Impact of Vitamin D Levels on Progression-Free Survival and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
- Primary objectives
- Assess the impact of VD levels on treatment response.
- Determine the influence of VD levels on PFS.
- Quality assessment
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tan, T.; Han, L.; Appelman, L.; Veltman, J.; Wessels, R.; Duvivier, K.M.; Loo, C.; Gao, Y.; Wang, X.; et al. Predicting Breast Cancer Types on and beyond Molecular Level in a Multi-Modal Fashion. NPJ Breast Cancer 2023, 9, 16. [Google Scholar] [CrossRef]
- Tarighati, E.; Keivan, H.; Mahani, H. A Review of Prognostic and Predictive Biomarkers in Breast Cancer. Clin. Exp. Med. 2023, 23, 1–16. [Google Scholar] [CrossRef]
- Capuozzo, M.; Celotto, V.; Santorsola, M.; Fabozzi, A.; Landi, L.; Ferrara, F.; Borzacchiello, A.; Granata, V.; Sabbatino, F.; Savarese, G.; et al. Emerging Treatment Approaches for Triple-Negative Breast Cancer. Med. Oncol. 2023, 41, 5. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.; Espié, M.; Petit, T. Neoadjuvant Therapy: Current Landscape and Future Horizons for ER-Positive/HER2-Negative and Triple-Negative Early Breast Cancer. Curr. Treat. Options Oncol. 2024, 25, 1210–1224. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Vitamin D Baseline Levels at Diagnosis of Breast Cancer: A Systematic Review and Meta-Analysis. Hematol./Oncol. Stem Cell Ther. 2021, 14, 16–26. [Google Scholar] [CrossRef]
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and Breast Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Callen, D.F.; Li, J.; Zheng, H. Circulating Vitamin D and Overall Survival in Breast Cancer Patients: A Dose-Response Meta-Analysis of Cohort Studies. Integr. Cancer Ther. 2018, 17, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Riondino, S.; Formica, V.; Valenzi, E.; Morelli, C.; Flaminio, V.; Portarena, I.; Torino, F.; Roselli, M. Obesity and Breast Cancer: Interaction or Interference with the Response to Therapy? Curr. Oncol. 2023, 30, 1220–1231. [Google Scholar] [CrossRef]
- Bollen, S.E.; Bass, J.J.; Fujita, S.; Wilkinson, D.; Hewison, M.; Atherton, P.J. The Vitamin D/Vitamin D Receptor (VDR) Axis in Muscle Atrophy and Sarcopenia. Cell Signal 2022, 96, 110355. [Google Scholar] [CrossRef]
- Khayami, R.; Goltzman, D.; Rabbani, S.A.; Kerachian, M.A. Epigenomic Effects of Vitamin D in Colorectal Cancer. Epigenomics 2022, 14, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Imran Ali Shah, S. Beneficial Role of Vitamin D in Common Cancers: Is the Evidence Compelling Enough? World Cancer Res. J. 2020, 7, e1574. [Google Scholar] [CrossRef]
- Berretta, M.; Quagliariello, V.; Bignucolo, A.; Facchini, S.; Maurea, N.; Di Francia, R.; Fiorica, F.; Sharifi, S.; Bressan, S.; Richter, S.N.; et al. The Multiple Effects of Vitamin D against Chronic Diseases: From Reduction of Lipid Peroxidation to Updated Evidence from Clinical Studies. Antioxidants 2022, 11, 1090. [Google Scholar] [CrossRef]
- Lawler, T.; Warren Andersen, S. Serum 25-Hydroxyvitamin D and Cancer Risk: A Systematic Review of Mendelian Randomization Studies. Nutrients 2023, 15, 422. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Facchini, S.; Santorsola, M.; Nasti, G.; Facchini, G.; Montella, L.; Maurea, N.; Cascella, M.; Iervolino, D.; Facchini, B.A.; et al. Circulating Vitamin D Level and Its Impact on Mortality and Recurrence in Stage III Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3012. [Google Scholar] [CrossRef] [PubMed]
- Mirzavandi, F.; Babaie, S.; Rahimpour, S.; Razmpoosh, E.; Talenezhad, N.; Aghaei Zarch, S.M.; Mozaffari-Khosravi, H. The Effect of High Dose of Intramuscular Vitamin D Supplement Injections on Depression in Patients with Type 2 Diabetes and Vitamin D Deficiency: A Randomized Controlled Clinical Trial. Obes. Med. 2020, 17, 100192. [Google Scholar] [CrossRef]
- Ottaiano, A.; Iacovino, M.L.; Santorsola, M.; Facchini, S.; Iervolino, D.; Perri, F.; Nasti, G.; Quagliariello, V.; Maurea, N.; Ronchi, A.; et al. Circulating Vitamin D Level before Initiating Chemotherapy Impacts on the Time-to-Outcome in Metastatic Colorectal Cancer Patients: Systematic Review and Meta-Analysis. J. Transl. Med. 2024, 22, 119. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-Randomized Studies (Minors): Development and Validation of a New Instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Jackson, D.; Bowden, J.; Baker, R. How Does the DerSimonian and Laird Procedure for Random Effects Meta-Analysis Compare with Its More Efficient but Harder to Compute Counterparts? J. Stat. Plan. Inference 2010, 140, 961–970. [Google Scholar] [CrossRef]
- Altman, D.G.; Bryant, T.N.; Gardner, M.J.; Machin, D. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000; Available online: https://www.wiley.com/en-us/Statistics+with+Confidence%3A+Confidence+Intervals+and+Statistical+Guidelines%2C+2nd+Edition-p-9780727913753 (accessed on 19 September 2024).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
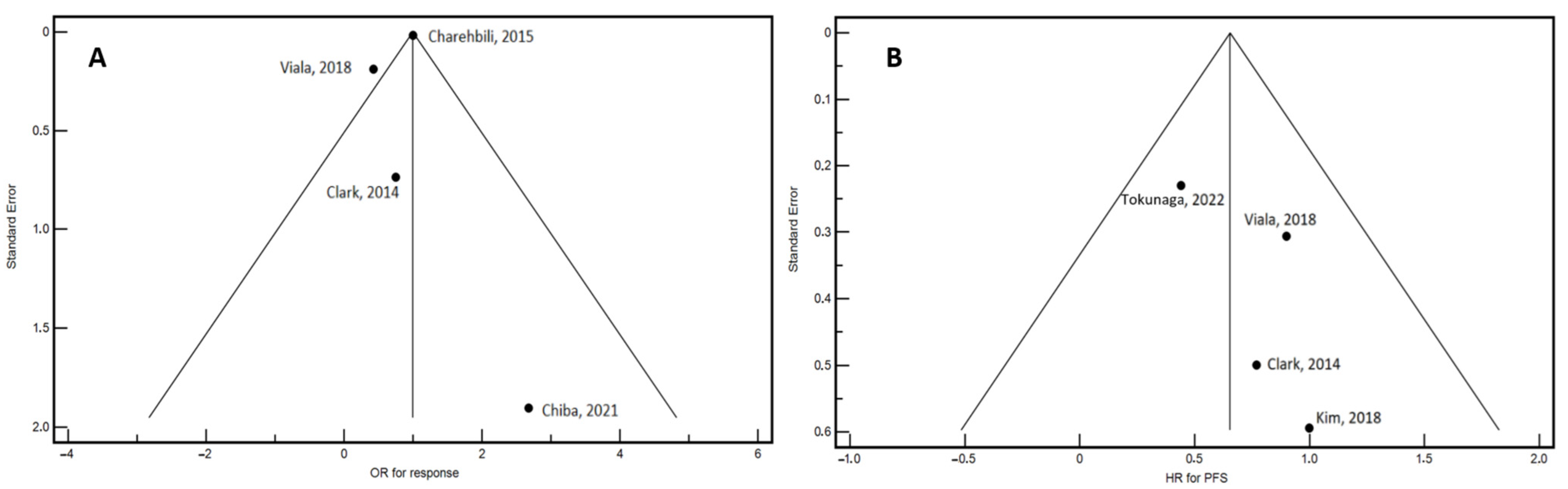
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Clark, A.S.; Chen, J.; Kapoor, S.; Friedman, C.; Mies, C.; Esserman, L.; DeMichele, A.; I-SPY1 Investigators. Pretreatment Vitamin D Level and Response to Neoadjuvant Chemotherapy in Women with Breast Cancer on the I-SPY Trial (CALGB 150007/150015/ACRIN6657). Cancer Med. 2014, 3, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Charehbili, A.; Hamdy, N.a.T.; Smit, V.T.H.B.M.; Kessels, L.; van Bochove, A.; van Laarhoven, H.W.; Putter, H.; Meershoek-Klein Kranenbarg, E.; van Leeuwen-Stok, A.E.; van der Hoeven, J.J.M.; et al. Vitamin D (25-0H D3) Status and Pathological Response to Neoadjuvant Chemotherapy in Stage II/III Breast Cancer: Data from the NEOZOTAC Trial (BOOG 10-01). Breast 2016, 25, 69–74. [Google Scholar] [CrossRef]
- Kim, J.S.; Haule, C.C.; Kim, J.H.; Lim, S.M.; Yoon, K.H.; Kim, J.Y.; Park, H.S.; Park, S.; Kim, S.I.; Cho, Y.U.; et al. Association between Changes in Serum 25-Hydroxyvitamin D Levels and Survival in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. J. Breast Cancer 2018, 21, 134–141. [Google Scholar] [CrossRef]
- Viala, M.; Chiba, A.; Thezenas, S.; Delmond, L.; Lamy, P.-J.; Mott, S.L.; Schroeder, M.C.; Thomas, A.; Jacot, W. Impact of Vitamin D on Pathological Complete Response and Survival Following Neoadjuvant Chemotherapy for Breast Cancer: A Retrospective Study. BMC Cancer 2018, 18, 770. [Google Scholar] [CrossRef] [PubMed]
- Chiba, A.; Raman, R.; Thomas, A.; Lamy, P.-J.; Viala, M.; Pouderoux, S.; Mott, S.L.; Schroeder, M.C.; Thezenas, S.; Jacot, W. Serum Vitamin D Levels Affect Pathologic Complete Response in Patients Undergoing Neoadjuvant Systemic Therapy for Operable Breast Cancer. Clin. Breast Cancer 2018, 18, 144–149. [Google Scholar] [CrossRef]
- Tokunaga, E.; Masuda, T.; Ijichi, H.; Tajiri, W.; Koga, C.; Koi, Y.; Nakamura, Y.; Ohno, S.; Taguchi, K.; Okamoto, M. Impact of Serum Vitamin D on the Response and Prognosis in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Breast Cancer 2022, 29, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Colston, K.W.; Hansen, C.M. Mechanisms Implicated in the Growth Regulatory Effects of Vitamin D in Breast Cancer. Endocr. Relat. Cancer 2002, 9, 45–59. [Google Scholar] [CrossRef]
- Mathiasen, I.S.; Lademann, U.; Jäättelä, M. Apoptosis Induced by Vitamin D Compounds in Breast Cancer Cells Is Inhibited by Bcl-2 but Does Not Involve Known Caspases or P53. Cancer Res. 1999, 59, 4848–4856. [Google Scholar] [PubMed]
- Cos, S.; Mediavilla, M.D.; Fernández, R.; González-Lamuño, D.; Sánchez-Barceló, E.J. Does Melatonin Induce Apoptosis in MCF-7 Human Breast Cancer Cells in Vitro? J. Pineal Res. 2002, 32, 90–96. [Google Scholar] [CrossRef]
- Li, J.; Luco, A.-L.; Camirand, A.; St-Arnaud, R.; Kremer, R. Vitamin D Regulates CXCL12/CXCR4 and Epithelial-to-Mesenchymal Transition in a Model of Breast Cancer Metastasis to Lung. Endocrinology 2021, 162, bqab049. [Google Scholar] [CrossRef]
- Bajbouj, K.; Al-Ali, A.; Shafarin, J.; Sahnoon, L.; Sawan, A.; Shehada, A.; Elkhalifa, W.; Saber-Ayad, M.; Muhammad, J.S.; Elmoselhi, A.B.; et al. Vitamin D Exerts Significant Antitumor Effects by Suppressing Vasculogenic Mimicry in Breast Cancer Cells. Front. Oncol. 2022, 12, 918340. [Google Scholar] [CrossRef]
- Łabędź, N.; Anisiewicz, A.; Stachowicz-Suhs, M.; Banach, J.; Kłopotowska, D.; Maciejczyk, A.; Gazińska, P.; Piotrowska, A.; Dzięgiel, P.; Matkowski, R.; et al. Dual Effect of Vitamin D3 on Breast Cancer-Associated Fibroblasts. BMC Cancer 2024, 24, 209. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, Z.; Slominski, A.T.; Li, W.; Żmijewski, M.A.; Liu, Y.; Chen, J. Vitamin D and Its Analogs as Anticancer and Anti-Inflammatory Agents. Eur. J. Med. Chem. 2020, 207, 112738. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Morin, S.O.; Bou Tayeh, B.; Goubard, A.; Josselin, E.; Castellano, R.; Fauriat, C.; Guittard, G.; Olive, D.; Nunès, J.A. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front. Immunol. 2019, 10, 1307. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef]
- Gewirtz, D.A. A Critical Evaluation of the Mechanisms of Action Proposed for the Antitumor Effects of the Anthracycline Antibiotics Adriamycin and Daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Ravid, A.; Rocker, D.; Machlenkin, A.; Rotem, C.; Hochman, A.; Kessler-Icekson, G.; Liberman, U.A.; Koren, R. 1,25-Dihydroxyvitamin D3 Enhances the Susceptibility of Breast Cancer Cells to Doxorubicin-Induced Oxidative Damage. Cancer Res. 1999, 59, 862–867. [Google Scholar]
- Ravid, A.; Koren, R. The Role of Reactive Oxygen Species in the Anticancer Activity of Vitamin D. Recent Results Cancer Res. 2003, 164, 357–367. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Chen, L.; Holder, R.; Porter, C.; Shah, Z. Vitamin D3 Attenuates Doxorubicin-Induced Senescence of Human Aortic Endothelial Cells by Upregulation of IL-10 via the pAMPKα/Sirt1/Foxo3a Signaling Pathway. PLoS ONE 2021, 16, e0252816. [Google Scholar] [CrossRef]
- Jennaro, T.S.; Fang, F.; Kidwell, K.M.; Smith, E.M.L.; Vangipuram, K.; Burness, M.L.; Griggs, J.J.; Van Poznak, C.; Hayes, D.F.; Henry, N.L.; et al. Vitamin D Deficiency Increases Severity of Paclitaxel-Induced Peripheral Neuropathy. Breast Cancer Res. Treat. 2020, 180, 707–714. [Google Scholar] [CrossRef]
- Chen, C.-S.; Zirpoli, G.; Barlow, W.E.; Budd, G.T.; McKiver, B.; Pusztai, L.; Hortobagyi, G.N.; Albain, K.S.; Damaj, M.I.; Godwin, A.K.; et al. Vitamin D Insufficiency as a Risk Factor for Paclitaxel-Induced Peripheral Neuropathy in SWOG S0221. J. Natl. Compr. Cancer Netw. 2023, 21, 1172–1180.e3. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.A.; Clor, Z.J.; Kelts, J.L. Effect of Vitamin D on Paclitaxel Efficacy in Triple-Negative Breast Cancer Cell Lines. Anticancer Res. 2018, 38, 5043–5048. [Google Scholar] [CrossRef] [PubMed]


| First Author, Year | Study Design | Stage | Vitamin D Assessment | VD Adjusted per Season | N. of Patients | TTO | Response to NACT | Comparison Modalities * | Other Biomarkers Evaluated |
|---|---|---|---|---|---|---|---|---|---|
| Clark, 2014 [27] | R | II, III | RIA | No | 82 | PFS | Yes (RCB 0–1) vs. No (RCB 2–3) | Deficient: <20 ng/mL Sufficient: ≥20 ng/mL | Ki67, Bcl2 and tumor grade |
| Charehbili, 2015 [28] | P | II, III | NA | Yes | 169 | NA | pCR vs. no-pCR | Low: vitamin D < the median of measured values (50.99 nmol/L) High: vitamin D > 50.99 nmol/L | ER, PR |
| Kim, 2018 [29] | R | II, III | RIA | Yes | 374 | PFS | pCR vs. no-pCR | Deficient: <20 ng/mL Sufficient: ≥20 ng/mL | ER, PR, HER-2, Ki67 |
| Viala, 2018 [30] | R | I, II, III | EBA, RIA | No | 327 | PFS, OS | pCR vs. no-pCR | Deficient: <20 ng/mL Sufficient: ≥20 ng/mL | ER, PR, HER-2 |
| Chiba, 2018 [31] | R | I, II, III | EBA, RIA | No | 144 | NA | pCR vs. no-pCR | Deficient: <20 ng/mL Sufficient: ≥20 ng/mL | ER, PR, HER-2 |
| Tokunaga, 2022 [32] | R | I, II, III | ELISA | No | 250 | PFS | pCR vs. no-pCR | Deficient: <20 ng/mL Sufficient: ≥20 ng/mL | ER, PR, HER-2, Ki67 |
| Author | Year | Age (Mean) | Hormone Receptor | Response to NACT | ||
|---|---|---|---|---|---|---|
| Positive, n | Negative, n | pCR, n | No pCR, n | |||
| Clark [27] | 2014 | 48.1 | 50 | 32 | 12 | 70 |
| Charehbili [28] | 2015 | 48.0 * | 142 | 27 | NR ** | NR ** |
| Kim [29] | 2018 | 48.7 | 240 | 134 | 97 | 277 |
| Viala [30] | 2018 | 50 | 143 | 184 | 107 | 220 |
| Chiba [31] | 2018 | NA | 82 | 62 | 48 | 96 |
| Tokunaga [32] | 2022 | 59 * | 178 | 72 | 60 | 190 |
| Author | Year | Median Follow-Up (Months) | Median PFS (Months) | Response to NACT | PFS | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI | P | HR | CI | P | ||||
| Clark [27] | 2014 | NA | NA | 0.75 | 0.14–2.19 | 0.535 | 0.77 | 0.34–1.75 | 0.535 |
| Charehbili [28] | 2015 | NA | NA | 1.00 | 0.97–1.03 | 0.76 | NA | NA | NA |
| Kim [29] | 2018 | 52.3 * | NA | NA | NA | 0.795 | 0.998 | 0.461–2.163 | 0.997 |
| Viala [30] | 2018 | 63.6 | NR | 0.43 | 0.2–0.8 | 0.01 | 0.9 | 0.6–1.5 | 0.8 |
| Chiba [31] | 2018 | NA | NA | 2.68 | 1.12–6.41 | 0.03 | NA | NA | NA |
| Tokunaga [32] | 2022 | NA | NA | NA | NA | 0.4133 | 2.28 | 1.12–5.03 | 0.0231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottaiano, A.; Facchini, B.A.; Iacovino, M.; Santorsola, M.; Facchini, S.; Di Mauro, G.; Toscano, E.; Montopoli, M.; Di Mauro, A.; Quagliariello, V.; et al. Impact of Vitamin D Levels on Progression-Free Survival and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 4206. https://doi.org/10.3390/cancers16244206
Ottaiano A, Facchini BA, Iacovino M, Santorsola M, Facchini S, Di Mauro G, Toscano E, Montopoli M, Di Mauro A, Quagliariello V, et al. Impact of Vitamin D Levels on Progression-Free Survival and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(24):4206. https://doi.org/10.3390/cancers16244206
Chicago/Turabian StyleOttaiano, Alessandro, Bianca Arianna Facchini, Marialucia Iacovino, Mariachiara Santorsola, Sergio Facchini, Giordana Di Mauro, Enrica Toscano, Monica Montopoli, Annabella Di Mauro, Vincenzo Quagliariello, and et al. 2024. "Impact of Vitamin D Levels on Progression-Free Survival and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients: A Systematic Review and Meta-Analysis" Cancers 16, no. 24: 4206. https://doi.org/10.3390/cancers16244206
APA StyleOttaiano, A., Facchini, B. A., Iacovino, M., Santorsola, M., Facchini, S., Di Mauro, G., Toscano, E., Montopoli, M., Di Mauro, A., Quagliariello, V., Maurea, N., Vanni, G., Bignucolo, A., Montella, L., Materazzo, M., Roselli, M., Buonomo, O. C., & Berretta, M. (2024). Impact of Vitamin D Levels on Progression-Free Survival and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers, 16(24), 4206. https://doi.org/10.3390/cancers16244206









