Phase I/II Trial of Urokinase Plasminogen Activator-Targeted Oncolytic Newcastle Disease Virus for Canine Intracranial Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. rLAS-uPA Viral Formulation and In Vitro Viral and Immunological Analytical Methods
2.1.1. Formulation of rLAS-uPA Recombinant NDV
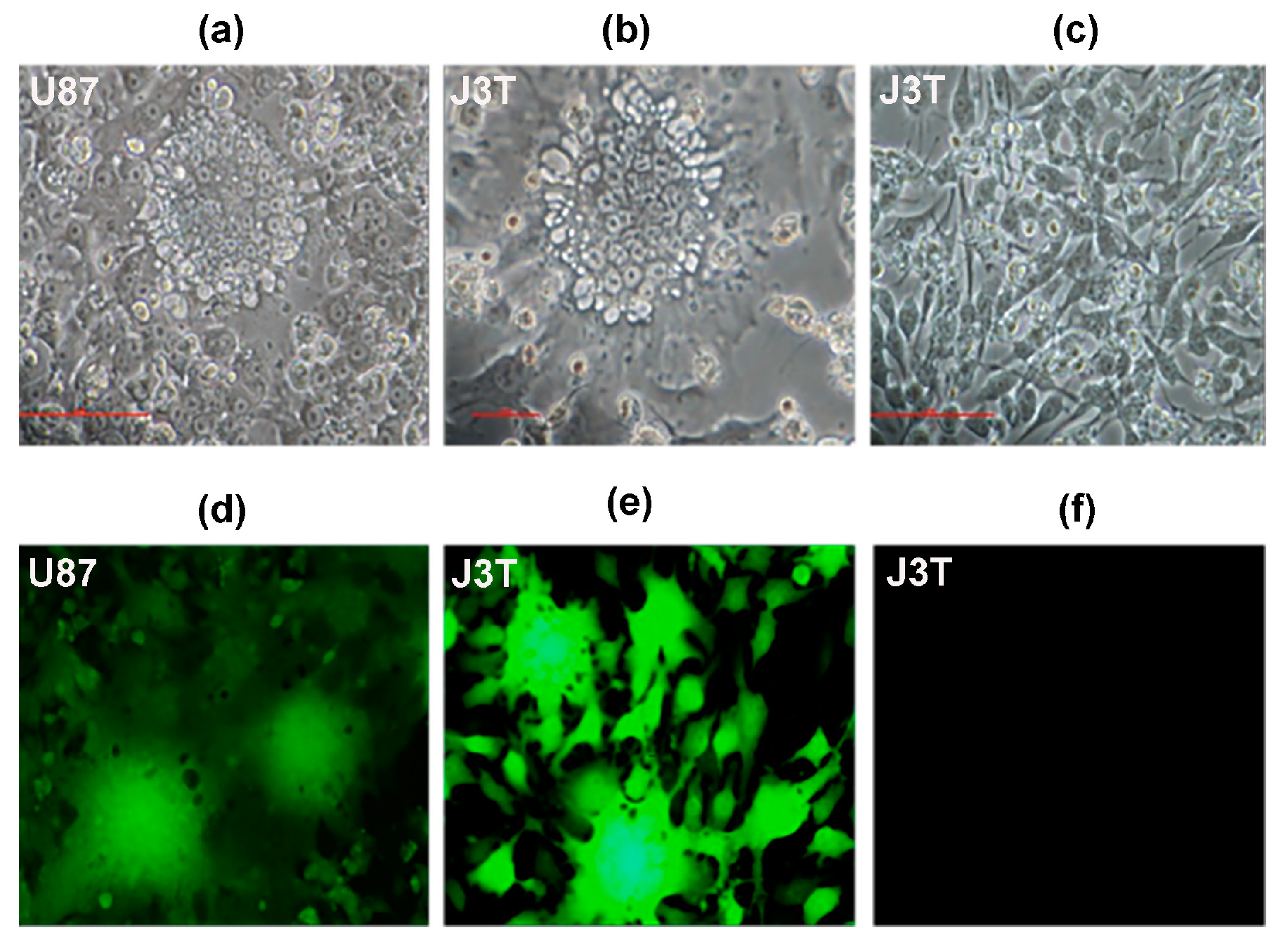
2.1.2. Cell Lines
2.1.3. Virus Neutralization Test
2.1.4. Cell Viability Assays and Syncytia Formation
2.1.5. Infectious Viral Recovery
2.1.6. rLAS-uPA qRT-PCR in Brain Tumor Samples
2.1.7. Serum Cytokine Analyses
2.2. In Vivo Canine Clinical Trial Protocol and Clinical Endpoints
2.3. Canine Tissue Collection and Preservation for In Vitro Viral and Immunologic Analyses
2.4. Statistical Analyses
3. Results
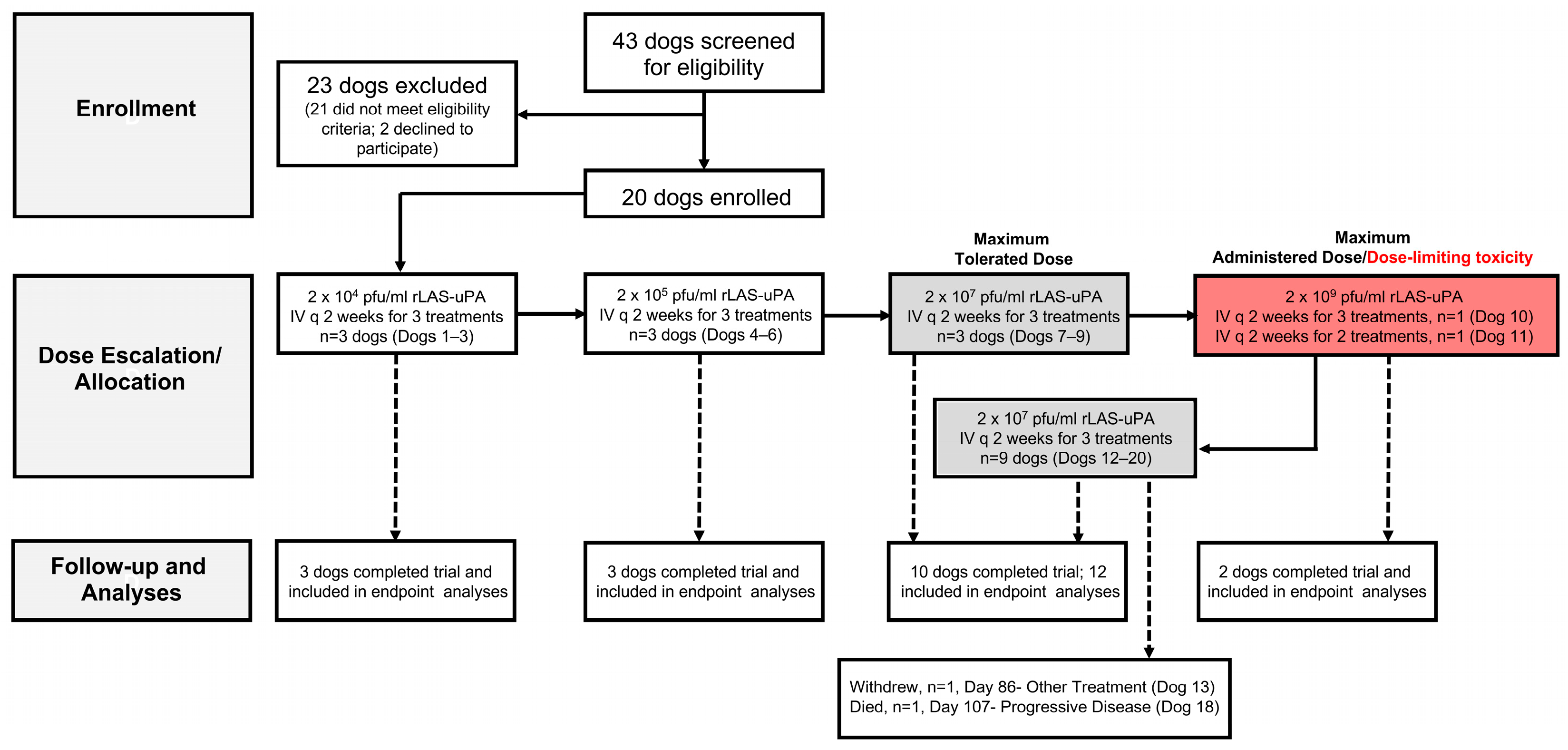
3.1. Canine Study Subjects and Tumor Characteristics
3.2. rLAS-uPA Viral Treatment, Safety and Neuroimaging Assessments, and Secondary Clinical Endpoints in Canine Subjects
3.2.1. Adverse Events in Canine Subjects
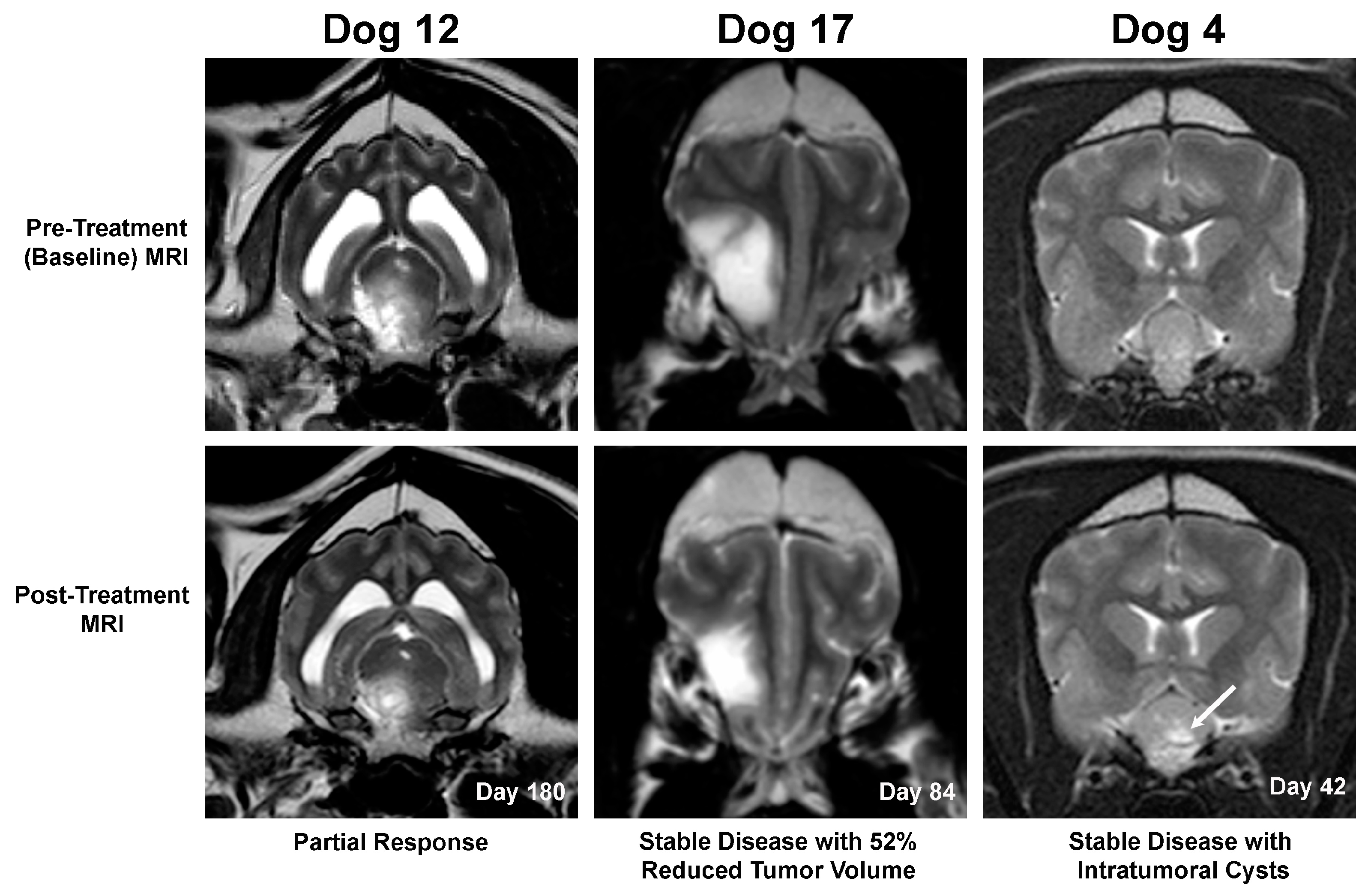
3.2.2. Neuroimaging Evaluation of Anti-Tumor Effects in Canine Subjects
3.2.3. Secondary Clinical Endpoints in Canine Subjects
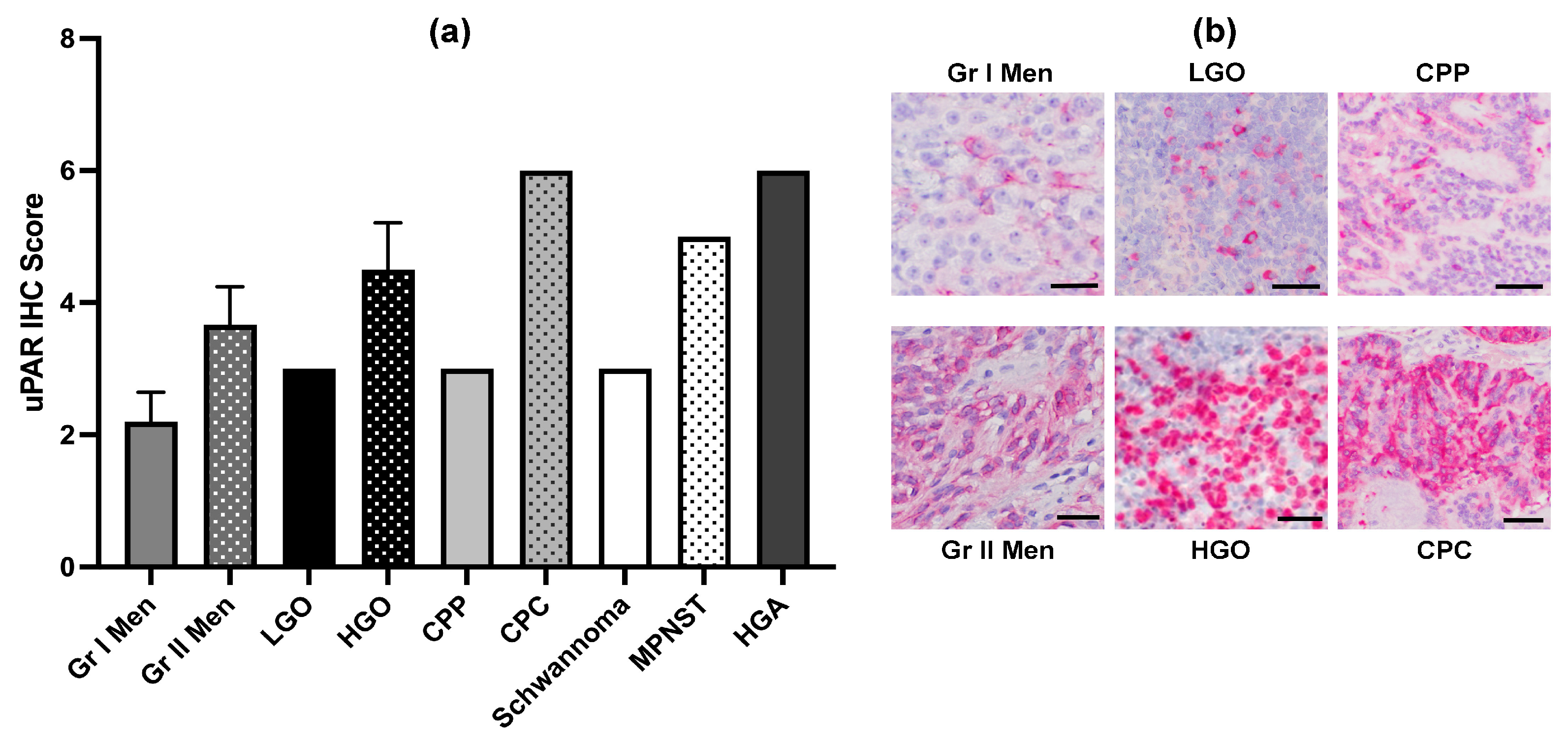
3.3. rLAS-uPA Viral and Immunologic Assays
3.3.1. Infectious Viral Recovery and Viral Neutralizing Antibodies
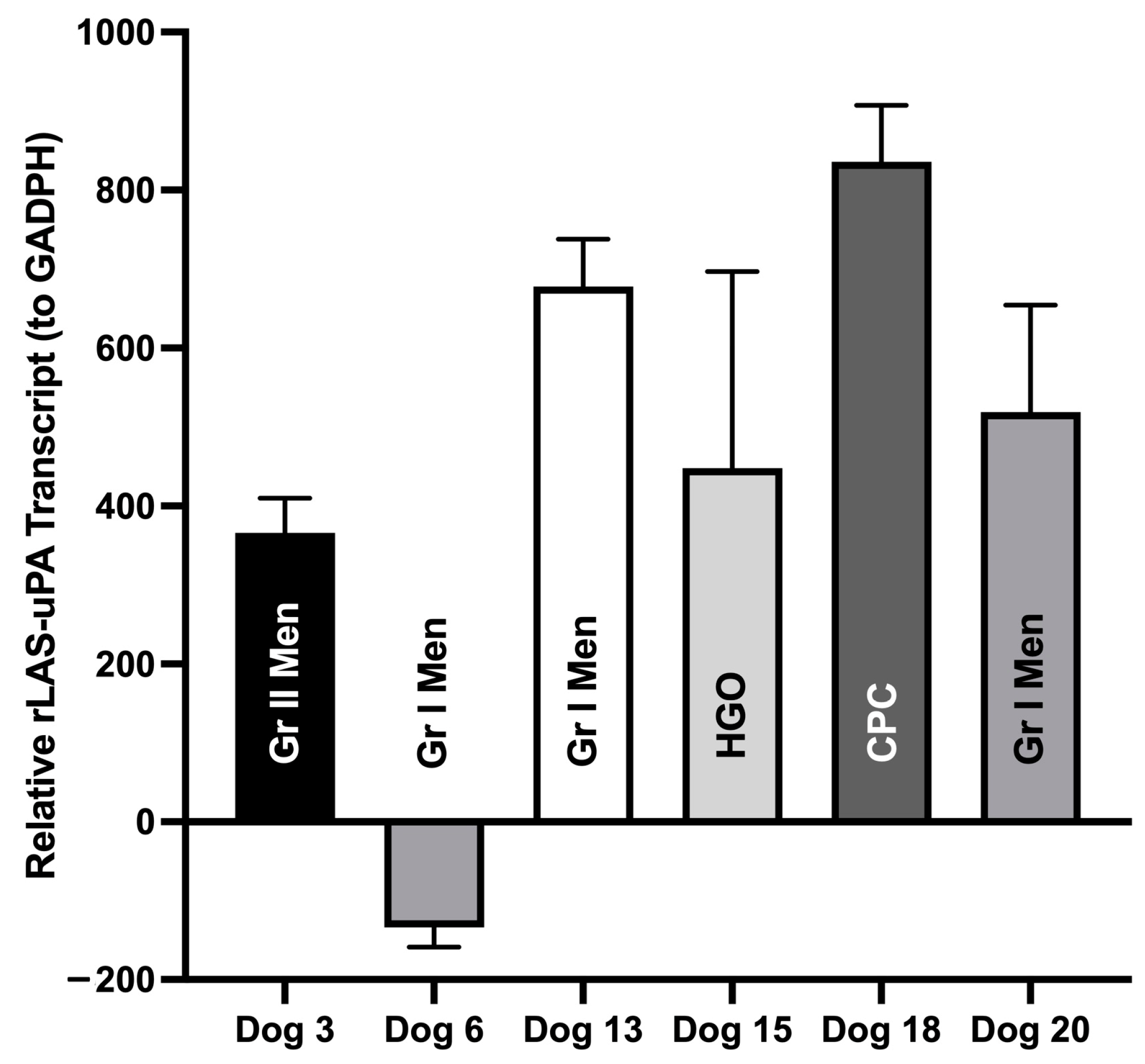
3.3.2. rLAS-uPA qRT-PCR in Brain Tumors
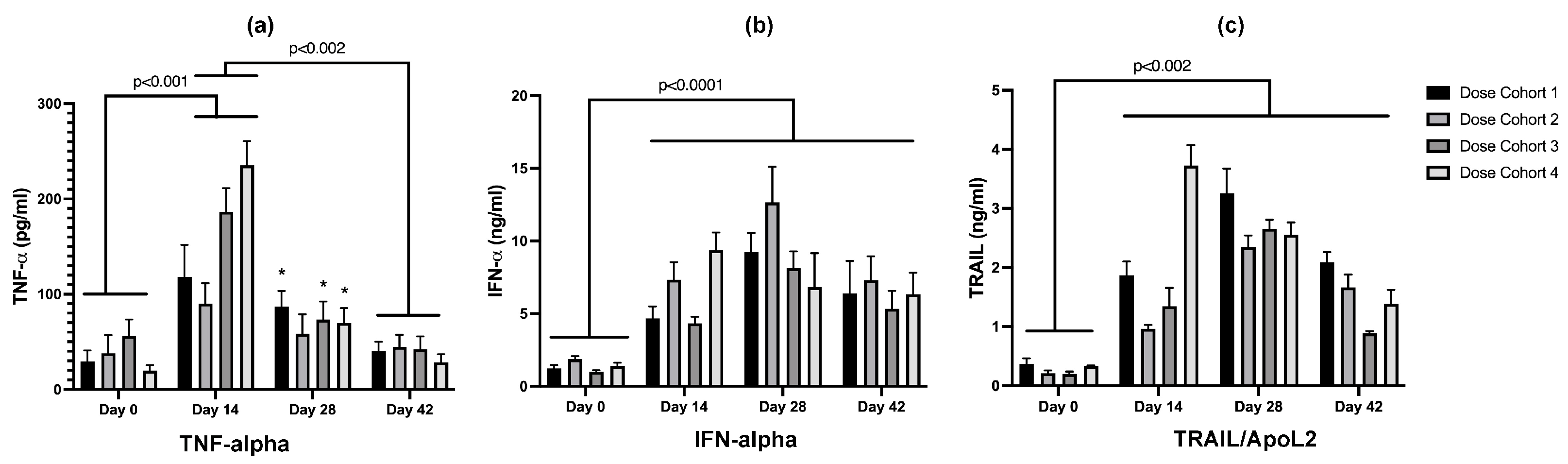
3.3.3. Serum Cytokine Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickinson, P.J. Advances in diagnostic and treatment modalities for intracranial tumors. J. Vet. Int. Med. 2014, 28, 1165–1185. [Google Scholar] [CrossRef]
- Snyder, J.M.; Shofer, F.S.; Van Winkle, T.J.; Massicotte, C. Canine intracranial primary neoplasia: 173 cases (1986–2003). J. Vet. Intern. Med. 2006, 20, 669–675. [Google Scholar] [CrossRef]
- Snyder, J.M.; Lipitz, L.; Skorupski, K.A.; Shofer, F.S.; Van Winkle, T.J. Secondary intracranial neoplasia in the dog: 177 cases (1986–2003). J. Vet. Int. Med. 2008, 22, 172–177. [Google Scholar] [CrossRef]
- Mier-García, J.F.; Ospina-Santa, S.; Orozco-Mera, J.; Ma, R.; Plaha, P. Supramaximal versus gross total resection in Glioblastoma, IDH wild-type and Astrocytoma, IDH-mutant, grade 4, effect on overall and progression free survival: Systematic review and meta-analysis. J. Neuro-Oncol. 2023, 164, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Barker, A.; Harcourt-Brown, T.; Jeffery, N. Systematic review of brain tumor treatment in dogs. J. Vet. Intern. Med. 2015, 29, 1456–1463. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Mazcko, C.; Brown, D.E.; Koehler, J.W.; Miller, A.D.; Miller, C.R.; Bentley, R.T.; Packer, R.A.; Breen, M.; Boudreau, C.E.; et al. Creation of an NCI comparative brain tumor consortium: Informing the translation of new knowledge from canine to human brain tumor patients. Neuro-Oncology 2016, 18, 1209–1218. [Google Scholar] [CrossRef]
- Sanchez, D.; Cesarman-Maus, G.; Amador-Molina, A.; Lizano, M. Oncolytic viruses for canine cancer treatment. Cancers 2018, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Palese, P. Oncolytic Newcastle Disease virus for cancer therapy: Old challenges and new directions. Future Microbiol. 2012, 7, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.I.; Zakay-Rones, Z.; Gomori, J.M.; Linetsky, E.; Rasooly, L.; Greenbaum, E.; Rozenman-Yair, S.; Panet, A.; Libson, E.; Irving, C.S.; et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006, 13, 221–228. [Google Scholar] [CrossRef]
- Ge, J.; Wang, X.; Tao, L.; Wen, Z.; Feng, N.; Yang, S.; Xia, X.; Yang, C.; Chen, H.; Bu, Z. Newcastle disease virus-vectored rabies vaccine is safe, highly immunogenic, and provides long-lasting protection in dogs and cats. J. Virol. 2011, 85, 8241–8252. [Google Scholar] [CrossRef]
- Peeters, B.P.; de Leeuw, O.S.; Koch, G.; Gielkens, A.L. Rescue of Newcastle disease virus from cloned cDNA: Evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 1999, 73, 5001–5009. [Google Scholar] [CrossRef]
- Rossmeisl, J.H.; Hall-Manning, K.; Robertson, J.L.; King, J.N.; Davalos, R.V.; Debinski, W.; Elankumaran, S. Expression and activity of the urokinase plasminogen activator system in canine primary brain tumors. OncoTargets Ther. 2017, 10, 2077–2085. [Google Scholar] [CrossRef]
- Shobana, R.; Samal, S.K.; Elankumaran, S. Prostate-specific antigen retargeted recombinant Newcastle disease virus for prostate cancer virotherapy. J. Virol. 2013, 87, 3792–3800. [Google Scholar] [CrossRef]
- Santry, L.A.; McAusland, T.M.; Susta, L.; Wood, G.A.; Major, P.P.; Petrick, J.J.; Bridle, B.W.; Wootton, S.K. Production and purification of high-titer Newcastle disease virus for use in preclinical mouse models of cancer. Mol. Ther. Methods Clin. Dev. 2018, 9, 181–191. [Google Scholar] [CrossRef]
- Rainov, N.G.; Koch, S.; Sena-Esteves, M.; Berens, M.E. Characterization of a canine glioma cell line as related to established experimental brain tumor models. J. Neuropathol. Exp. Neurol. 2000, 59, 607–613. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.S.; King, D.J.; Bennett, J.D. Characterization of Newcastle disease virus isolates by reverse transcription PCR coupled to direct nucleotide sequencing and development of sequence database for pathotype prediction and molecular epidemiological analysis. J. Clin. Microbiol. 1995, 33, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Lee, J.J.; Siu, L.L. Dose escalation methods in phase I cancer clinical trials. J. Natl. Cancer Inst. 2009, 101, 708–720. [Google Scholar] [CrossRef]
- Dickinson, P.J.; LeCouteur, R.A.; Higgins, R.J.; Bringas, J.R.; Larson, R.F.; Yamashita, Y.; Krauze, M.T.; Forsayeth, J.; Noble, C.O.; Drummond, D.C.; et al. Canine spontaneous glioma: A translational model system for convection-enhanced delivery. Neuro-Oncology 2010, 12, 928–940. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef] [PubMed]
- Ammons, D.T.; Guth, A.; Rozenthal, A.J.; Kurihara, J.; Marolf, A.J.; Chow, L.; Griffin, J.F., IV; Makii, R.; MacQuiddy, B.; Boss, M.K.; et al. Reprogamming the canine glioma microenvironment with tumor vaccination plus oral losaratan and propranolol induced objectives responses. Cancer Res. Commun. 2022, 2, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Weiske, R.; Sroufe, M.; Quigley, M.; Pancotto, T.; Werre, S.; Rossmeisl, J.H. Development and evaluation of a caregiver reported quality of life assessment instrument in dogs with intracranial disease. Front. Vet. Sci. 2020, 7, 537. [Google Scholar] [CrossRef]
- Di Terlizzi, R.; Platt, S.R. The function, composition, and analysis of cerebrospinal fluid in companion animals: Part II—Analysis. Vet. J. 2009, 1, 15–32. [Google Scholar] [CrossRef]
- Calderón, N.L.; Galundo-Muñiz, F.; Ortiz, M.; Lomniczi, B.; Fehervari, T.; Paasch, L.H. Thrombocytopenia in Newcastle disease: Haematological evaluation and histological study of bone marrow. Acta Vet. Hung. 2005, 53, 507–513. [Google Scholar] [CrossRef]
- Piguet, P.F.; Vesin, C.; Donati, Y.; Tacchini-Cottier, F.; Belin, D.; Barazzone, C. Urokinase receptor (uPAR, CD87) is a platelet receptor important for kinetics and TNF-induced endothelial adhesion in mice. Circulation 1999, 99, 3315–3321. [Google Scholar] [CrossRef]
- Shinno, K.; Banno, Y.; Kamimaki, I. Severe immune thrombocytopenia that developed immediately after COVID-19 in a school-aged patient: A case report. Front. Pediatr. 2023, 11, 1120093. [Google Scholar] [CrossRef]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Ruf, B.; Lauer, U.M. Assessment of current virotherapeutic application schemes: “Hit hard and early” versus “killing softly”? Mol. Ther. Oncolytics 2015, 2, 15018. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Johnson, J.B.; Kumar, S.R.; Parks, G.D.; Elankumarana, S. Incorporation of host complement regulatory proteins into Newcastle disease virus enhances complement evasion. J. Virol. 2012, 86, 12708–12716. [Google Scholar] [CrossRef]
- Burman, B.; Pesci, G.; Zamarin, D. Newcastle disease virus at the forefront of cancer immunotherapy. Cancers 2020, 12, 3552. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Krishnamurthy, S.; Panda, A.; Samal, S.K. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J. Virol. 2003, 77, 8676–8685. [Google Scholar] [CrossRef]


| Monitoring Parameter | Methods | Time Point (Trial Day) | ||||||
|---|---|---|---|---|---|---|---|---|
| −14 to 0 | 1 | 14 | 28 | 42 | 84 | 180 | ||
| Owner | Observation | ✓ Daily Days 1–42 | X Daily Days 43–180 | |||||
| Rectal temperature | ||||||||
| Adverse events | ||||||||
| −14 to 0 | 1 | 14 | 28 | 42 | 84 | 180 | ||
| Clinical | Physical/Neurological Exams | ✓ | ✓ | ✓ | ✓ | ✓ | X | X |
| Karnofsky Performance Score | ✓ | ✓ | ✓ | ✓ | ✓ | X | X | |
| Adverse events | ✓ | ✓ | ✓ | ✓ | ✓ | X | X | |
| Laboratory | CBC, Chemistry, Urinalysis | ✓ | ✓ | ✓ | ✓ | ✓ | X | X |
| Cerebrospinal fluid | ✓ | ✓ | X | X | ||||
| Viral Shedding | Blood and urine infectious viral recovery | † | † | † | † | |||
| Cerebrospinal fluid infectious viral recovery | † | |||||||
| Viral Immune Response | Anti-NDV antibodies; plaque neutralization assay | ✓ | ✓ | ✓ | ✓ | ✓ | X | X |
| Serum TNF-α, IFN-α, TRAIL; ELISA | † | † | † | † | ||||
| Tumor Response | Brain MRI | ✓ | ✓ | X | X | |||
| Quality of Life Assessment | CanBrain-24 QOL Caregiver Survey | X | X | X | X | |||
| Intervention | rLAS-uPA NDV IV Infusion | ✓ | ✓ | ✓ | ||||
| Adverse Events Attributable to rLAS-uPA Treatment | |||
|---|---|---|---|
| Adverse Event | Category | Grade | Canine Subject (n, Dog Identification) |
| Fever | Constitutional | 3/DLT | n = 2, Dog 10 ^, Dog 11 ◊ |
| Anorexia | Gastrointestinal | 3/DLT | n = 2, Dog 10 ^, Dog 11 ◊ |
| Thrombocytopenia | Blood/Bone Marrow | 4/DLT | n = 1, Dog 10 ^ |
| Cerebral edema | Neurology | 3/DLT | n = 1, Dog 11 ◊ |
| Shivering/tremor | Administration Reaction | 1 | n = 14, Dogs 1 •, 2 •, 3 •, 4 •, 5 *, 7 •, 9 *, 10 *, 11 ◊, 12 *, 13 •, 15 •, 17 •, 18 *, 20 • |
| Diarrhea | Gastrointestinal | 1 | n = 6, Dogs 1 *, 2 ^, 7 *, 15 *, 18 *, 20 • |
| Diarrhea | Gastrointestinal | 2 | n = 2, Dogs 12 ^, 14 * |
| Fever | Constitutional | 1 | n = 4, Dogs 7 *, 9 *, 17 *, 19 * |
| Fever | Constitutional | 2 | n = 1, Dog 1 * |
| Appetite, altered | Gastrointestinal | 1 | n = 3, Dogs 10 *, 14 •, 17 * |
| Conjunctivitis | Ocular | 2 | n = 1, Dog 14 * |
| Adverse Events Attributable to Concomitant Medication | |||
| High alanine aminotransferase | Metabolic | 1 | n = 4, Dogs 1, 2, 15, 20 (prednisone, phenobarbital) |
| High alkaline phosphatase | Metabolic | 1 | n = 6, Dogs 1, 2, 3, 5, 13, 20 (prednisone, phenobarbital) |
| High alkaline phosphatase | Metabolic | 2 | n = 1, Dog 15 (prednisone, phenobarbital) |
| Adverse Events Attributable to Non-Investigational Protocol Procedures | |||
| Cystitis/hematuria | Genitourinary | 2 | n = 1, Dog 15 (cystocentesis) |
| Hematoma | Hemorrhage/Bleeding | 1 | n = 1, Dog 7 (jugular venipuncture) |
| Adverse Events Attributable to Pre-existing Disease | |||
| Seizures | Neurology | 2 | n = 8, Dogs 2, 3, 7, 8, 13, 14, 15, 19 (structural epilepsy) |
| Seizures | Neurology | 4 | n = 1, Dog 1 (structural epilepsy) |
| Depressed level of consciousness | Neurology | 4 | n = 1, Dog 18 (tumor progression) |
| Hydrocephalus | Neurology | 3 | n = 1, Dog 18 (tumor progression) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossmeisl, J.H.; King, J.N.; Robertson, J.L.; Weger-Lucarelli, J.; Elankumaran, S. Phase I/II Trial of Urokinase Plasminogen Activator-Targeted Oncolytic Newcastle Disease Virus for Canine Intracranial Tumors. Cancers 2024, 16, 564. https://doi.org/10.3390/cancers16030564
Rossmeisl JH, King JN, Robertson JL, Weger-Lucarelli J, Elankumaran S. Phase I/II Trial of Urokinase Plasminogen Activator-Targeted Oncolytic Newcastle Disease Virus for Canine Intracranial Tumors. Cancers. 2024; 16(3):564. https://doi.org/10.3390/cancers16030564
Chicago/Turabian StyleRossmeisl, John H., Jamie N. King, John L. Robertson, James Weger-Lucarelli, and Subbiah Elankumaran. 2024. "Phase I/II Trial of Urokinase Plasminogen Activator-Targeted Oncolytic Newcastle Disease Virus for Canine Intracranial Tumors" Cancers 16, no. 3: 564. https://doi.org/10.3390/cancers16030564
APA StyleRossmeisl, J. H., King, J. N., Robertson, J. L., Weger-Lucarelli, J., & Elankumaran, S. (2024). Phase I/II Trial of Urokinase Plasminogen Activator-Targeted Oncolytic Newcastle Disease Virus for Canine Intracranial Tumors. Cancers, 16(3), 564. https://doi.org/10.3390/cancers16030564






