Preoperative High C-Reactive Protein to Albumin Ratio Predicts Short- and Long-Term Postoperative Outcomes in Elderly Gastric Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Procedure and Follow-Up
2.3. Preoperative Assessments
2.4. Preoperative Predictive Scoring Model
2.5. Statistical Analysis
3. Results
3.1. Comparison of Clinicopathological Characteristics between Young and Elderly Patient Groups
3.2. Identification and Comparison of Risk Factors for Postoperative Complications between Young and Elderly Patient Groups
3.3. Prognostic Significance of Factors Identified as Risk Factors for Postoperative Complications
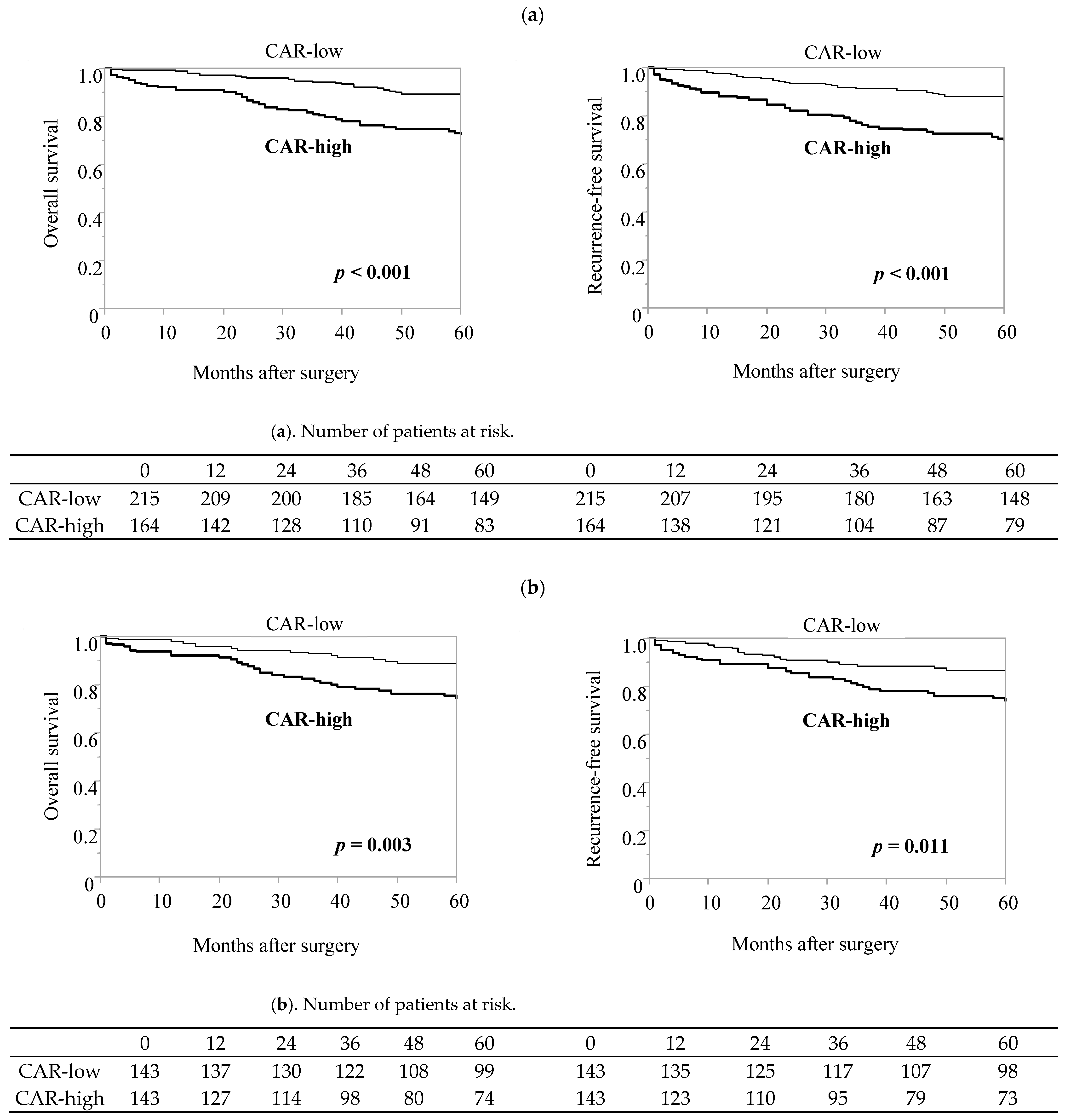
3.4. Efficacy of CAR in Predicting the Incidence of Postoperative Complications and Long-Term Prognosis in the Elderly Patient Group after Propensity Score Matching
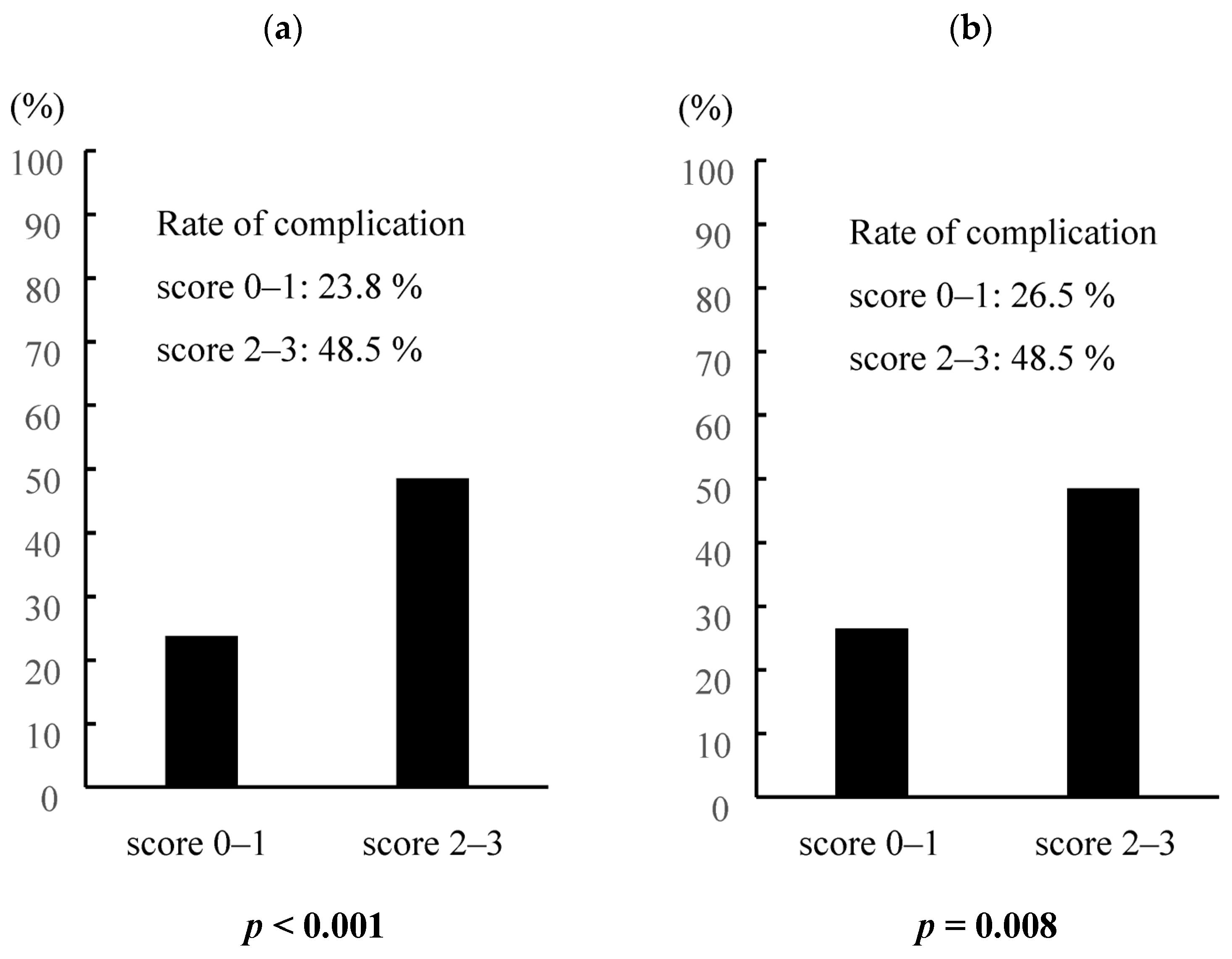
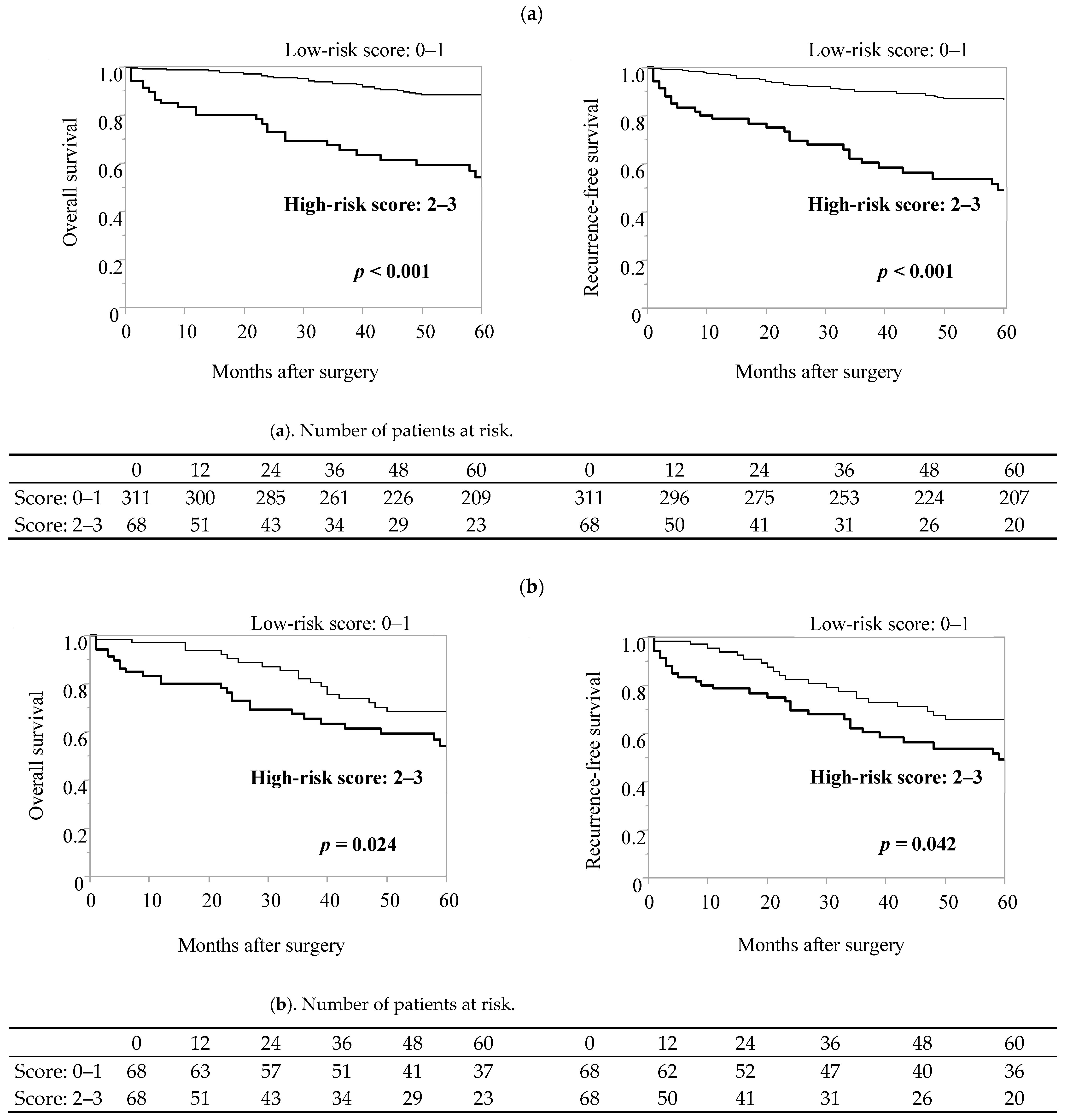
3.5. Preoperative Predictive Scoring Model for Elderly Gastric Cancer Patients Based on CAR, ASA-PS, Surgical Procedures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nashimoto, A.; Akazawa, K.; Isobe, Y.; Miyashiro, I.; Katai, H.; Kodera, Y.; Tsujitani, S.; Seto, Y.; Furukawa, H.; Oda, I.; et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer 2013, 16, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Sawada, N.; Oae, S.; Seki, J.; Takano, Y.; Ishiyama, Y.; Nakahara, K.; Maeda, C.; Hidaka, E.; Ishida, F.; et al. Safety and curability of laparoscopic gastrectomy in elderly patients with gastric cancer. Surg. Endosc. 2018, 32, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, T.; Hiki, N.; Nunobe, S.; Honda, M.; Ohashi, M.; Sano, T.; Yamaguchi, T. Feasibility of Gastrectomy with Standard Lymphadenectomy for Patients Over 85 Years Old with Gastric Cancer. Ann. Surg. Oncol. 2015, 22, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Lee, H.J.; Kim, T.H.; Huh, Y.J.; Son, Y.G.; Park, J.H.; Ahn, H.S.; Suh, Y.S.; Kong, S.H.; Yang, H.K. Short- and Long-Term Outcomes After Gastrectomy in Elderly Gastric Cancer Patients. Ann. Surg. Oncol. 2017, 24, 469–477. [Google Scholar] [CrossRef]
- Hayashi, T.; Yoshikawa, T.; Aoyama, T.; Ogata, T.; Cho, H.; Tsuburaya, A. Severity of complications after gastrectomy in elderly patients with gastric cancer. World J. Surg. 2012, 36, 2139–2145. [Google Scholar] [CrossRef]
- Takeuchi, D.; Koide, N.; Suzuki, A.; Ishizone, S.; Shimizu, F.; Tsuchiya, T.; Kumeda, S.; Miyagawa, S. Postoperative complications in elderly patients with gastric cancer. J. Surg. Res. 2015, 198, 317–326. [Google Scholar] [CrossRef]
- Liang, Y.X.; Deng, J.Y.; Guo, H.H.; Ding, X.W.; Wang, X.N.; Wang, B.G.; Zhang, L.; Liang, H. Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World J. Gastroenterol. 2013, 19, 6568–6578. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, X.; Gao, P.; Song, Y.; Huang, X.; Yang, Y.; Zhao, J.; Ma, B.; Gao, X.; Wang, Z. Can the Neutrophil to Lymphocyte Ratio Be Used to Determine Gastric Cancer Treatment Outcomes? A Systematic Review and Meta-Analysis. Dis. Markers 2016, 2016, 7862469. [Google Scholar] [CrossRef]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hong, D.; Zhai, Y.; Shen, P. Meta-analysis of associations between neutrophil-to-lymphocyte ratio and prognosis of gastric cancer. World J. Surg. Oncol. 2015, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.T.; Wang, C.C.; Le, P.H.; Chen, T.H.; Kuo, C.J.; Lin, C.J.; Chou, W.C.; Yeh, T.S. Lymphocyte-to-monocyte ratios predict gastric cancer surgical outcomes. J. Surg. Res. 2016, 202, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Liu, Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: A meta-analysis. Int. J. Surg. 2018, 50, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.C.; Jia, Z.F.; Cao, D.H.; Wu, Y.H.; Jiang, J.; Wen, S.M.; Zhao, D.; Zhang, S.L.; Cao, X.Y. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine 2018, 97, e13896. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Kono, Y.; Murakami, Y.; Shishido, Y.; Kuroda, H.; Matsunaga, T.; Fukumoto, Y.; Osaki, T.; Ashida, K.; Fujiwara, Y. Prognostic Significance of the Preoperative Ratio of C-Reactive Protein to Albumin and Neutrophil-Lymphocyte Ratio in Gastric Cancer Patients. World J. Surg. 2018, 42, 1819–1825. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Liu, J.; Kong, P.; Chen, S.; Zhan, Y.; Xu, D. Preoperative C-Reactive Protein/Albumin Ratio Predicts Prognosis of Patients after Curative Resection for Gastric Cancer. Transl. Oncol. 2015, 8, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, C.; Liu, Z.; Ai, S.; Guan, W.; Liu, S. Controlling Nutritional Status (CONUT) score as a predictive marker for short-term complications following gastrectomy of gastric cancer: A retrospective study. BMC Gastroenterol. 2021, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, D.; Sawayama, H.; Kurashige, J.; Iwatsuki, M.; Eto, T.; Tokunaga, R.; Kitano, Y.; Yamamura, K.; Ouchi, M.; Nakamura, K.; et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 2018, 21, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Jia, Z.; Chen, D.; Xu, C.; Yang, P. The prognostic value of the C-reactive protein to albumin ratio in cancer: An updated meta-analysis. Medicine 2020, 99, e19165. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Y.; Wang, H.; Ding, C.; Tian, H.; Zhang, X.; Gong, J.; Zhu, W.; Li, N. Diagnostic accuracy of the postoperative ratio of C-reactive protein to albumin for complications after colorectal surgery. World J. Surg. Oncol. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yamasaki, N.; Tsuchiya, T.; Matsumoto, K.; Kunizaki, M.; Kamohara, R.; Hatachi, G.; Doi, R.; Obata, T.; Nagayasu, T. Ratio of C-reactive protein to albumin is a prognostic factor for operable non-small-cell lung cancer in elderly patients. Surg. Today 2017, 47, 836–843. [Google Scholar] [CrossRef]
- Hashimoto, S.; Tominaga, T.; Nonaka, T.; Hamada, K.; Araki, M.; Takeshita, H.; Fukuoka, H.; Wada, H.; To, K.; Komatsu, H.; et al. The C-reactive protein to albumin ratio predicts postoperative complications in oldest-old patients with colorectal cancer. Int. J. Colorectal Dis. 2020, 35, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, X.; Chen, J.; Xing, J.; Hei, Z.; Zhang, Q.; Liu, Z.; Zhou, S. Association between Preoperative hs-crp/Albumin Ratio and Postoperative sirs in Elderly Patients: A Retrospective Observational Cohort Study. J. Nutr. Health Aging 2022, 26, 352–359. [Google Scholar] [CrossRef]
- Kim, M.H.; Ahn, J.Y.; Song, J.E.; Choi, H.; Ann, H.W.; Kim, J.K.; Kim, J.H.; Jeon, Y.D.; Kim, S.B.; Jeong, S.J.; et al. The C-Reactive Protein/Albumin Ratio as an Independent Predictor of Mortality in Patients with Severe Sepsis or Septic Shock Treated with Early Goal-Directed Therapy. PLoS ONE 2015, 10, e0132109. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ge, X.; Liu, Z.; Du, S.; Ai, S.; Guan, W. Postoperative C-reactive protein/albumin ratio as a novel predictor for short-term complications following gastrectomy of gastric cancer. World J. Surg. Oncol. 2017, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liang, Z.; Lu, F.; Fang, X.; Liu, S.; Zeng, Y.; Zhu, F.; Chen, X.; Shen, T.; Li, J.; et al. Toll-like receptors and cytokines/cytokine receptors polymorphisms associate with non-response to hepatitis B vaccine. Vaccine 2011, 29, 706–711. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 15th ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2022, 26, 1–25. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Li, H.; Manwani, B.; Leng, S.X. Frailty, inflammation, and immunity. Aging Dis. 2011, 2, 466–473. [Google Scholar] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Bain, C.R.; Myles, P.S.; Corcoran, T.; Dieleman, J.M. Postoperative systemic inflammatory dysregulation and corticosteroids: A narrative review. Anaesthesia 2023, 78, 356–370. [Google Scholar] [CrossRef]
- Lahiri, R.; Derwa, Y.; Bashir, Z.; Giles, E.; Torrance, H.D.; Owen, H.C.; O’Dwyer, M.J.; O’Brien, A.; Stagg, A.J.; Bhattacharya, S.; et al. Systemic Inflammatory Response Syndrome After Major Abdominal Surgery Predicted by Early Upregulation of TLR4 and TLR5. Ann. Surg. 2016, 263, 1028–1037. [Google Scholar] [CrossRef]
- Irvin, T.T. Effects of malnutrition and hyperalimentation on wound healing. Surg. Gynecol. Obstet. 1978, 146, 33–37. [Google Scholar]
- Fukuda, Y.; Yamamoto, K.; Hirao, M.; Nishikawa, K.; Maeda, S.; Haraguchi, N.; Miyake, M.; Hama, N.; Miyamoto, A.; Ikeda, M.; et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S778–S785. [Google Scholar] [CrossRef]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef]
- Senjo, H.; Onozawa, M.; Hidaka, D.; Yokoyama, S.; Yamamoto, S.; Tsutsumi, Y.; Haseyama, Y.; Nagashima, T.; Mori, A.; Ota, S.; et al. High CRP-albumin ratio predicts poor prognosis in transplant ineligible elderly patients with newly diagnosed acute myeloid leukemia. Sci. Rep. 2022, 12, 8885. [Google Scholar] [CrossRef]
- Xu, X.L.; Yu, H.Q.; Hu, W.; Song, Q.; Mao, W.M. A Novel Inflammation-Based Prognostic Score, the C-Reactive Protein/Albumin Ratio Predicts the Prognosis of Patients with Operable Esophageal Squamous Cell Carcinoma. PLoS ONE 2015, 10, e0138657. [Google Scholar] [CrossRef]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Tanaka, K.; Fushiya, N.; Koike, K.; Nishino, H.; Matsushima, M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann. Surg. Oncol. 2015, 22, 803–810. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef]
- Chojkier, M. Inhibition of albumin synthesis in chronic diseases: Molecular mechanisms. J. Clin. Gastroenterol. 2005, 39, S143–S146. [Google Scholar] [CrossRef]
- Piazuelo, M.B.; Riechelmann, R.P.; Wilson, K.T.; Algood, H.M.S. Resolution of Gastric Cancer-Promoting Inflammation: A Novel Strategy for Anti-cancer Therapy. Curr. Top. Microbiol. Immunol. 2019, 421, 319–359. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef]
- Argilés, J.M. Cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. S2), S39–S50. [Google Scholar] [CrossRef]
- Matsunaga, T.; Saito, H.; Osaki, T.; Takahashi, S.; Iwamoto, A.; Fukuda, K.; Taniguchi, K.; Kuroda, H.; Takeuchi, T.; Sugamura, K.; et al. Impact of geriatric nutritional risk index on outcomes after gastrectomy in elderly patients with gastric cancer: A retrospective multicenter study in Japan. BMC Cancer 2022, 22, 540. [Google Scholar] [CrossRef]
- Forget, P.; Machiels, J.P.; Coulie, P.G.; Berliere, M.; Poncelet, A.J.; Tombal, B.; Stainier, A.; Legrand, C.; Canon, J.L.; Kremer, Y.; et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann. Surg. Oncol. 2013, 20 (Suppl. S3), S650–S660. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Fowkes, F.G.; Belch, J.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.; Meade, T.W.; Mehta, Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef]
- Ham, I.H.; Oh, H.J.; Jin, H.; Bae, C.A.; Jeon, S.M.; Choi, K.S.; Son, S.Y.; Han, S.U.; Brekken, R.A.; Lee, D.; et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, 68. [Google Scholar] [CrossRef]
- Ishizuka, M.; Nagata, H.; Takagi, K.; Iwasaki, Y.; Shibuya, N.; Kubota, K. Clinical Significance of the C-Reactive Protein to Albumin Ratio for Survival After Surgery for Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 900–907. [Google Scholar] [CrossRef] [PubMed]

| All Patients (N = 571) | Elderly (≥65) Patient Group (n = 379) | Young (<65) Patient Group (n = 192) | p-Value | |
|---|---|---|---|---|
| Sex | 0.240 | |||
| Male | 393 (68.8) | 267 (70.5) | 126 (65.6) | |
| Female | 178 (31.2) | 112 (29.5) | 66 (34.4) | |
| BMI | 22.1 (14.5–32.9) | 22.3 (14.5–31.5) | 22.0 (15.1–32.9) | 0.670 |
| ASA-PS | <0.001 | |||
| 1 | 61 (10.7) | 32 (8.5) | 29 (15.1) | |
| 2 | 470 (82.3) | 309 (81.5) | 161 (83.9) | |
| 3 | 40 (7.0) | 38 (10.0) | 2 (1.0) | |
| Charlson Comorbidity Index * | <0.001 | |||
| Low: 0 | 326 (57.1) | 167 (44.1) | 159 (82.8) | |
| Medium: 1–2 | 187 (32.8) | 158 (41.7) | 29 (15.1) | |
| High: 3–4 | 47 (8.2) | 44 (11.6) | 3 (1.6) | |
| Very high: ≥5 | 11 (1.9) | 10 (2.6) | 1 (0.5) | |
| Preoperative evaluation | ||||
| WBC (μL) | 5600 (2300–15,200) | 5590 (2300–15,200) | 5650 (2420–11,960) | 0.512 |
| Ne (μL) | 3339 (930–12,070) | 3317 (930–12,070) | 3401 (1111–11,362) | 0.277 |
| Ly (μL) | 1670 (120–5426) | 1679 (280–5426) | 1651 (120–3902) | 0.987 |
| Mo (μL) | 319 (69–1376) | 333 (99–984) | 288 (69–1376) | <0.001 |
| Alb (g/dL) | 4.1 (2.2–5.3) | 3.6 (2.2–5.1) | 3.9 (2.3–5.3) | <0.001 |
| CRP (mg/L) | 0.07 (0.02–11.90) | 0.07 (0.02–11.90) | 0.05 (0.02–3.93) | <0.001 |
| Total cholesterol (mg/mL) | 191 (92–321) | 189 (92–320) | 198 (116–321) | 0.003 |
| CEA (ng/mL) | 2.2 (0.5–64.0) | 2.3 (0.5–64.0) | 1.8 (0.5–14.7) | <0.001 |
| CA19-9 (U/mL) | 5.0 (0.04–7775.0) | 5.0 (0.04–7775.0) | 5.0 (2.0–182.0) | 0.213 |
| Preoperative nutrition and inflammation markers | ||||
| NLR | 1.99 (0.51–95.00) | 1.97 (0.51–13.80) | 2.01 (0.65–95.00) | 0.435 |
| LMR | 5.15 (0.25–22.07) | 4.97 (0.86–17.19) | 5.80 (0.25–22.08) | 0.004 |
| CAR | 0.016 (0.004–3.838) | 0.019 (0.004–3.838) | 0.012 (0.004–0.914) | <0.001 |
| CONUT score | 0.001 | |||
| Normal (0–1) | 351 (61.5) | 213 (56.2) | 138 (71.9) | |
| Light malnutrition (2–4) | 182 (31.9) | 134 (35.4) | 48 (25.0) | |
| Moderate malnutrition (5–8) | 34 (5.9) | 30 (7.9) | 4 (2.1) | |
| Severe malnutrition (9–12) | 4 (0.7) | 2 (0.5) | 2 (1.0) | |
| Procedure | 0.569 | |||
| DG | 378 (66.2) | 247 (65.2) | 131 (68.2) | |
| TG | 131 (22.9) | 92 (24.3) | 39 (20.3) | |
| PG | 54 (9.5) | 36 (9.5) | 18 (9.4) | |
| PPG | 8 (1.4) | 4 (1.0) | 4 (2.1) | |
| Approach | 0.101 | |||
| Laparoscopy | 357 (62.5) | 228 (60.2) | 129 (67.2) | |
| Open | 214 (37.5) | 151 (39.8) | 63 (32.8) | |
| Operative time (min) | 298 (142–885) | 301 (142–749) | 293 (147–885) | 0.598 |
| Intraoperative bleeding (mL) | 56 (0–6882) | 60 (0–6882) | 50 (4–1550) | 0.280 |
| Intraoperative blood transfusion | 0.130 | |||
| No | 542 (94.9) | 356 (93.9) | 186 (96.9) | |
| Yes | 29 (5.1) | 23 (6.1) | 6 (3.1) | |
| Postoperative complications (≥CD II) | 0.006 | |||
| Absent | 430 (75.3) | 272 (71.8) | 158 (82.3) | |
| Present | 141 (24.7) | 107 (28.2) | 34 (17.7) | |
| Hospital stays (days) | 12 (7–344) | 13 (8–136) | 11 (7–344) | <0.001 |
| Location of tumor | 0.065 | |||
| Upper | 143 (25.0) | 102 (26.9) | 41 (21.4) | |
| Middle | 207 (36.3) | 125 (33.0) | 82 (42.7) | |
| Low | 221 (38.7) | 152 (40.1) | 69 (35.9) | |
| Histopathological type ** | <0.001 | |||
| Differentiated | 281 (49.2) | 218 (57.5) | 63 (32.8) | |
| Undifferentiated | 290 (50.8) | 161 (42.5) | 129 (67.2) | |
| Depth of tumor *** | 0.550 | |||
| T1a,b | 393 (68.8) | 254 (67.0) | 139 (72.4) | |
| T2 | 65 (11.4) | 45 (11.9) | 20 (10.4) | |
| T3 | 60 (10.5) | 41 (10.8) | 19 (9.9) | |
| T4a,b | 53 (9.3) | 39 (10.3) | 14 (7.3) | |
| Lymph node metastasis *** | 0.281 | |||
| N0 | 430 (75.3) | 279 (73.6) | 151 (78.6) | |
| N1 | 61 (10.7) | 44 (11.6) | 17 (8.9) | |
| N2 | 40 (7.0) | 31 (8.2) | 9 (4.7) | |
| N3 | 40 (7.0) | 25 (6.6) | 15 (7.8) | |
| Pathological stage *** | 0.536 | |||
| I | 429 (75.1) | 280 (73.9) | 149 (77.6) | |
| II | 61 (10.7) | 41 (10.8) | 20 (10.4) | |
| III | 81 (14.2) | 58 (15.3) | 23 (12.0) |
| All Patients (N = 571) | Complication (−) (n = 430) | Complication (+) (n = 141) | p-Value | |
|---|---|---|---|---|
| Age | 69 (21–89) | 68 (21–89) | 71 834–89) | 0.001 |
| Sex | 0.407 | |||
| Male | 393 (68.8) | 292 (67.9) | 101 (71.6) | |
| Female | 178 (31.2) | 138 (32.1) | 40 (28.4) | |
| BMI | 22.1 (14.5–32.9) | 22.1 (14.5–32.9) | 22.1 (15.5–31.5) | 0.711 |
| ASA-PS | 0.005 | |||
| 1 | 61 (10.7) | 50 (11.6) | 11 (7.8) | |
| 2 | 470 (82.3) | 358 (83.3) | 112 (79.4) | |
| 3 | 40 (7.0) | 22 (5.1) | 18 (12.8) | |
| Charlson Comorbidity Index * | <0.001 | |||
| Low: 0 | 326 (57.1) | 262 (60.9) | 64 (45.4) | |
| Medium: 1–2 | 187 (32.8) | 134 (31.2) | 53 (37.6) | |
| High: 3–4 | 47 (8.2) | 25 (5.8) | 22 (15.6) | |
| Very high: ≥5 | 11 (1.9) | 9 (2.1) | 2 (1.4) | |
| Preoperative evaluation | ||||
| WBC (μL) | 5600 (2300–15,200) | 5600 (2300–13,890) | 5620 (2420–15,200) | 0.299 |
| Ne (μL) | 3339 (930–12,070) | 3329 (979–12,070) | 3347 (930–9634) | 0.749 |
| Ly (μL) | 1670 (120–5426) | 1711 (120–4681) | 1592 (266–5426) | 0.031 |
| Mo (μL) | 319 (69–1376) | 315 (69–1376) | 332 (110–983) | 0.099 |
| Alb (g/dL) | 4.1 (2.2–5.3) | 4.2 (2.4–5.3) | 4.0 (2.2–5.1) | <0.001 |
| CRP (mg/L) | 0.07 (0.02–11.90) | 0.06 (0.02–11.90) | 0.10 (0.02–4.04) | 0.020 |
| Total cholesterol (mg/mL) | 191 (92–321) | 193 (112–321) | 185 (92–286) | 0.003 |
| CEA (ng/mL) | 2.2 (0.5–64.0) | 2.0 (0.5–64.0) | 2.4 (0.5–23.5) | 0.005 |
| CA19-9 (U/mL) | 5.0 (0.04–7775.0) | 5.0 (0.04–7775.0) | 6.0 (2.0–429.0) | 0.328 |
| Preoperative nutrition and inflammation markers | ||||
| NLR | 1.99 (0.51–95.00) | 1.96 (0.52–95.00) | 2.05 (0.51–13.83) | 0.196 |
| LMR | 5.15 (0.25–22.07) | 5.41 (0.25–22.07) | 4.72 (0.48–11.26) | 0.005 |
| CAR | 0.016 (0.004–3.838) | 0.014 (0.004–3.839) | 0.024(0.004–1.154) | 0.010 |
| CONUT score | 0.012 | |||
| Normal (0–1) | 351 (61.5) | 277 (64.4) | 74 (52.5) | |
| Light malnutrition (2–4) | 182 (31.9) | 129 (30.0) | 53 (37.6) | |
| Moderate malnutrition (5–8) | 34 (5.9) | 23 (5.4) | 11 (7.8) | |
| Severe malnutrition (9–12) | 4 (0.7) | 1 (0.2) | 3 (2.1) | |
| Procedure | <0.001 | |||
| DG | 378 (66.2) | 299 (69.5) | 79 (56.0) | |
| TG | 131 (22.9) | 82 (19.1) | 49 (34.8) | |
| PG | 54 (9.5) | 45 (10.5) | 9 (6.4) | |
| PPG | 8 (1.4) | 4 (0.9) | 4 (2.8) | |
| Approach | 0.005 | |||
| Laparoscopy | 357 (62.5) | 283 (65.8) | 74 (52.5) | |
| Open | 214 (37.5) | 147 (34.2) | 67 (47.5) | |
| Operation time (min) | 298 (142–885) | 298 (147–885) | 301 (142–755) | 0.209 |
| Intraoperative bleeding (mL) | 56 (0–6882) | 50 (0–2870) | 80 (8–6882) | <0.001 |
| Intraoperative blood transfusion | <0.001 | |||
| No | 542 (94.9) | 418 (97.2) | 124 (87.9) | |
| Yes | 29 (5.1) | 12 (2.8) | 17 (12.1) | |
| Hospital stays (days) | 12 (7–344) | 11 (7–35) | 22 (9–61) | <0.001 |
| Location of tumor | 0.532 | |||
| Upper | 143 (25.0) | 103 (24.0) | 40 (28.4) | |
| Middle | 207 (36.3) | 160 (37.2) | 47 (33.3) | |
| Low | 221 (38.7) | 167 (38.8) | 54 (38.3) | |
| Histopathological type ** | 0.371 | |||
| Differentiated | 281 (49.2) | 207 (48.1) | 74 (52.5) | |
| Undifferentiated | 290 (50.8) | 223 (51.9) | 67 (47.5) | |
| Depth of tumor *** | 0.010 | |||
| T1a,b | 393 (68.8) | 310 (72.1) | 83 (58.9) | |
| T2 | 65 (11.4) | 47 (10.9) | 18 (12.8) | |
| T3 | 60 (10.5) | 36 (8.4) | 24 (17.0) | |
| T4a,b | 53 (9.3) | 37 (8.6) | 16 (11.4) | |
| Lymph node metastasis *** | 0.232 | |||
| N0 | 430 (75.3) | 332 (77.2) | 98 (69.5) | |
| N1 | 61 (10.7) | 40 (9.3) | 21 (14.9) | |
| N2 | 40 (7.0) | 29 (6.7) | 11 (7.8) | |
| N3 | 40 (7.0) | 29 (6.7) | 11 (7.8) | |
| Pathological stage *** | 0.040 | |||
| I | 429 (75.1) | 334 (77.7) | 95 (67.4) | |
| II | 61 (10.7) | 39 (9.1) | 22 (15.6) | |
| III | 81 (14.2) | 57 (13.3) | 24 (17.0) | |
| (a) | ||||||||||
| Elderly Patient Group: n = 379 | Univariate Analysis | Multivariate Analysis | ||||||||
| Variables | Categories | Number of Patients with Complication (%) | 95% CI of OR | 95% CI of OR | ||||||
| OR | Low | High | p-Value | OR | Low | High | p-Value | |||
| ASA-PS | 3 | 17 (46.0) | 2.38 | 1.19 | 4.74 | 0.012 | 1.49 | 0.71 | 3.15 | 0.291 |
| 1, 2 | 90 (26.3) | |||||||||
| Charlson Comorbidity Index * | ≥Medium risk | 70 (33.0) | 1.73 | 1.09 | 2.75 | 0.020 | 1.48 | 0.90 | 2.43 | 0.118 |
| Low | 37 (22.2) | |||||||||
| CEA (ng/mL) | ≥5.0 | 18 (36.7) | 1.52 | 0.81 | 2.85 | 0.195 | ||||
| <5.0 | 87 (27.7) | |||||||||
| LMR *** | <5.08 | 63 (32.0) | 1.47 | 0.94 | 2.32 | 0.092 | ||||
| ≥5.08 | 44 (24.2) | |||||||||
| CAR *** | ≥0.024 | 59 (36.0) | 1.95 | 1.24 | 3.07 | 0.003 | 1.62 | 1.01 | 2.62 | 0.046 |
| <0.024 | 48 (22.3) | |||||||||
| CONUT score | ≥Light malnutrition | 52 (31.3) | 1.31 | 0.84 | 2.05 | 0.238 | ||||
| Normal | 55 (25.8) | |||||||||
| Procedure | TG | 37 (40.2) | 2.09 | 1.27 | 3.43 | 0.003 | 1.62 | 0.92 | 2.84 | 0.096 |
| DG, PG, PPG | 70 (24.4) | |||||||||
| Approach | Open | 51 (33.8) | 1.56 | 1.01 | 2.46 | 0.034 | 0.80 | 0.45 | 1.43 | 0.455 |
| Laparoscopy | 56 (24.6) | |||||||||
| Intraoperative blood transfusion | Yes | 13 (56.5) | 3.62 | 1.54 | 8.54 | 0.002 | 2.11 | 0.83 | 5.42 | 0.118 |
| No | 94 (26.4) | |||||||||
| Pathological stage ** | II, III | 39 (39.8) | 2.07 | 1.27 | 3.37 | 0.003 | 1.70 | 0.95 | 3.05 | 0.076 |
| I | 68 (24.2) | |||||||||
| (b) | ||||||||||
| Young Patient Group: n = 192 | Univariate Analysis | Multivariate Analysis | ||||||||
| Variables | Categories | Number of Patients with Complication (%) | 95% CI of OR | 95% CI of OR | ||||||
| OR | Low | High | p-Value | OR | Low | High | p-Value | |||
| ASA-PS | 3 | 1 (50.0) | 4.75 | 0.29 | 0.32 | 0.229 | ||||
| 1, 2 | 33 (17.4) | |||||||||
| Charlson Comorbidity Index * | ≥Medium risk | 7 (21.2) | 1.32 | 0.52 | 3.34 | 0.562 | ||||
| Low | 27 (17.0) | |||||||||
| CEA (ng/mL) | ≥5.0 | 1 (6.3) | 0.29 | 0.04 | 2.27 | 0.210 | ||||
| <5.0 | 33 (18.8) | |||||||||
| LMR *** | <5.08 | 20 (25.0) | 2.56 | 1.10 | 4.96 | 0.025 | 1.63 | 0.71 | 3.74 | 0.254 |
| ≥5.08 | 14 (12.5) | |||||||||
| CAR *** | ≥0.024 | 13 (25.5) | 1.95 | 0.89 | 4.27 | 0.089 | ||||
| <0.024 | 21 (14.9) | |||||||||
| CONUT score | ≥Light malnutrition | 15 (27.8) | 2.41 | 1.12 | 5.19 | 0.022 | 1.81 | 0.75 | 4.36 | 0.184 |
| Normal | 19 (13.8) | |||||||||
| Procedure | TG | 12 (30.8) | 2.65 | 1.17 | 5.99 | 0.017 | 2.00 | 0.74 | 5.39 | 0.171 |
| DG, PG, PPG | 22 (14.4) | |||||||||
| Approach | Open | 16 (25.4) | 2.10 | 1.06 | 4.47 | 0.042 | 1.15 | 0.46 | 2.84 | 0.768 |
| Laparoscopy | 18 (14.0) | |||||||||
| Intraoperative blood transfusion | Yes | 4 (66.7) | 10.40 | 1.82 | 59.36 | 0.001 | 3.90 | 0.57 | 26.80 | 0.166 |
| No | 30 (16.1) | |||||||||
| Pathological stage *** | II, III | 7 (16.3) | 0.89 | 0.35 | 2.18 | 0.781 | ||||
| I | 27 (18.1) | |||||||||
| (a) | |||||||||||||||
| Elderly Patient Group (n = 379) | Young Patient Group (n = 192) | ||||||||||||||
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||||||||
| 5-Year | 95% CI of OR | 5-Year | 95% CI of OR | ||||||||||||
| Variable | Categories | n (%) | Survival | p-Value | HR | Low | High | p-Value | n (%) | Survival | p-Value | HR | Low | High | p-Value |
| ASA-PS | 3 | 37 (9.8) | 51.5% | <0.001 | 2.34 | 1.21 | 4.55 | 0.012 | 2 (1.0) | 50.0% | 0.014 | 2.97 | 0.23 | 38.70 | 0.407 |
| 1, 2 | 342 (90.2) | 85.1% | 190 (99.0) | 92.4% | |||||||||||
| CCI * | ≥Medium risk | 212 (55.9) | 73.6% | <0.001 | 2.90 | 1.45 | 5.79 | 0.003 | 33 (17.2) | 84.5% | 0.047 | 2.64 | 0.64 | 10.93 | 0.181 |
| Low | 167 (44.1) | 92.7% | 159 (82.8) | 93.5% | |||||||||||
| CEA (ng/mL) | ≥5.0 | 49 (13.5) | 69.8% | 0.012 | 1.67 | 0.87 | 3.2 | 0.121 | 16 (8.3) | 87.1% | 0.427 | ||||
| <5.0 | 314 (86.5) | 84.6% | 16 (91.7) | 92.4% | |||||||||||
| LMR ** | <5.08 | 197 (52.0) | 75.4% | <0.001 | 1.67 | 0.90 | 3.07 | 0.102 | 80 (41.7) | 90.9% | 0.530 | ||||
| ≥5.08 | 182 (48.0) | 89.0% | 112 (58.3) | 92.7% | |||||||||||
| CAR ** | ≥0.024 | 164 (43.3) | 72.1% | <0.001 | 2.02 | 1.15 | 3.56 | 0.015 | 51 (26.6) | 86.2% | 0.070 | ||||
| <0.024 | 215 (56.7) | 89.3% | 141 (73.4) | 94.0% | |||||||||||
| CONUT score | ≥Light malnutrition | 166 (43.8) | 76.1% | 0.004 | 1.08 | 0.61 | 1.91 | 0.787 | 54 (28.1) | 92.6% | 0.564 | ||||
| Normal | 213 (56.2) | 86.7% | 138 (71.9) | 97.1% | |||||||||||
| Procedure | TG | 92 (24.3) | 67.2% | <0.001 | 1.92 | 1.06 | 3.50 | 0.033 | 39 (20.3) | 71.1% | <0.001 | 4.28 | 1.20 | 15.24 | 0.025 |
| DG, PG, PPG | 287 (75.7) | 86.7% | 153 (79.7) | 97.3% | |||||||||||
| Approach | Open | 151 (39.8) | 71.0% | <0.001 | 1.92 | 1.00 | 3.69 | 0.049 | 63 (32.8) | 75.2% | <0.001 | NA | NA | NA | 0.999 |
| Laparoscopy | 228 (60.2) | 89.1% | 129 (67.2) | 100.0% | |||||||||||
| Transfusion | Yes | 23 (6.1) | 60.2% | 0.003 | 0.90 | 0.39 | 2.09 | 0.812 | 6 (3.1) | 33.3% | <0.001 | 6.41 | 1.31 | 31.44 | 0.022 |
| No | 356 (93.9) | 83.4% | 129 (96.9) | 93.9% | |||||||||||
| pStage *** | II, III | 98 (25.9) | 69.7% | <0.001 | 1.11 | 0.6 | 2.04 | 0.740 | 43 (22.4) | 70.2% | <0.001 | 12.58 | 2.19 | 72.18 | 0.005 |
| I | 281 (74.1) | 86.0% | 149 (77.6) | 97.9% | |||||||||||
| (b) | |||||||||||||||
| Elderly Patient Group (n = 379) | Young Patient Group (n = 192) | ||||||||||||||
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||||||||
| 5-Year | 95% CI of OR | 5-Year | 95% CI of OR | ||||||||||||
| Variable | Categories | n (%) | Survival | p-Value | HR | Low | High | p-Value | n (%) | Survival | p-Value | HR | Low | High | p-Value |
| ASA-PS | 3 | 37 (9.8) | 44.7% | <0.001 | 2.51 | 1.34 | 4.67 | 0.004 | 2 (1.0) | 50.0% | 0.048 | 1.59 | 0.15 | 16.52 | 0.699 |
| 1, 2 | 342 (90.2) | 83.8% | 190 (99.0) | 90.4% | |||||||||||
| CCI * | ≥Medium risk | 212 (55.9) | 71.8% | <0.001 | 2.42 | 1.29 | 4.52 | 0.006 | 33 (17.2) | 78.8% | 0.014 | 2.13 | 0.64 | 7.04 | 0.217 |
| Low | 167 (44.1) | 91.1% | 159 (82.8) | 92.3% | |||||||||||
| CEA (ng/mL) | ≥5.0 | 49 (13.5) | 66.3% | 0.005 | 1.65 | 0.90 | 3.04 | 0.104 | 16 (8.3) | 87.5% | 0.175 | ||||
| <5.0 | 314 (86.5) | 83.1% | 16 (91.7) | 90.2% | |||||||||||
| LMR ** | <5.08 | 92 (24.3) | 64.1% | <0.001 | 1.72 | 0.97 | 3.05 | 0.064 | 80 (41.7) | 88.5% | 0.374 | ||||
| ≥5.08 | 287 (75.7) | 85.5% | 112 (58.3) | 91.0% | |||||||||||
| CAR ** | ≥0.024 | 151 (39.8) | 64.1% | <0.001 | 2.51 | 1.34 | 4.67 | 0.035 | 51 (26.6) | 84.3% | 0.107 | ||||
| <0.024 | 228 (60.2) | 85.5% | 141 (73.4) | 92.0% | |||||||||||
| CONUT score | ≥Light malnutrition | 23 (6.1) | 46.7% | <0.001 | 1.33 | 0.63 | 2.82 | 0.451 | 54 (28.1) | 88.7% | 0.684 | ||||
| Normal | 356 (93.9) | 82.5% | 138 (71.9) | 90.4% | |||||||||||
| Procedure | TG | 98 (25.9) | 64.2% | <0.001 | 1.48 | 0.83 | 2.62 | 0.104 | 39 (20.3) | 65.9& | <0.001 | 3.42 | 1.14 | 10.26 | 0.028 |
| DG, PG, PPG | 281 (74.1) | 85.6% | 153 (79.7) | 96.1% | |||||||||||
| Approach | Open | 151 (39.8) | 64.1% | <0.001 | 2.51 | 1.34 | 4.67 | 0.035 | 63 (32.8) | 65.9% | <0.001 | 7.49 | 0.84 | 67.05 | 0.072 |
| Laparoscopy | 228 (60.2) | 85.5% | 129 (67.2) | 96.5% | |||||||||||
| Transfusion | Yes | 23 (6.1) | 46.7% | <0.001 | 1.33 | 0.63 | 2.82 | 0.451 | 6 (3.1) | 16.7% | <0.001 | 4.82 | 1.16 | 20.07 | 0.031 |
| No | 356 (93.9) | 82.5% | 129 (96.9) | 92.4% | |||||||||||
| pStage *** | II, III | 98 (25.9) | 64.2% | <0.001 | 1.48 | 0.83 | 2.62 | 0.104 | 43 (22.4) | 66.7% | <0.001 | 6.58 | 1.91 | 22.72 | 0.003 |
| I | 281 (74.1) | 85.6% | 149 (77.6) | 96.6% | |||||||||||
| Elderly (≥65) Patient Group: N = 379 | Overall Cohort | Propensity Score-Matched Pairs | |||||
| Variables | Categories | CAR-Low (n = 215) | CAR-High (n = 164) | p-Value | CAR-Low (n = 143) | CAR-High (n = 143) | p-Value |
| Sex | male | 146 (67.9) | 121 (73.8) | 0.214 | 99 (69.2) | 102 (71.3) | 0.698 |
| Female | 69 (32.1) | 43 (26.2) | 44 (30.8) | 41 (28.7) | |||
| BMI | <18.5 | 23 (10.7) | 22 (13.4) | 0.418 | 18 (12.6) | 18 (12.6) | 1.000 |
| ≥18.5 | 192 (89.3) | 142 (86.6) | 0.420 | 125 (87.4) | 125 (87.4) | ||
| ASA-PS | 3 | 12 (5.6) | 25 (15.2) | 0.002 | 11 (7.7) | 15 (10.5) | 0.410 |
| 1, 2 | 203 (94.4) | 139 (84.8) | 132 (92.3) | 128 (89.5) | |||
| Charlson Comorbidity Index * | ≥Medium risk | 107 (49.8) | 105 (64.0) | 0.006 | 89 (62.2) | 85 (59.4) | 0.628 |
| Low risk | 108 (50.2) | 59 (36.0) | 54 (37.7) | 58 (40.6) | |||
| Diabetes mellitus | present | 31 (14.4) | 33 (20.1) | 0.142 | 24 (16.8) | 26 (18.2) | 0.756 |
| absent | 184 (85.6) | 131 (79.9) | 119 (83.2) | 117 (81.8) | |||
| Location of tumor | Upper | 53 (24.7) | 49 (29.9) | 0.256 | 40 (28.0) | 44 (30.8) | 0.603 |
| Middle/Low | 162 (75.4) | 115 (70.1) | 103 (72.0) | 99 (69.2) | |||
| Depth of tumor ** | T2-4 | 65 (30.2) | 61 (37.2) | 0.154 | 58 (40.6) | 46 (32.1) | 0.140 |
| T1a, T1b | 150 (69.8) | 103 (62.8) | 85 (59.4) | 97 (67.8) | |||
| Lymph node metastasis ** | N1-3 | 49 (22.8) | 51 (31.1) | 0.069 | 42 (29.4) | 39 (27.3) | 0.155 |
| N0 | 166 (77.2) | 113 (68.9) | 101 (70.6) | 104 (72.7) | |||
| Microscopic lymph duct invasion | ly (+) | 76 (35.4) | 70 (42.7) | 0.146 | 62 (43.4) | 58 (40.6) | 0.632 |
| ly (−) | 139 (64.7) | 94 (57.3) | 81 (56.6) | 85 (59.4) | |||
| Microvascular invasion | v (+) | 52 (24.2) | 53 (32.3) | 0.080 | 39 (27.3) | 42 (29.4) | 0.694 |
| v (−) | 163 (75.8) | 111 (67.7) | 104 (72.7) | 101 (70.6) | |||
| Pathological stage ** | II, III | 45 (20.9) | 53 (32.3) | 0.012 | 42 (26.6) | 38 (26.6) | 0.598 |
| I | 170 (79.1) | 111 (67.7) | 101 (73.4) | 105 (73.4) | |||
| Postoperative complication (≥CD II) | present | 48 (22.3) | 59 (36.0) | 0.004 | 35 (24.5) | 51 (35.7) | 0.039 |
| absent | 167 (77.7) | 105 (64.0) | 108 (75.5) | 92 (64.3) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takemoto, Y.; Tanabe, K.; Chikuie, E.; Saeki, Y.; Ota, H.; Karakuchi, N.; Kohata, A.; Ohdan, H. Preoperative High C-Reactive Protein to Albumin Ratio Predicts Short- and Long-Term Postoperative Outcomes in Elderly Gastric Cancer Patients. Cancers 2024, 16, 616. https://doi.org/10.3390/cancers16030616
Takemoto Y, Tanabe K, Chikuie E, Saeki Y, Ota H, Karakuchi N, Kohata A, Ohdan H. Preoperative High C-Reactive Protein to Albumin Ratio Predicts Short- and Long-Term Postoperative Outcomes in Elderly Gastric Cancer Patients. Cancers. 2024; 16(3):616. https://doi.org/10.3390/cancers16030616
Chicago/Turabian StyleTakemoto, Yuki, Kazuaki Tanabe, Emi Chikuie, Yoshihiro Saeki, Hiroshi Ota, Nozomi Karakuchi, Akihiro Kohata, and Hideki Ohdan. 2024. "Preoperative High C-Reactive Protein to Albumin Ratio Predicts Short- and Long-Term Postoperative Outcomes in Elderly Gastric Cancer Patients" Cancers 16, no. 3: 616. https://doi.org/10.3390/cancers16030616
APA StyleTakemoto, Y., Tanabe, K., Chikuie, E., Saeki, Y., Ota, H., Karakuchi, N., Kohata, A., & Ohdan, H. (2024). Preoperative High C-Reactive Protein to Albumin Ratio Predicts Short- and Long-Term Postoperative Outcomes in Elderly Gastric Cancer Patients. Cancers, 16(3), 616. https://doi.org/10.3390/cancers16030616






