Lymphedema and Trismus after Head and Neck Cancer, and the Impact on Body Image and Quality of Life
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
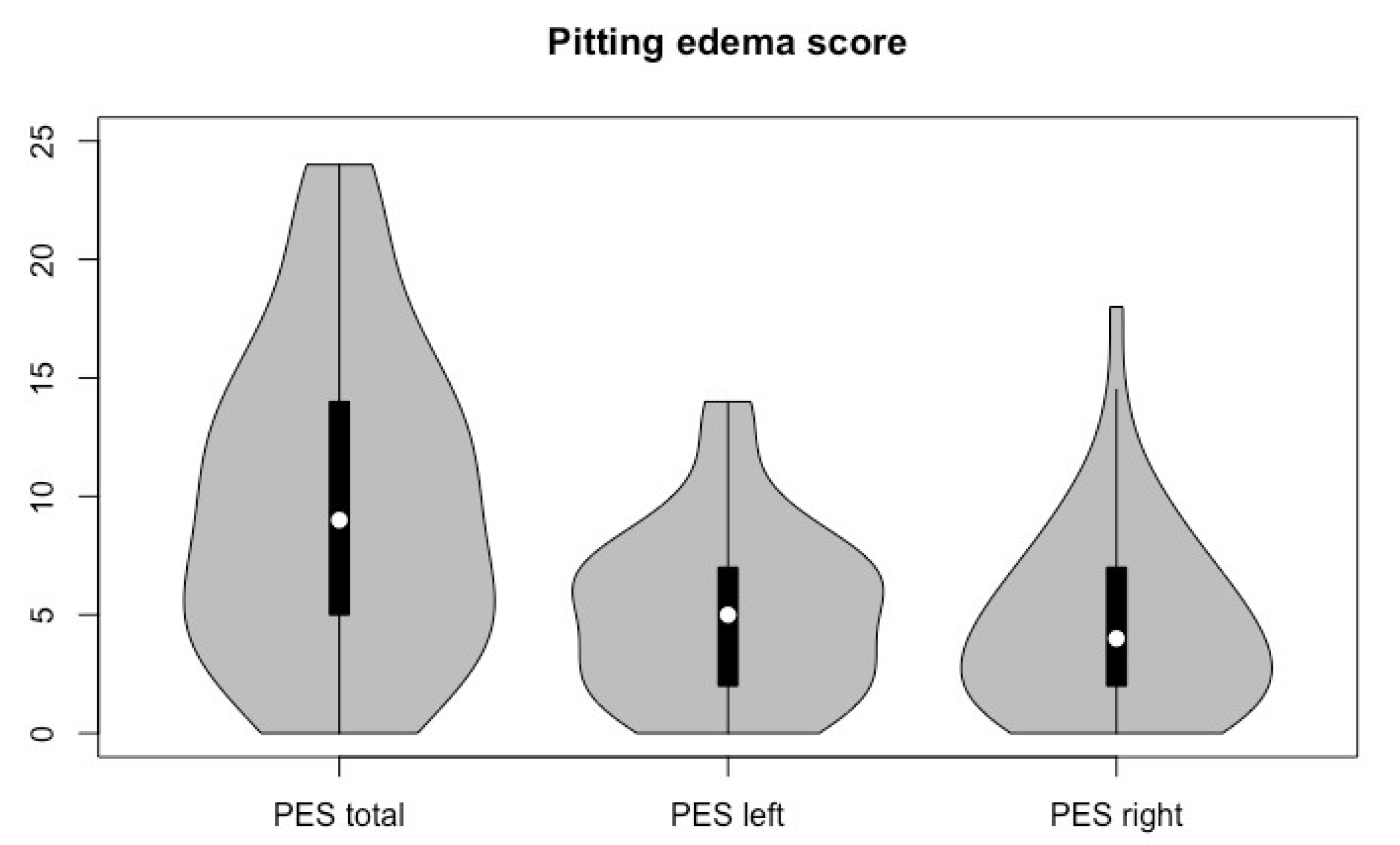
3.2. Lymphedema and Trismus
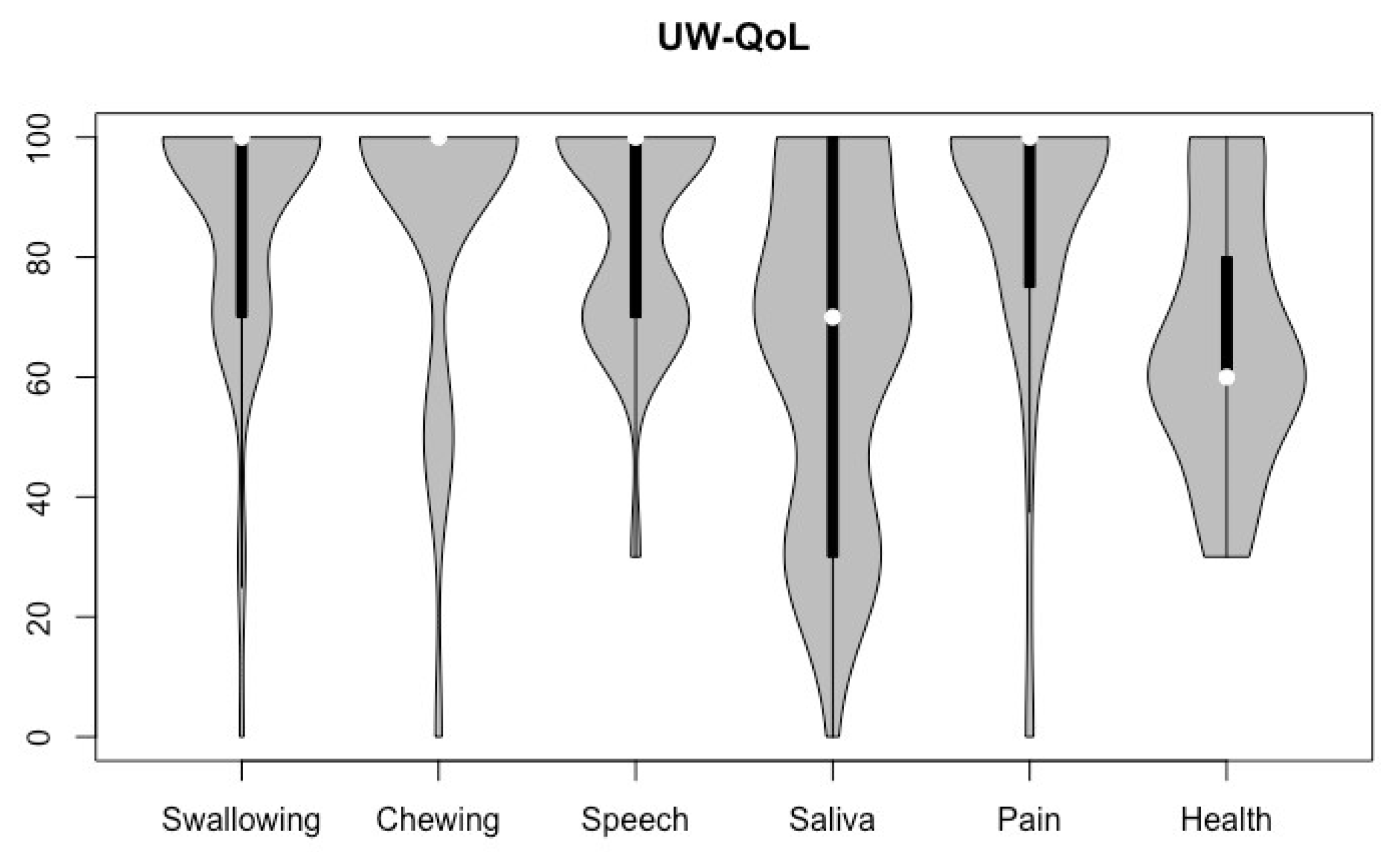
3.3. Body Image and Quality of Life
3.4. Correlation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, J.; Wulff-Burchfield, E.M.; Murphy, B.A. Late Soft Tissue Complications of Head and Neck Cancer Therapy: Lymphedema and Fibrosis. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz005. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Thankappan, K.; Janakiram, C.; Iyer, S.; Mathew, A. Etiopathogenesis of Trismus in Patients with Head and Neck Cancer: An Exploratory Literature Review. Craniomaxillofacial Trauma Reconstr. 2020, 13, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Karsten, R.T.; van der Molen, L.; Hamming-Vrieze, O.; van Son, R.J.J.H.; Hilgers, F.J.M.; van den Brekel, M.W.M.; Stuiver, M.M.; Smeele, L.E. Long-term swallowing, trismus, and speech, outcomes after combined chemoradiotherapy and preventive rehabilitation for head and neck cancer; 10-year plus update. Head Neck 2020, 42, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.G.; Lewin, J.S. The role of Lymphedema Management in Head And Neck Cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.G.; Hutcheson, K.A.; Little, L.G.; Skoracki, R.J.; Rosenthal, D.I.; Lai, S.Y.; Lewin, J.S. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol. Head Neck Surg. 2015, 152, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Jeans, C.; Brown, B.; Ward, E.C.; Vertigan, A.E.; Pigott, A.E.; Nixon, J.L.; Wratten, C. Comparing the prevalence, location, and severity of head and neck lymphedema after postoperative radiotherapy for oral cavity cancers and definitive chemoradiotherapy for oropharyngeal, laryngeal, and hypopharyngeal cancers. Head Neck 2020, 42, 3364–3374. [Google Scholar] [CrossRef]
- Deng, J.; Ridner, S.H.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Murphy, B.A. Prevalence of secondary lymphedema in patients with head and neck cancer. J. Pain Symptom Manag. 2012, 43, 244–252. [Google Scholar] [CrossRef]
- Deng, J.; Murphy, B.A.; Dietrich, M.S.; Sinard, R.J.; Mannion, K.; Ridner, S.H. Differences of symptoms in head and neck cancer patients with and without lymphedema. Support. Care Cancer 2016, 24, 1305–1316. [Google Scholar] [CrossRef]
- Johnson, J.; Johansson, M.; Ryden, A.; Houltz, E.; Finizia, C. Impact of trismus on health-related quality of life and mental health. Head Neck 2015, 37, 1672–1679. [Google Scholar] [CrossRef]
- van der Geer, S.J.; Kamstra, J.I.; Roodenburg, J.L.; van Leeuwen, M.; Reintsema, H.; Langendijk, J.A.; Dijkstra, P.U. Predictors for trismus in patients receiving radiotherapy. Acta Oncol. 2016, 55, 1318–1323. [Google Scholar] [CrossRef]
- Loorents, V.; Rosell, J.; Salgado Willner, H.; Borjeson, S. Health-related quality of life up to 1 year after radiotherapy in patients with head and neck cancer (HNC). Springerplus 2016, 5, 669. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Murphy, B.A.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Gilbert, J.; Ridner, S.H. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck 2013, 35, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ridner, S.H.; Aulino, J.M.; Murphy, B.A. Assessment and measurement of head and neck lymphedema: State-of-the-science and future directions. Oral Oncol. 2015, 51, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Dietrich, M.S.; Ridner, S.H.; Fleischer, A.C.; Wells, N.; Murphy, B.A. Preliminary evaluation of reliability and validity of head and neck external lymphedema and fibrosis assessment criteria. Eur. J. Oncol. Nurs. 2016, 22, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Saund, D.S.; Pearson, D.; Dietrich, T. Reliability and validity of self-assessment of mouth opening: A validation study. BMC Oral Health 2012, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Jager-Wittenaar, H.; Dijkstra, P.U.; Vissink, A.; van Oort, R.P.; Roodenburg, J.L. Variation in repeated mouth-opening measurements in head and neck cancer patients with and without trismus. Int. J. Oral Maxillofac. Surg. 2009, 38, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.U.; Huisman, P.M.; Roodenburg, J.L. Criteria for trismus in head and neck oncology. Int. J. Oral Maxillofac. Surg. 2006, 35, 337–342. [Google Scholar] [CrossRef]
- Hopwood, P.; Fletcher, I.; Lee, A.; Al Ghazal, S. A body image scale for use with cancer patients. Eur. J. Cancer 2001, 37, 189–197. [Google Scholar] [CrossRef]
- van Verschuer, V.M.; Vrijland, W.W.; Mares-Engelberts, I.; Klem, T.M. Reliability and validity of the Dutch-translated Body Image Scale. Qual. Life Res. 2015, 24, 1629–1633. [Google Scholar] [CrossRef]
- Melissant, H.C.; Neijenhuijs, K.I.; Jansen, F.; Aaronson, N.K.; Groenvold, M.; Holzner, B.; Terwee, C.B.; van Uden-Kraan, C.F.; Cuijpers, P.; Verdonck-de Leeuw, I.M. A systematic review of the measurement properties of the Body Image Scale (BIS) in cancer patients. Support. Care Cancer 2018, 26, 1715–1726. [Google Scholar] [CrossRef]
- Weymuller, E.A.; Alsarraf, R.; Yue, B. Analysis of the Performance Characteristics of the University of Washington Quality of Life Instrument and Its Modification (UW-QOL-R). Arch. Otolaryngol. Head Neck Surg. 2001, 127, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Watters, A.L.; Cope, S.; Keller, M.N.; Padilla, M.; Enciso, R. Prevalence of trimsus in patients with head and neck cancer: A systematic review with meta-analysis. Head Neck 2019, 41, 3408–3421. [Google Scholar] [CrossRef] [PubMed]
- Bensadoun, R.J.; Riesenbeck, D.; Lockhart, P.B.; Elting, L.S.; Spijkervet, F.K.; Brennan, M.T.; Trismus Section, Oral Care Study Group, Multinational Association for Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support. Care Cancer 2010, 18, 1033–1038. [Google Scholar] [CrossRef]
- Weber, C.; Dommerich, S.; Pau, H.W.; Kramp, B. Limited mouth opening after primary therapy of head and neck cancer. Oral Maxillofac. Surg. 2010, 14, 169–173. [Google Scholar] [CrossRef]
- Kraaijenga, S.A.C.; van der Molen, L.; Joacobi, I.; Hamming-Vrieze, O.; Hilgers, F.J.; van den Brekel, M.W.M. Prospective clinical study on long-term swallowing function and voice quality in advanced head and neck cancer patients treated with concurrent chemoradiotherapy and preventive swallowing exercises. Eur. Arch. Otorhinolaryngol. 2015, 272, 3521–3531. [Google Scholar] [CrossRef]
- Kraaijenga, S.A.; Hamming-Vrieze, O.; Verheijen, S.; Lamers, E.; van der Molen, L.; Hilgers, F.J.; van den Brekel, M.W.M.; Heemsbergen, W.D. Radiation dose to the masseter and medial pterygoid muscle in relation to trismus after chemoradiotherapy for advanced head and neck cancer. Head Neck 2019, 41, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.; McSharry, L.; Lawson, S.; Regan, J. The impact of dysphagia prehabilitation on swallowing outcomes post-chemoradiation therapy in head and neck cancer: A systematic review. Eur. J. Cancer Care 2022, 31, e13549. [Google Scholar] [CrossRef]
- Hashemipour, M.A.; Pooyafard, A.; Navabi, N.; Kakoie, S.; Rahbanian, N. Quality of life in Iranian patients with head-and-neck cancer. J. Educ. Health Promot. 2020, 9, 358. [Google Scholar] [CrossRef]
| Characteristics | (N = 59) |
|---|---|
| Sex (Male) | 41 (69.5%) |
| Age | 60.6 (10) 1 |
| BMI | 24.3 (22–25.9) 2 |
| Time since treatment (months) | 12.9 (3.3) 1 |
| Primary site | |
| Skin | 4 (6.8%) |
| Hypopharynx | 3 (5.1%) |
| Larynx | 7 (11.9%) |
| Oral cavity | 7 (11.9%) |
| Nasopharynx | 5 (8.5%) |
| Oropharynx | 19 (32%) |
| Thyroid | 1 (1.7%) |
| Salivary glad | 7 (11.9%) |
| Other | 3 (5.1%) |
| Unknown primary | 3 (5.1%) |
| T-class | |
| X | 6 (10.2%) |
| 1 | 18 (30.5%) |
| 2 | 14 (23.7%) |
| 3 | 7 (11.9%) |
| 4 | 13 (22%) |
| NA | 1 (1.7%) |
| N-class | |
| 0 | 21 (35.6%) |
| 1 | 17 (28.8%) |
| 2 | 16 (27.1%) |
| 3 | 4 (6.8%) |
| NA | 1 (1.7%) |
| M-class | |
| 0 | 58 (96.6%) |
| 1 | 1 (1.7%) |
| NA | 1 (1.7%) |
| Treatment received | |
| Radiotherapy | |
| Concomitant | 13 (22%) |
| Postoperative | 22 (37.3%) |
| Primary | 24 (40.7%) |
| Chemotherapy (concomintant Cisplatin) | 12 (20.3%) |
| Surgery (selevtive/comprehensive neck dissection) | |
| No | 44 (74.6%) |
| Unilateral | 13 (22%) |
| Bilateral | 2 (3.4%) |
| Immunotherapy | 4 (7%) |
| Avelumab | 1 (1.7%) |
| Ipilimumab | 3 (5.1%) |
| Nivolumab | 3 (5.1%) |
| Correlates | Spearman rho | 95% CI | p-Value |
|---|---|---|---|
| BIS | 0.08 | −0.19–0.35 | 0.544 |
| Swallowing | 0.02 | −0.26–0.26 | 0.868 |
| Chewing | −0.19 | −0.46–0.1 | 0.143 |
| Speech | −0.39 | −0.61–−0.14 | 0.003 |
| Saliva | 0.03 | −0.21–0.31 | 0.830 |
| Pain | 0.06 | −0.24–0.32 | 0.639 |
| Overall health | −0.09 | −0.24–0.32 | 0.516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arends, C.R.; van der Molen, L.; Lindhout, J.E.; Bragante, K.; Navran, A.; van den Brekel, M.W.M.; Stuiver, M.M. Lymphedema and Trismus after Head and Neck Cancer, and the Impact on Body Image and Quality of Life. Cancers 2024, 16, 653. https://doi.org/10.3390/cancers16030653
Arends CR, van der Molen L, Lindhout JE, Bragante K, Navran A, van den Brekel MWM, Stuiver MM. Lymphedema and Trismus after Head and Neck Cancer, and the Impact on Body Image and Quality of Life. Cancers. 2024; 16(3):653. https://doi.org/10.3390/cancers16030653
Chicago/Turabian StyleArends, Coralie R., Lisette van der Molen, Josephine E. Lindhout, Karoline Bragante, Arash Navran, Michiel W. M. van den Brekel, and Martijn M. Stuiver. 2024. "Lymphedema and Trismus after Head and Neck Cancer, and the Impact on Body Image and Quality of Life" Cancers 16, no. 3: 653. https://doi.org/10.3390/cancers16030653
APA StyleArends, C. R., van der Molen, L., Lindhout, J. E., Bragante, K., Navran, A., van den Brekel, M. W. M., & Stuiver, M. M. (2024). Lymphedema and Trismus after Head and Neck Cancer, and the Impact on Body Image and Quality of Life. Cancers, 16(3), 653. https://doi.org/10.3390/cancers16030653







