M.P.; Parker, L.A.; Carson, S.S. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch. Intern. Med. 2009, 169, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.C.; Mali, W.P.; Grobbee, D.E.; van der Graaf, Y. Prevalence of incidental findings in computed tomographic screening of the chest: A systematic review. J. Comput. Assist. Tomogr. 2008, 32, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, R.; Marjański, T.; Rzyman, W. A narrative review of invasive diagnostics and treatment of early lung cancer. Transl. Lung Cancer Res. 2021, 10, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, T.; Bölükbas, S.; Beqiri, S.; Trainer, S.; Schirren, J. Solitary pulmonary nodule. Assessment and therapy. Chirurg 2007, 78, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.J.; Silverstein, M.D.; Ilstrup, D.M.; Schleck, C.D.; Edell, E.S. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch. Intern. Med. 1997, 157, 849–855. [Google Scholar] [CrossRef]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M.; et al. Probability of cancer in pulmonary nodules detected on first screening CT. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef]
- Zhang, M.; Zhuo, N.; Guo, Z.; Zhang, X.; Liang, W.; Zhao, S.; He, J. Establishment of a mathematic model for predicting malignancy in solitary pulmonary nodules. J. Thorac. Dis. 2015, 7, 1833–1841. [Google Scholar] [CrossRef]
- González Maldonado, S.; Delorme, S.; Hüsing, A.; Motsch, E.; Kauczor, H.U.; Heussel, C.P.; Kaaks, R. Evaluation of Prediction Models for Identifying Malignancy in Pulmonary Nodules Detected via Low-Dose Computed Tomography. JAMA Netw. Open 2020, 3, e1921221. [Google Scholar] [CrossRef]
- Erasmus, J.J.; Connolly, J.E.; McAdams, H.P.; Roggli, V.L. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000, 20, 43–58. [Google Scholar] [CrossRef]
- MacMahon, H.; Austin, J.H.; Gamsu, G.; Herold, C.J.; Jett, J.R.; Naidich, D.P.; Patz, E.F., Jr.; Swensen, S.J.; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: A statement from the Fleischner Society. Radiology 2005, 237, 395–400. [Google Scholar] [CrossRef]
- Predicting outcome in ICU patients. 2nd European Consensus Conference in Intensive Care Medicine. Intensive Care Med. 1994, 20, 390–397. [CrossRef]
- Jin, R.; Grunkemeier, G.L. Does the logistic EuroSCORE offer an advantage over the additive model? Interact. Cardiovasc. Thorac. Surg. 2006, 5, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Waydhas, C.; Nast-Kolb, D.; Ruchholtz, S.; Schweiberer, L. Practical and theoretical limits of score systems. Unfallchirurg 1994, 97, 185–190. [Google Scholar] [PubMed]
- Neugebauer, E.; Bouillon, B. What is the value of score systems? Unfallchirurg 1994, 97, 172–176. [Google Scholar] [PubMed]
- Poschenrieder, F.; Beyer, L.; Rehbock, B.; Diederich, S.; Wormanns, D.; Stroszczynski, C.; Hamer, O.W. Management of solid pulmonary nodules. Radiologe 2014, 54, 436–448. [Google Scholar] [CrossRef]
- Grundmann, R.T.; Meyer, F. Second primary malignancy among cancer survivors-epidemiology, prognosis and clinical relevance. Zentralbl Chir. 2012, 137, 565–574. [Google Scholar] [CrossRef]
- National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health. Reports of the Surgeon General. In The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014. [Google Scholar]
- Patel, V.K.; Naik, S.K.; Naidich, D.P.; Travis, W.D.; Weingarten, J.A.; Lazzaro, R.; Gutterman, D.D.; Wentowski, C.; Grosu, H.B.; Raoof, S. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: Part 1: Radiologic characteristics and imaging modalities. Chest 2013, 143, 825–839. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Furuya, K.; Murayama, S.; Soeda, H.; Murakami, J.; Ichinose, Y.; Yabuuchi, H.; Katsuda, Y.; Koga, M.; Masuda, K. New classification of small pulmonary nodules by margin characteristics on high-resolution CT. Acta Radiol. 1999, 40, 496–504. [Google Scholar] [CrossRef]
- Menzel, C.; Hamer, O.W. Characterization and management of incidentally detected solitary pulmonary nodules. Radiologe 2010, 50, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.B.; Im, J.G.; Goo, J.M.; Chung, M.J.; Kim, M.Y. Atypical pulmonary metastases: Spectrum of radiologic findings. Radiographics 2001, 21, 403–417. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeong, W.G.; Kim, Y.H. Differentiation of primary lung cancer from solitary lung metastasis in patients with colorectal cancer: A retrospective cohort study. World J. Surg. Oncol. 2021, 19, 28. [Google Scholar] [CrossRef]
- Choromańska, A.; Macura, K.J. Evaluation of solitary pulmonary nodule detected during computed tomography examination. Pol. J. Radiol. 2012, 77, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Fletcher, J.; Iannettoni, M.D.; Lynch, W.R.; Midthun, D.E.; Naidich, D.P.; Ost, D.E.; American College of Chest Physicians. Evaluation of patients with pulmonary nodules: When is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007, 132, 108s–130s. [Google Scholar] [CrossRef]
- D’Amico, T.A.; Wong, T.Z.; Harpole, D.H.; Brown, S.D.; Coleman, R.E. Impact of computed tomography-positron emission tomography fusion in staging patients with thoracic malignancies. Ann. Thorac. Surg. 2002, 74, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Herder, G.J.; van Tinteren, H.; Golding, R.P.; Kostense, P.J.; Comans, E.F.; Smit, E.F.; Hoekstra, O.S. Clinical prediction model to characterize pulmonary nodules: Validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005, 128, 2490–2496. [Google Scholar] [CrossRef] [PubMed]


| Predictors | All Patients (n = 386) | Malignant (n = 238) | Benign (n = 148) | p Value |
|---|---|---|---|---|
| Age in years (mean ± SD) | 63.4 ± 11.8 | 64.5 ± 10.2 | 61.6 ± 13.8 | 0.021 |
| Male (%) | 53.9 | 54.6 | 52.7 | 0.713 |
| FEV1 (mean ± SD) | 77.1 ± 21.6 | 81.1 ± 21.4 | 71.6 ± 12.7 | 0.162 |
| TLCO (mean ± SD) | 78.8 ± 47.0 | 78.1 ± 53.7 | 80.7 ± 21.4 | 0.718 |
| COPD (%) | 61.1 | 63.9 | 56.8 | 0.164 |
| History of cancer (%) | 30.8 | 34.9 | 24.3 | 0.030 |
| Family history of cancer (%) | 15.7 | 11.3 | 20.3 | 0.249 |
| Family history of lung cancer (%) | 3.4 | 1.3 | 6.1 | 0.074 |
| Current or former smoker (%) | 73.6 | 92.0 | 43.9 | <0.0001 |
| Pack years (mean ± SD) | 29.8 ± 20.1 | 35.1 ± 19.1 | 21.3 ± 18.8 | <0.0001 |
| Quitting smoking (%) | 24.2 | 22.3 | 28.7 | 0.473 |
| Diameter in mm (mean ± SD) | 20.4 ± 7.8 | 21.8 ± 7.5 | 18.3 ± 7.9 | <0.0001 |
| Spiculation (%) | 61.1 | 73.1 | 41.9 | <0.0001 |
| Clear border (%) | 38.9 | 26.9 | 58.1 | <0.0001 |
| Solid (%) | 76.4 | 84.9 | 62.8 | <0.0001 |
| Upper lobe (%) | 60.6 | 61.3 | 59.5 | 0.712 |
| Emphysema (%) | 28.5 | 34.5 | 18.9 | 0.061 |
| Calcification (%) | 2.6 | 0.5 | 6.1 | 0.010 |
| Nodule count | 1.2 ± 0.7 | 1.2 ± 0.6 | 1.3 ± 0.8 | 0.229 |
| Hounsfield units (mean ± SD) | −21.7 ± 117.0 | −19.8 ± 131.6 | −24.7 ± 117.0 | 0.709 |
| Dynamics in mm/3 months (mean ± SD) | 4.0 ± 6.8 | 6.4 ± 7.7 | 0.2 ± 0.9 | <0.0001 |
| SUVmax (mean ± SD) | 5.7 ± 6.5 | 9.3 ± 6.0 | 1.3 ± 0.4 | <0.0001 |
| Predictors | p-Value | Beta Coefficient | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Age in years | 0.054 | 0.028 | 1.029 | 1.0–1.059 |
| Diameter in mm | 0.051 | 0.046 | 1.047 | 1.0–1.097 |
| Spiculation | 0.024 | 0.818 | 2.267 | 1.114–4.613 |
| Solid | 0.004 | 1.141 | 3.130 | 1.443–6.790 |
| Dynamics in mm/3 months | <0.0001 | 0.556 | 1.744 | 1.511–2.014 |
| Current or former smoker | <0.0001 | 2.163 | 8.695 | 3.497–21.622 |
| Pack years | 0.079 | 0.017 | 1.017 | 0.998–1.036 |
| History of cancer | 0.052 | 0.726 | 2.068 | 0.993–4.305 |
| Constant | −6.964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doerr, F.; Giese, A.; Höpker, K.; Menghesha, H.; Schlachtenberger, G.; Grapatsas, K.; Baldes, N.; Baldus, C.J.; Hagmeyer, L.; Fallouh, H.; et al. LIONS PREY: A New Logistic Scoring System for the Prediction of Malignant Pulmonary Nodules. Cancers 2024, 16, 729. https://doi.org/10.3390/cancers16040729
Doerr F, Giese A, Höpker K, Menghesha H, Schlachtenberger G, Grapatsas K, Baldes N, Baldus CJ, Hagmeyer L, Fallouh H, et al. LIONS PREY: A New Logistic Scoring System for the Prediction of Malignant Pulmonary Nodules. Cancers. 2024; 16(4):729. https://doi.org/10.3390/cancers16040729
Chicago/Turabian StyleDoerr, Fabian, Annika Giese, Katja Höpker, Hruy Menghesha, Georg Schlachtenberger, Konstantinos Grapatsas, Natalie Baldes, Christian J. Baldus, Lars Hagmeyer, Hazem Fallouh, and et al. 2024. "LIONS PREY: A New Logistic Scoring System for the Prediction of Malignant Pulmonary Nodules" Cancers 16, no. 4: 729. https://doi.org/10.3390/cancers16040729
APA StyleDoerr, F., Giese, A., Höpker, K., Menghesha, H., Schlachtenberger, G., Grapatsas, K., Baldes, N., Baldus, C. J., Hagmeyer, L., Fallouh, H., Pinto dos Santos, D., Bender, E. M., Quaas, A., Heldwein, M., Wahlers, T., Hautzel, H., Darwiche, K., Taube, C., Schuler, M., ... Bölükbas, S. (2024). LIONS PREY: A New Logistic Scoring System for the Prediction of Malignant Pulmonary Nodules. Cancers, 16(4), 729. https://doi.org/10.3390/cancers16040729









