Biomarker Analysis from a Phase I/Ib Study of Regorafenib and Nivolumab in Mismatch Repair-Proficient Advanced Refractory Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunohistochemical Staining
2.3. Cytokine/Chemokine Analysis
2.4. RNA Sequencing
2.5. Statistical Methods
2.6. RNAseq Analysis
3. Results
3.1. Baseline Clinicopathological Characteristics and Clinical Outcome
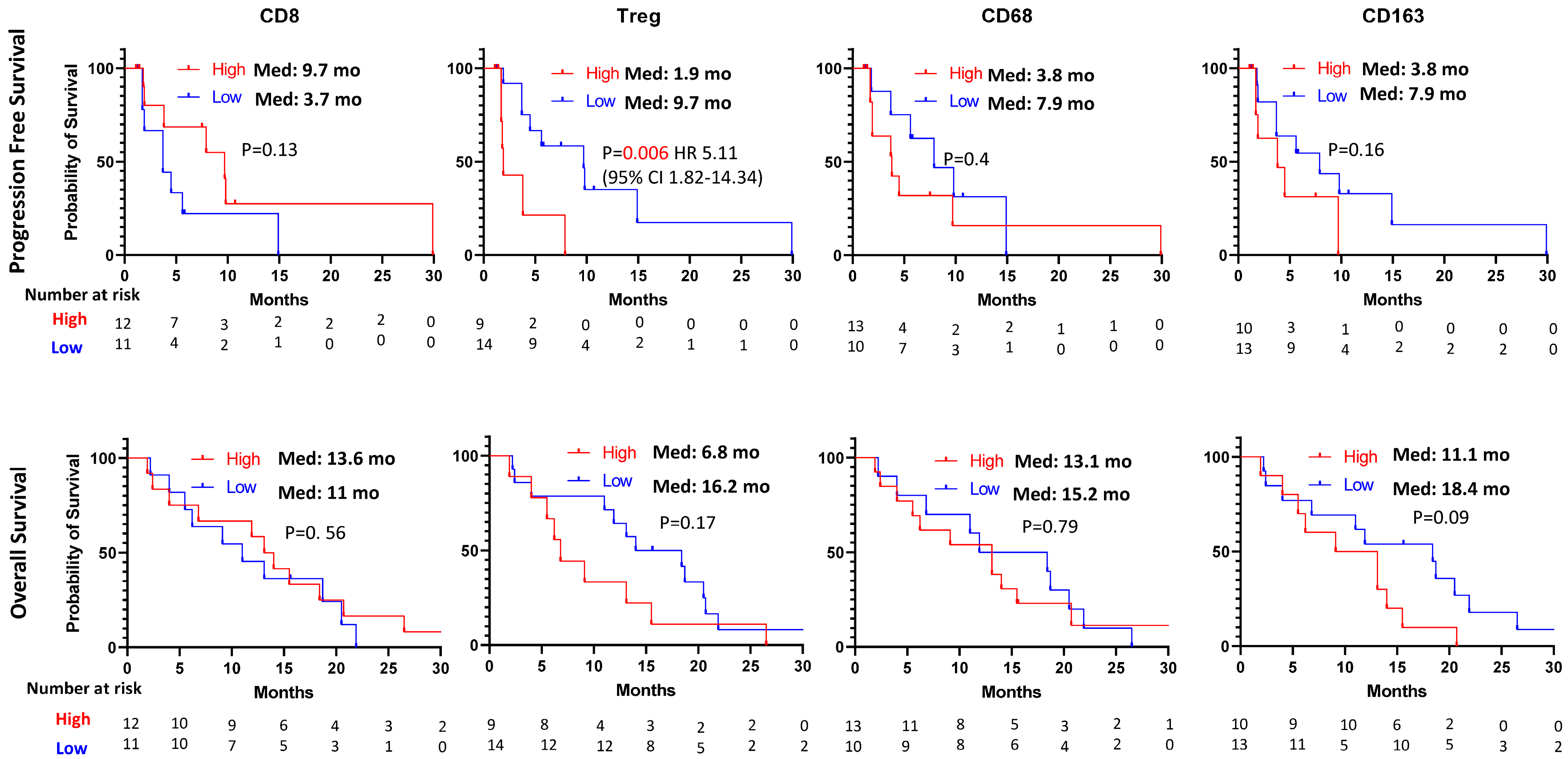
3.2. Immunohistochemical Staining
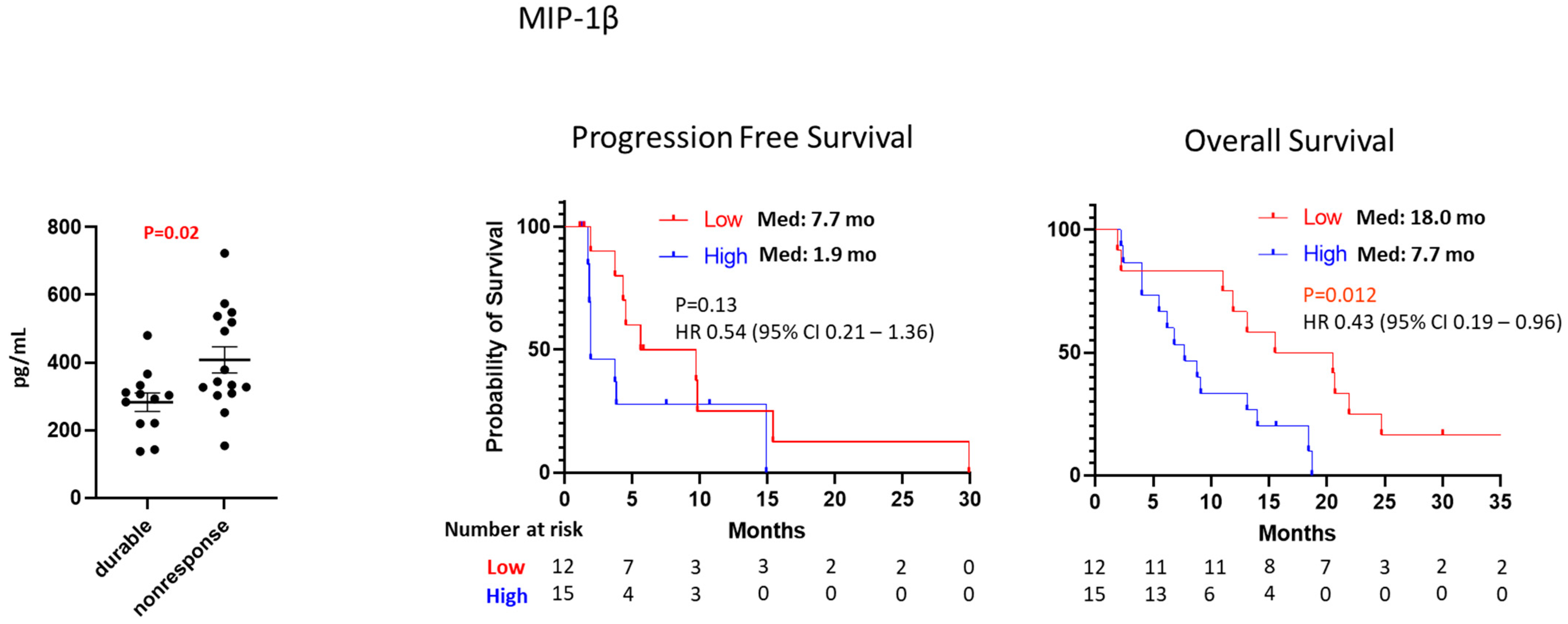
3.3. Cytokine/Chemokine Analysis
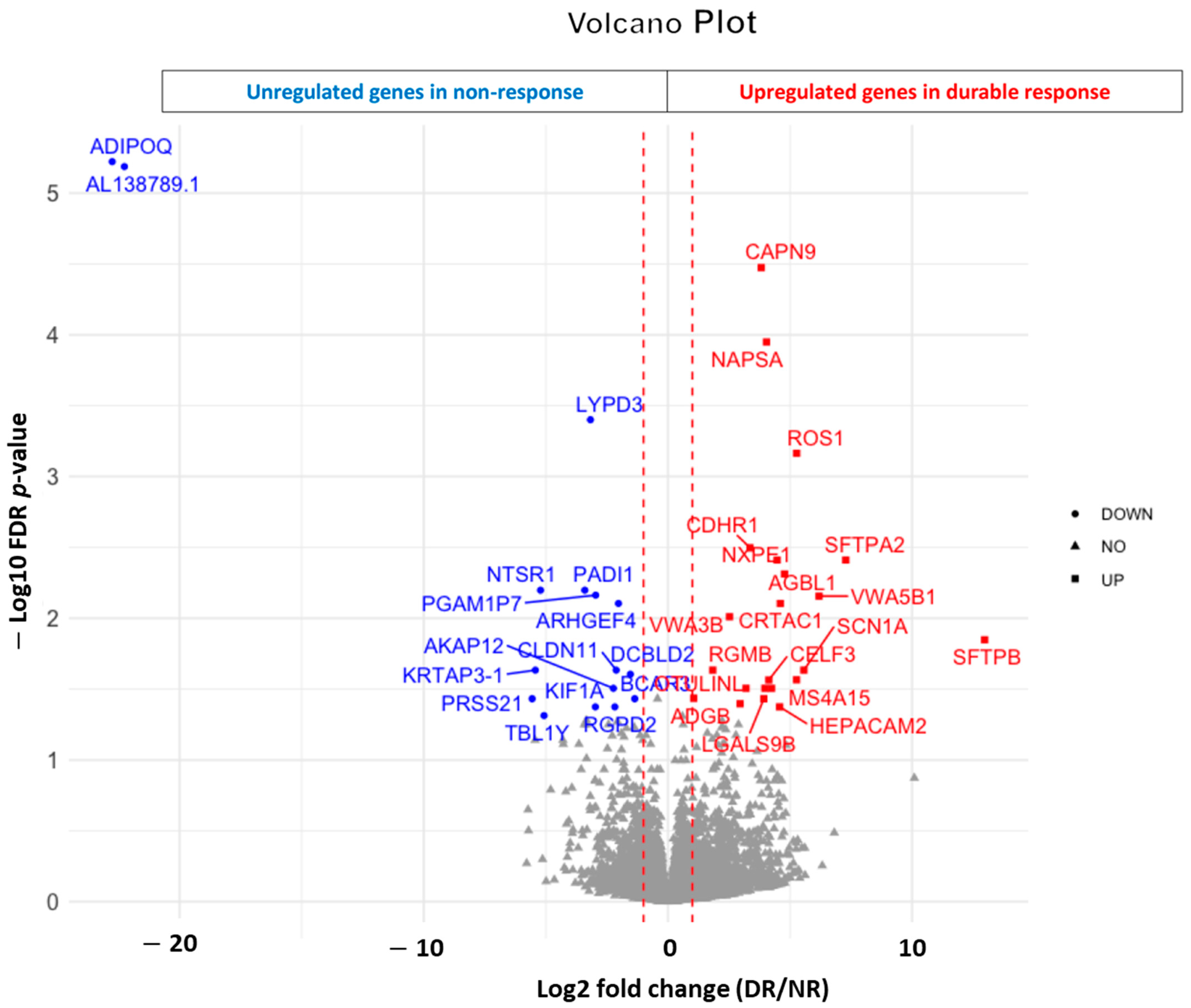
3.4. RNAseq Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andre, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jager, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Tan, E.; Zhou, J.M.; Schell, M.J.; Martinez, M.; Yu, J.; Carballido, E.; Mehta, R.; Strosberg, J.; Imanirad, I.; et al. A phase 1/2 trial of ibrutinib in combination with pembrolizumab in patients with mismatch repair proficient metastatic colorectal cancer. Br. J. Cancer 2021, 124, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Kovari, B.P.; Martinez, M.; Xie, H.; Sahin, I.H.; Mehta, R.; Strosberg, J.; Imanirad, I.; Ghayouri, M.; Kim, Y.C.; et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur. J. Cancer 2022, 169, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, Y.C.; Kovari, B.P.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Park, W.; Kim, R.D. Biomarker analysis from a phase II multi-institutional study of nivolumab in patients with advanced refractory biliary tract cancer. Eur. J. Cancer 2022, 176, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Shah, K.; Steinmetz, T.; Abraham, A.; Jensen, H.; Bouton, A.; Marullo, K.; Shi, S. Development and validation of a multiplexed drug level assay in support of combination biologics therapy clinical studies. J. Pharm. Biomed. Anal. 2019, 171, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.K.; Chowell, D.; Valero, C.; Morris, L.G.T.; Chan, T.A. Pre-treatment serum albumin and mutational burden as biomarkers of response to immune checkpoint blockade. NPJ Precis. Oncol. 2022, 6, 23. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Rizzo, A.; Ricci, A.D.; Aksoy, S.; Yalcin, S. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Mol. Biosci. 2022, 9, 1039121. [Google Scholar] [CrossRef]
- Yeun, J.Y.; Kaysen, G.A. Factors influencing serum albumin in dialysis patients. Am. J. Kidney Dis. 1998, 32, S118–S125. [Google Scholar] [CrossRef]
- Svaton, M.; Zemanova, M.; Skrickova, J.; Jakubikova, L.; Kolek, V.; Kultan, J.; Koubkova, L.; Bejckova, A.; Salajka, F.; Hrnciarik, M.; et al. Chronic Inflammation as a Potential Predictive Factor of Nivolumab Therapy in Non-small Cell Lung Cancer. Anticancer. Res. 2018, 38, 6771–6782. [Google Scholar] [CrossRef]
- Gouez, M.; Delrieu, L.; Bouleuc, C.; Girard, N.; Raynard, B.; Marchal, T. Association between Nutritional Status and Treatment Response and Survival in Patients Treated with Immunotherapy for Lung Cancer: A Retrospective French Study. Cancers 2022, 14, 3439. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Fakih, M.; Raghav, K.P.S.; Chang, D.Z.; Larson, T.; Cohn, A.L.; Huyck, T.K.; Cosgrove, D.; Fiorillo, J.A.; Tam, R.; D’Adamo, D.; et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: A single-arm, open-label, multicentre phase 2 study. EClinicalMedicine 2023, 58, 101917. [Google Scholar] [CrossRef]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef]
- Lee, J.C.; Mehdizadeh, S.; Smith, J.; Young, A.; Mufazalov, I.A.; Mowery, C.T.; Daud, A.; Bluestone, J.A. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci. Immunol. 2020, 5, eaba0759. [Google Scholar] [CrossRef]
- Cousin, S.; Cantarel, C.; Guegan, J.P.; Gomez-Roca, C.; Metges, J.P.; Adenis, A.; Pernot, S.; Bellera, C.; Kind, M.; Auzanneau, C.; et al. Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-arm, Open-label, Phase II Trial. Clin. Cancer Res. 2021, 27, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Frey, D.M.; Droeser, R.A.; Viehl, C.T.; Zlobec, I.; Lugli, A.; Zingg, U.; Oertli, D.; Kettelhack, C.; Terracciano, L.; Tornillo, L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int. J. Cancer 2010, 126, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.E.; Crome, S.Q.; Crellin, N.K.; Passerini, L.; Steiner, T.S.; Bacchetta, R.; Roncarolo, M.G.; Levings, M.K. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007, 19, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, N.; Sasaki, S.I.; Baba, T. CCL4 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 23–32. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente Lopez, M.; Landskron, G.; Parada, D.; Dubois-Camacho, K.; Simian, D.; Martinez, M.; Romero, D.; Roa, J.C.; Chahuan, I.; Gutierrez, R.; et al. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumour Biol. 2018, 40, 1010428318810059. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Wang, L.; Li, J.; Yang, T.; Shao, Q.; Liang, X.; Ma, M.; Zhang, N.; Jing, M.; et al. Identification of CCL4 as an Immune-Related Prognostic Biomarker Associated With Tumor Proliferation and the Tumor Microenvironment in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 694664. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, X.; Zhang, L.; Yang, M.; Wang, X.; Huang, W.; Pan, H.; Wu, Y.; Huang, J.; Liang, W.; et al. SFTPA1 is a potential prognostic biomarker correlated with immune cell infiltration and response to immunotherapy in lung adenocarcinoma. Cancer Immunol. Immunother. 2022, 71, 399–415. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, H.; Du, Y.; Peng, D.; Zhou, Y.; Li, Y.; Wang, H.; Zhou, Y.; Nie, Y. Association of DCBLD2 upregulation with tumor progression and poor survival in colorectal cancer. Cell Oncol. 2020, 43, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lv, Y.; Xu, K.; Wang, X.; Zhao, Y.; Li, J.; Qin, X.; Shi, Y.; Wang, L.; Chang, A.; et al. DCBLD2 Mediates Epithelial-Mesenchymal Transition-Induced Metastasis by Cisplatin in Lung Adenocarcinoma. Cancers 2021, 13, 1403. [Google Scholar] [CrossRef]
- Xie, P.; Liu, J.Y.; Yan, H.; Wang, Z.B.; Jiang, S.L.; Li, X.; Liu, Z.Q. Pan-cancer analyses identify DCBLD2 as an oncogenic, immunological, and prognostic biomarker. Front. Pharmacol. 2022, 13, 950831. [Google Scholar] [CrossRef]
- Zhao, Z.; Mei, Y.; Wang, Z.; He, W. The Effect of Oxidative Phosphorylation on Cancer Drug Resistance. Cancers 2022, 15, 62. [Google Scholar] [CrossRef]
| n = 51 | Median PFS (Months) | Median OS (Months) | |||
|---|---|---|---|---|---|
| Age | |||||
| Median (range) | 56 (31–79) | 4.3 | 95% CI 1.6–7.0 | 11.1 | 95% CI 8.1–14.1 |
| Gender | |||||
| Male | 27 (53.8%) | 3.8 | HR 1.04 (95% CI 0.53–2.05) | 9.7 | HR 1.17 (95% CI 0.65–2.10) |
| Female | 24 (46.2%) | 4.3 | p = 0.9 | 14.3 | p = 0.61 |
| Race | |||||
| White | 42 (80.8%) | 4.3 | p = 0.25 | 11.5 | p = 0.36 |
| Hispanic | 3 (5.8%) | not reached | 10 | ||
| African | 2 (5.8%) | 3 | 20.9 | ||
| Asian | 4 (7.7%) | 3.8 | 19 | ||
| Obesity | |||||
| Yes | 15 (29.4%) | 3.7 | HR 0.7 (95% CI 0.35–1.40) | 18.4 | HR 0.72 (95% CI 0.39–1.35) |
| No | 36 (70.6%) | 4.3 | p = 0.32 | 10.5 | p = 0.33 |
| Albumin | |||||
| High | 37 (71.2%) | 5.6 | HR 0.34 (95% CI 0.16–0.72) | 15.5 | HR 0.20 (95% CI 0.10–0.37) |
| Low | 14 (28.8%) | 1.9 | p = 0.025 | 3.1 | p = 0.0001 |
| ECOG PS | |||||
| 0 | 20 (38.5%) | 4 | HR 1.28 (95% CI 0.66–2.45) | 11.6 | HR 0.92 (95% CI 0.51–1.68) |
| 1 | 31 (61.5%) | 5.6 | p = 0.6 | 7.9 | p = 0.068 |
| Primary tumor | |||||
| Left-sided | 21 (42.3%) | 3.8 | HR 1.15 (95% CI 0.57–2.28) | 14 | HR 0.65 (95% CI 0.36–1.17) |
| Right-sided | 30 (57.7%) | 4.3 | p = 0.7 | 10.5 | p = 0.16 |
| Previous systemic treatment | |||||
| 2nd line | 30 (57.7%) | 5.6 | HR 0.72 (95% CI 0.37–1.40) | 13.1 | HR 1.0 (95% CI 0.55–1.83) |
| ≥3rd line | 21 (42.3%) | 3 | p = 0.31 | 11 | p = 0.9 |
| RAS mutation status | |||||
| WT | 14 (28.8%) | 2.9 | HR 1.35 (95% CI 0.66–2.77) | 11 | HR 1.03 (95% CI 0.54–1.97) |
| Mutant | 37 (71.2%) | 5.6 | p = 0.38 | 11.9 | p = 0.9 |
| Sites of disease | |||||
| Liver involvement | |||||
| No | 14 (27.5%) | 11.7 | HR 0.37 (95% CI 0.19–0.73) | 19.5 | HR 0.40 (95% CI 0.22–0.74) |
| Yes | 37 (72.5%) | 2.3 | p = 0.006 | 9.1 | p = 0.01 |
| Lung involvement | |||||
| No | 19 (37.3%) | 2.3 | HR 2.3 (95% CI 1.07–4.76) | 10 | HR 1.03 (95% CI 0.56–1.91) |
| Yes | 32 (62.7%) | 5.6 | p = 0.009 | 12.1 | p = 0.9 |
| Peritoneal involvement | |||||
| No | 41 (80.4%) | 4.5 | HR 0.97 (95% CI 0.34–2.79) | 13.1 | HR 0.54 (95% CI 0.22–1.33) |
| Yes | 10 (19.6%) | 1.9 | p = 0.9 | 5.9 | p = 0.09 |
| n = 40 (Evaluable Patients) | PR (n = 4) | SD (n = 21) | ORR | p Value | DCR | p Value | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Median (range) | 56 (31–78) | 75 (54–78) | 57 (31–70) | ||||
| Gender | |||||||
| Male | 24 | 1 (4.2%) | 13 (54.2%) | 4.2% | p = 0.2 | 58.4% | p = 0.9 |
| Female | 16 | 3 (18.8%) | 8 (50.0%) | 18.8% | 68.8% | ||
| Race | |||||||
| White | 34 | 4 (11.8%) | 18 (52.9%) | 11.8% | p = 0.6 | 64.7% | p = 0.7 |
| Hispanic | 0 | 0 | 0 | 0 | 0 | ||
| African | 2 | 0 | 1 (50.0%) | 0 | 50.0% | ||
| Asian | 4 | 0 | 2 (50.0%) | 0 | 50.0% | ||
| Obesity | |||||||
| Yes | 13 | 2 (15.4%) | 6 (46.2%) | 15.4% | p = 0.4 | 61.5% | p = 0.9 |
| No | 27 | 2 (7.4%) | 15 (55.6%) | 7.4% | 63.0% | ||
| Albumin | |||||||
| High | 32 | 4 (12.5%) | 19 (59.4%) | 12.5% | p = 0.2 | 71.9% | p = 0.01 |
| Low | 8 | 0 | 2 (25%) | 0 | 25.0% | ||
| ECOG PS | |||||||
| 0 | 18 | 1 (5.6%) | 9 (50%) | 5.6% | p = 0.4 | 55.6% | p = 0.4 |
| 1 | 22 | 3 (13.6%) | 12 (54.5%) | 13.6% | 68.2% | ||
| Primary tumor | |||||||
| Left-sided | 18 | 3 (16.7%) | 9 (37.5%) | 16.7% | p = 0.2 | 66.7% | p = 0.6 |
| Right-sided | 22 | 1 (4.5%) | 12 (54.5%) | 4.5% | 59.1% | ||
| Previous systemic treatment | |||||||
| 2nd line | 21 | 3 (14.3%) | 12 (57.1%) | 14.3% | p = 0.3 | 71.4% | p = 0.2 |
| ≥3rd line | 19 | 1 (5.3%) | 9 (47.3%) | 5.3% | 52.6% | ||
| RAS mutation status | |||||||
| WT | 13 | 2 (15.4%) | 5 (38.5%) | 15.4% | p = 0.4 | 53.9% | p = 0.4 |
| Mutant | 27 | 2 (7.4%) | 16 (59.3%) | 7.4% | 66.7% | ||
| Sites of disease | |||||||
| Liver involvement | |||||||
| No | 12 | 3 (25.0%) | 7 (58.3%) | 25% | p = 0.04 | 83.3% | p = 0.07 |
| Yes | 28 | 1 (3.6%) | 14 (50.0%) | 3.6% | 53.6% | ||
| Lung involvement | |||||||
| No | 15 | 1 (6.7%) | 6 (40.0%) | 6.7% | p = 0.6 | 46.7% | p = 0.1 |
| Yes | 25 | 3 (12.0%) | 15 (60.0%) | 12.0% | 72.0% | ||
| Peritoneal involvement | |||||||
| No | 36 | 3 (8.3%) | 21 (58.3%) | 8.3% | p = 0.3 | 66.7% | p = 0.1 |
| Yes | 4 | 1 (25.0%) | 0 | 25.0% | 25.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.W.; Kim, Y.-C.; Kovari, B.P.; Martinez, M.; Miao, R.; Yu, J.; Mehta, R.; Strosberg, J.; Imanirad, I.; Kim, R.D. Biomarker Analysis from a Phase I/Ib Study of Regorafenib and Nivolumab in Mismatch Repair-Proficient Advanced Refractory Colorectal Cancer. Cancers 2024, 16, 556. https://doi.org/10.3390/cancers16030556
Kim DW, Kim Y-C, Kovari BP, Martinez M, Miao R, Yu J, Mehta R, Strosberg J, Imanirad I, Kim RD. Biomarker Analysis from a Phase I/Ib Study of Regorafenib and Nivolumab in Mismatch Repair-Proficient Advanced Refractory Colorectal Cancer. Cancers. 2024; 16(3):556. https://doi.org/10.3390/cancers16030556
Chicago/Turabian StyleKim, Dae Won, Young-Chul Kim, Bence P. Kovari, Maria Martinez, Ruoyu Miao, James Yu, Rutika Mehta, Jonathan Strosberg, Iman Imanirad, and Richard D. Kim. 2024. "Biomarker Analysis from a Phase I/Ib Study of Regorafenib and Nivolumab in Mismatch Repair-Proficient Advanced Refractory Colorectal Cancer" Cancers 16, no. 3: 556. https://doi.org/10.3390/cancers16030556
APA StyleKim, D. W., Kim, Y.-C., Kovari, B. P., Martinez, M., Miao, R., Yu, J., Mehta, R., Strosberg, J., Imanirad, I., & Kim, R. D. (2024). Biomarker Analysis from a Phase I/Ib Study of Regorafenib and Nivolumab in Mismatch Repair-Proficient Advanced Refractory Colorectal Cancer. Cancers, 16(3), 556. https://doi.org/10.3390/cancers16030556








