Radiomics Features from Positron Emission Tomography with [18F] Fluorodeoxyglucose Can Help Predict Cervical Nodal Status in Patients with Head and Neck Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Image Acquisition
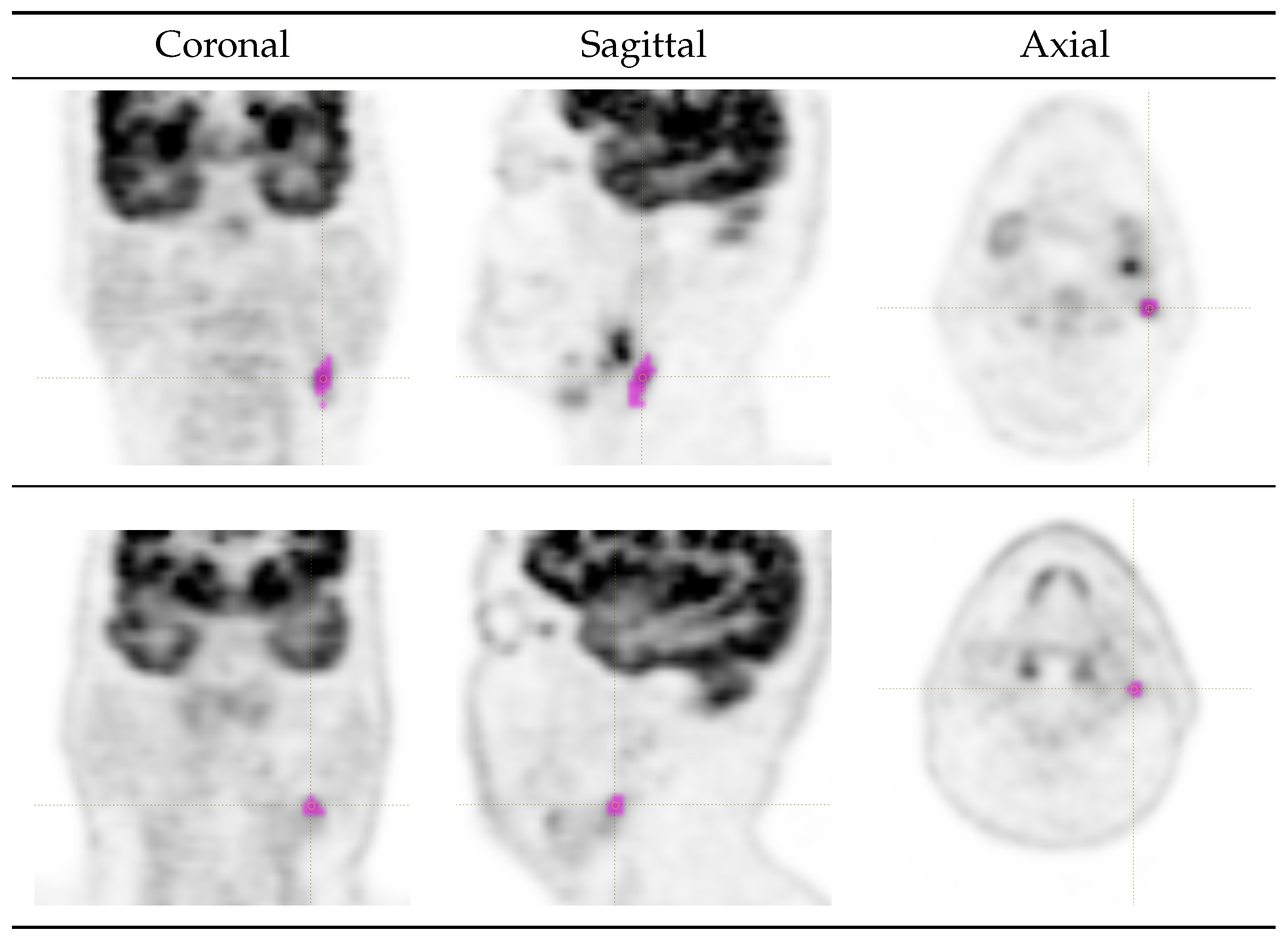
2.3. Lesion Delineation
2.4. Feature Extraction
Feature Selection
- (i)
- Some features are equivalent by definition (e.g., 50th intensity percentile and intensity median); in this case, we only retained one feature per group.
- (ii)
- Some groups of morphological features can be obtained from one another by simple mathematical transformations, and therefore are strongly correlated [30]. Hence, for each of such groups, we retained just one feature. For instance, the morphological features compactness 1, compactness 2, sphericity and spherical disproportion (respectively, corresponding to IBSI codes [26,27]: SKGS, BQWJ, QCFX and KRCK) can be obtained from one another through mathematical transformations. We therefore kept only one feature of this group, i.e., sphericity.
- (iii)
- Under fixed-bin-width absolute quantisation, first-order histogram-based features are a discretised approximation of their first-order intensity-based counterparts. Samples of such pairs of features are intensity-based skewness (KE2A) and histogram-based skewness (88K1), as well as intensity-based kurtosis (IPH6) and histogram-based kurtosis (C317). For all of these pairs, we only retained the intensity-based feature.
2.5. Statistical Analyses
2.5.1. Univariate Analysis
2.5.2. Cut-Off Analysis
2.5.3. Machine Learning Analysis
3. Results
3.1. Univariate and Cut-Off Analysis
3.2. Machine Learning Analysis
4. Discussion
5. Limitations and Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIMN | Associazione italiana di medicina nucleare, imaging molecolare e terapia |
| AUC | Area under the curve |
| CI | Confidence interval |
| CT | Computed tomography |
| FDG PET | Positron emission tomography/computed tomography with [18F] fluorodeoxyglucose |
| FNAC | Fine needle aspiration cytology |
| HNC | Head and neck cancer |
| HNSCC | Head and neck squamous-cell carcinoma |
| IBSI | Image biomarker standardisation initiative |
| LN | Lymph node(s) |
| LOOCV | Leave-one-out cross-validation |
| MRI | Magnetic resonance imaging |
| MTV | Metabolic tumour volume |
| ROI | Region(s) of interest |
| SVM | Support vector machine(s) |
| SUV | Standardised uptake value |
| TLG | Total lesion glycolysis |
| US | Ultrasound |
References
- Peng, Z.; Wang, Y.; Wang, Y.; Jiang, S.; Fan, R.; Zhang, H.; Jiang, W. Application of radiomics and machine learning in head and neck cancers. Int. J. Biol. Sci. 2021, 17, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana di Oncologia Medica; Associazione Italiana dei Registri Tumori. I Numeri del Cancro in Italia. 2023. Available online: https://www.aiom.it/wp-content/uploads/2024/02/2023_AIOM_NDC-web_def.pdf (accessed on 6 October 2024).
- Machiels, J.P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Reprint of “Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up”. Oral Oncol. 2021, 113, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Uozumi, H.; Hirai, T.; Nishimura, R.; Shiraishi, S.; Ota, K.; Murakami, D.; Tomiguchi, S.; Oya, N.; Katsuragawa, S.; et al. Impact of FDG-PET/CT Imaging on Nodal Staging for Head-And-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Ryu, K.H.; Baek, H.J.; Kim, T.H.; Moon, J.I.; Choi, B.H.; Park, S.E.; Ha, J.Y.; Song, D.H.; An, H.J.; et al. Cervical lymph nodes detected by F-18 FDG PET/CT in oncology patients: Added value of subsequent ultrasonography for determining nodal metastasis. Medicina 2020, 56, 16. [Google Scholar] [CrossRef]
- Peng, H.; Chen, L.; Tang, L.L.; Li, W.F.; Mao, Y.P.; Guo, R.; Zhang, Y.; Liu, L.Z.; Tian, L.; Zhang, X.; et al. Significant value of 18F-FDG-PET/CT in diagnosing small cervical lymph node metastases in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Chin. J. Cancer 2017, 36, 95. [Google Scholar] [CrossRef]
- Kikuchi, M.; Johnson, M.K.; Lee, J.Y.; Kim, J.H. Head and neck imaging. In Clinical PET/MRI; Catalano, O.A., Ed.; Academic Press: Cambridge, MA, USA, 2023; Chapter 3; pp. 69–88. [Google Scholar]
- Nakagawa, T.; Yamada, M.; Suzuki, Y. 18F-FDG uptake in reactive neck lymph nodes of oral cancer: Relationship to lymphoid follicles. J. Nucl. Med. 2008, 49, 1053–1059. [Google Scholar] [CrossRef]
- Filippi, L.; Bianconi, F.; Schillaci, O.; Spanu, A.; Palumbo, B. The role and potential of 18F-FDG PET/CT in malignant melanoma: Prognostication, monitoring response to targeted and immunotherapy, and radiomics. Diagnostics 2022, 12, 929. [Google Scholar] [CrossRef]
- Miccichè, F.; Rizzo, G.; Casè, C.; Leone, M.; Quero, G.; Boldrini, L.; Bulajic, M.; Corsi, D.C.; Tondolo, V. Role of radiomics in predicting lymph node metastasis in gastric cancer: A systematic review. Front. Med. 2023, 10, 1189740. [Google Scholar] [CrossRef]
- Xie, H.; Song, C.; Jian, L.; Guo, Y.; Li, M.; Luo, J.; Li, Q.; Tan, T. A deep learning-based radiomics model for predicting lymph node status from lung adenocarcinoma. BMC Med. Imaging 2024, 24, 121. [Google Scholar] [CrossRef]
- Santer, M.; Kloppenburg, M.; Gottfried, T.M.; Runge, A.; Schmutzhard, J.; Vorbach, S.M.; Mangesius, J.; Riedl, D.; Mangesius, S.; Widmann, G.; et al. Current applications of artificial intelligence to classify cervical lymph nodes in patients with head and neck squamous cell carcinoma–A systematic review. Cancers 2022, 14, 5397. [Google Scholar] [CrossRef] [PubMed]
- Giannitto, C.; Mercante, G.; Ammirabile, A.; Cerri, L.; De Giorgi, T.; Lofino, L.; Vatteroni, G.; Casiraghi, E.; Marra, S.; Esposito, A.A.; et al. Radiomics-based machine learning for the diagnosis of lymph node metastases in patients with head and neck cancer: Systematic review. Head Neck 2023, 45, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of tumor grade and nodal status in oropharyngeal and oral cavity squamous-cell carcinoma using a radiomic approach. Anticancer Res. 2020, 40, 271–280. [Google Scholar] [CrossRef]
- Bardosi, Z.R.; Dejaco, D.; Santer, M.; Kloppenburg, M.; Mangesius, S.; Widmann, G.; Ganswindt, U.; Rumpold, G.; Riechelmann, H.; Freysinger, W. Benchmarking eliminative radiomic feature selection for head and neck lymph node classification. Cancers 2022, 14, 477. [Google Scholar] [CrossRef]
- Zheng, B.; Wu, J.; Zhao, Z.; Ou, X.; Cao, P.; Ma, X. Distinguishing lymphomatous and cancerous lymph nodes in 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography by radiomics analysis. Contrast Media Mol. Imaging 2020, 2020, 3959236. [Google Scholar] [CrossRef]
- Dohopolski, M.; Chen, L.; Sher, D.; Wang, J. Predicting lymph node metastasis in patients with oropharyngeal cancer by using a convolutional neural network with associated epistemic and aleatoric uncertainty. Phys. Med. Biol. 2020, 65, 225002. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Chen, S.W.; Kao, C.H.; Cheng, D.C. Neck lymph node recurrence in HNC patients might be predicted before radiotherapy using radiomics extracted from CT images and XGBoost algorithm. J. Pers. Med. 2022, 12, 1377. [Google Scholar] [CrossRef]
- van Staalduinen, E.K.; Matthews, R.; Khan, A.; Punn, I.; Cattell, R.F.; Li, H.; Franceschi, A.; Samara, G.J.; Czerwonka, L.; Bangiyev, L.; et al. Improved cervical lymph node characterization among patients with head and neck squamous cell carcinoma using MR texture analysis compared to traditional FDG-PET/MR features alone. Diagnostics 2024, 14, 71. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Nardone, V.; D’Onofrio, I.; Alessandro, A.; Salvia, A.A.H.; D’Ippolito, E.; Gallo, L.; Caliendo, V.; Gatta, G.; Fasano, M.; et al. Diffusion-weighted imaging and apparent diffusion coefficient mapping of head and neck lymph node metastasis: A systematic review. Explor. Target. Anti-Tumor Ther. 2022, 3, 734–745. [Google Scholar] [CrossRef]
- Nioche, C.; Orhlac, F.; Buvat, I. Local Image Feature Extraction—LIFEx. User Guide. 2023. Available online: https://www.lifexsoft.org/images/phocagallery/documentation/LIFEx/UserGuide/LIFExUserGuide.pdf (accessed on 31 May 2023).
- Zhang, W.; Guo, Y.; Jin, Q. Radiomics and its feature selection: A review. Symmetry 2023, 15, 1834. [Google Scholar] [CrossRef]
- Picchio, M.; Crippa, F. Raccomandazioni Procedurali per L’imaging Oncologico con 18F-FDG PET/TC; Technical Report; Italian Society for Nuclear Medicine and Molecular Imaging (AIMN), Oncology Study Group: Milan, Italy, 2017; Revision 4/2017. [Google Scholar]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuze, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.E.; Buvat, I. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. The Image Biomarker Standardisation Initiative. v11, Last Revised 17 December 2019. Available online: https://arxiv.org/abs/1612.07003 (accessed on 20 October 2020).
- Hatt, M.; Vallieres, M.; Visvikis, D.; Zwanenburg, A. IBSI: An international community radiomics standardization initiative. J. Nucl. Med. 2018, 59, 287. [Google Scholar]
- Orlhac, F.; Nioche, C.; Klyuzhin, I.; Rahmim, A.; Buvat, I. Radiomics in PET imaging: A practical guide for newcomers. PET Clin. 2021, 16, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Hatt, M.; Krizsan, A.K.; Rahmim, A.; Bradshaw, T.J.; Costa, P.F.; Forgacs, A.; Seifert, R.; Zwanenburg, A.; El Naqa, I.; Kinahan, P.E.; et al. Joint EANM/SNMMI guideline on radiomics in nuclear medicine: Jointly supported by the EANM Physics Committee and the SNMMI Physics, Instrumentation and Data Sciences Council. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 352–375. [Google Scholar] [CrossRef]
- Bianconi, F.; Fravolini, M.L.; Pascoletti, G.; Palumbo, I.; Scialpi, M.; Aristei, C.; Palumbo, B. Correlation between IBSI morphological features and manually-annotated shape attributes on lung lesions at CT. In Proceedings of the 26th Annual Conference on Medical Image Understanding and Analysis, MIUA 2022, Cambridge, UK, 27–29 July 2022; Yang, G., Aviles-Rivero, A., Roberts, M., Schönlieb, C.B., Eds.; Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer: Cham, Switzerland, 2022; Volume 13413, pp. 767–777, Code 281309. [Google Scholar]
- Schisterman, E.F.; Faraggi, D.; Reiser, B.; Hu, J. Youden index and the optimal threshold for markers with mass at zero. Stat. Med. 2008, 27, 297–315. [Google Scholar] [CrossRef]
- Brown, L.D.; Cai, T.T.; Das Gupta, A. Interval estimation for a binomial proportion. Stat. Sci. 2001, 16, 101–117. [Google Scholar] [CrossRef]
- Lim, R.S.M.; Ramdave, S.; Beech, P.; Billah, B.; Karim, M.N.; Smith, J.A.; Safdar, A.; Sigston, E. Utility of SUVmax on 18F-FDG PET in detecting cervical nodal metastases. Cancer Imaging 2016, 16, 1–8. [Google Scholar] [CrossRef]
- de Koekkoek-Doll, P.K.; Vogel, W.; Maas, M.; Castelijns, J.; Smit, L.; Zavrakidis, J.; Beets-Tan, R.; van den Brekel, M. SUVmax values at FDG PET-CT to predict malignancy in lymph nodes aspirated by real time image fused USgFNAC in head and neck squamous cell carcinoma. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 178–187. [Google Scholar]
- Bianchini, C.; Caracciolo, M.; Urso, L.; Ciorba, A.; Bonsembiante, A.; Migliorelli, A.; Corazzi, V.; Carandina, I.; Ortolan, N.; Cittanti, C.; et al. Role of 18F-FDG PET/CT in evaluating lymph node status in patients with head and neck squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2023, 43, 235–244. [Google Scholar] [CrossRef]
- Huang, B.; Chan, T.; Kwong, D.L.W.; Chan, W.K.S.; Khong, P.L. Nasopharyngeal carcinoma: Investigation of intratumoral heterogeneity with FDG PET/CT. Am. J. Roentgenol. 2012, 199, 169–174. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Q.; Zhang, Y.; Pan, H.; Yao, Z.; Hu, S.; Shi, W.; Zhu, B.; Zhang, Y.; Hu, C. Pretreatment 18F-FDG uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma—A retrospective study. Radiat. Oncol. 2015, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Bodet-Milin, C.; Bourgeois, M.; Gouard, S.; Ansquer, C.; Barbaud, M.; Sébille, J.C.; Chérel, M.; Kraeber-Bodéré, F.; Carlier, T. Exploring tumor heterogeneity using PET imaging: The big picture. Cancers 2019, 11, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, K.W.; Kim, J.S.; Gil, Y.C.; Tanvaa, T.; Shin, D.; Kim, H.J. Regional thickness of facial skin and superficial fat: Application to the minimally invasive procedures. Clin. Anat. 2019, 32, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
| Class | Name | Mean N | Mean P | -Value |
|---|---|---|---|---|
| MORPHOLOGICAL | Volume (= MTV) [mm3] | 813.84 | 8509.69 | 0.007 |
| MORPHOLOGICAL | SurfaceArea [mm2] | 494.51 | 1970.33 | 0.015 |
| MORPHOLOGICAL | Sphericity [*] | 0.84 | 0.82 | 1.000 |
| MORPHOLOGICAL | CentreOfMassShift [mm] | 0.52 | 0.64 | 1.000 |
| MORPHOLOGICAL | Maximum3DDiameter [mm] | 16.85 | 27.56 | 0.093 |
| MORPHOLOGICAL | IntegratedIntensity (=TLG) [mm3 × SUVbw] | 2034.21 | 60,074.73 | 0.002 |
| INTENSITY-BASED | Mean [SUVbw] | 2.34 | 4.78 | 0.001 |
| INTENSITY-BASED | Variance [SUVbw] | 0.37 | 3.16 | 0.001 |
| INTENSITY-BASED | Skewness [*] | 0.11 | 0.42 | 0.251 |
| INTENSITY-BASED | Kurtosis [*] | −0.78 | −0.19 | 0.002 |
| INTENSITY-BASED | Median [SUVbw] | 2.32 | 4.62 | 0.001 |
| INTENSITY-BASED | MinimumGreyLevel (=SUVmin) [SUVbw] | 1.44 | 2.07 | 1.000 |
| INTENSITY-BASED | 10thPercentile [SUVbw] | 1.69 | 2.96 | 0.013 |
| INTENSITY-BASED | 90thPercentile [SUVbw] | 3.04 | 6.93 | 0.001 |
| INTENSITY-BASED | MaximumGreyLevel (=SUVmax) [SUVbw] | 3.37 | 8.98 | 0.000 |
| INTENSITY-BASED | InterquartileRange [SUVbw] | 0.74 | 2.14 | 0.002 |
| INTENSITY-BASED | Range [SUVbw] | 1.93 | 6.91 | 0.001 |
| INTENSITY-BASED | MeanAbsoluteDeviation [SUVbw] | 0.42 | 1.24 | 0.001 |
| INTENSITY-BASED | RobustMeanAbsoluteDeviation [SUVbw] | 0.33 | 0.91 | 0.002 |
| INTENSITY-BASED | MedianAbsoluteDeviation [SUVbw] | 0.41 | 1.22 | 0.001 |
| INTENSITY-BASED | CoefficientOfVariation [*] | 0.21 | 0.31 | 0.201 |
| INTENSITY-BASED | QuartileCoefficientOfDispersion [*] | 0.16 | 0.23 | 0.479 |
| INTENSITY-BASED | Energy [SUVbw2] | 280.85 | 20,566.06 | 0.001 |
| INTENSITY-BASED | RootMeanSquare [SUVbw] | 2.41 | 5.05 | 0.001 |
| INTENSITY-HISTOGRAM | IntensityHistogramMode [a.u.] | 7.54 | 14.20 | 0.004 |
| INTENSITY-HISTOGRAM | IntensityHistogramEntropyLog2 [bits] | 2.37 | 3.68 | 0.001 |
| INTENSITY-HISTOGRAM | Uniformity [*] | 0.24 | 0.12 | 0.002 |
| INTENSITY-HISTOGRAM | MaximumHistogramGradient [a.u.] | 4.06 | 7.52 | 1.000 |
| INTENSITY-HISTOGRAM | MaximumHistogramGradientGreyLevel [a.u.] | 5.81 | 10.60 | 0.085 |
| INTENSITY-HISTOGRAM | MinimumHistogramGradient [a.u.] | −3.94 | −7.04 | 1.000 |
| INTENSITY-HISTOGRAM | MinimumHistogramGradientGreyLevel [a.u.] | 9.19 | 17.04 | 0.001 |
| Class | Name | Cut-Off |
|---|---|---|
| MORPHOLOGICAL | Volume | >496.76 |
| MORPHOLOGICAL | SurfaceArea | >362.38 |
| MORPHOLOGICAL | IntegratedIntensity | >1100.02 |
| INTENSITY-BASED | Mean | >2.82 |
| INTENSITY-BASED | Variance | >0.72 |
| INTENSITY-BASED | Kurtosis | >−0.91 |
| INTENSITY-BASED | Median | >3.00 |
| INTENSITY-BASED | 10thPercentile | >1.61 |
| INTENSITY-BASED | 90thPercentile | >3.91 |
| INTENSITY-BASED | MaximumGreyLevel | >4.56 |
| INTENSITY-BASED | InterquartileRange | >1.45 |
| INTENSITY-BASED | Range | >3.59 |
| INTENSITY-BASED | MeanAbsoluteDeviation | >0.71 |
| INTENSITY-BASED | RobustMeanAbsoluteDeviation | >0.56 |
| INTENSITY-BASED | MedianAbsoluteDeviation | >0.71 |
| INTENSITY-BASED | Energy | >52.52 |
| INTENSITY-BASED | RootMeanSquare | >2.80 |
| INTENSITY-HISTOGRAM | IntensityHistogramMode | >9.16 |
| INTENSITY-HISTOGRAM | IntensityHistogramEntropyLog2 | >3.23 |
| INTENSITY-HISTOGRAM | Uniformity | <0.11 |
| INTENSITY-HISTOGRAM | MinimumHistogramGradientGreyLevel | >11.20 |
| Feature Set | Classifier | AUC | Sens. | Spec. | Acc. | Acc. CI |
|---|---|---|---|---|---|---|
| PETBase | Logistic regression | 0.840 | 68.0% | 89.5% | 80.4% | 69.5–91.3% |
| PETRad | 0.880 | 72.0% | 90.0% | 82.4% | 71.9–92.8% | |
| PETBase | SVM | 0.811 | 72.0% | 90.0% | 82.4% | 71.9–92.8% |
| PETRad | 0.823 | 76.0% | 90.5% | 84.3% | 74.3–94.3% | |
| PETBase | Gaussian NB | 0.798 | 56.0% | 87.5% | 74.5% | 62.5–86.5% |
| PETRad | 0.846 | 64.0% | 88.9% | 78.4% | 67.1–89.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianconi, F.; Salis, R.; Fravolini, M.L.; Khan, M.U.; Filippi, L.; Marongiu, A.; Nuvoli, S.; Spanu, A.; Palumbo, B. Radiomics Features from Positron Emission Tomography with [18F] Fluorodeoxyglucose Can Help Predict Cervical Nodal Status in Patients with Head and Neck Cancer. Cancers 2024, 16, 3759. https://doi.org/10.3390/cancers16223759
Bianconi F, Salis R, Fravolini ML, Khan MU, Filippi L, Marongiu A, Nuvoli S, Spanu A, Palumbo B. Radiomics Features from Positron Emission Tomography with [18F] Fluorodeoxyglucose Can Help Predict Cervical Nodal Status in Patients with Head and Neck Cancer. Cancers. 2024; 16(22):3759. https://doi.org/10.3390/cancers16223759
Chicago/Turabian StyleBianconi, Francesco, Roberto Salis, Mario Luca Fravolini, Muhammad Usama Khan, Luca Filippi, Andrea Marongiu, Susanna Nuvoli, Angela Spanu, and Barbara Palumbo. 2024. "Radiomics Features from Positron Emission Tomography with [18F] Fluorodeoxyglucose Can Help Predict Cervical Nodal Status in Patients with Head and Neck Cancer" Cancers 16, no. 22: 3759. https://doi.org/10.3390/cancers16223759
APA StyleBianconi, F., Salis, R., Fravolini, M. L., Khan, M. U., Filippi, L., Marongiu, A., Nuvoli, S., Spanu, A., & Palumbo, B. (2024). Radiomics Features from Positron Emission Tomography with [18F] Fluorodeoxyglucose Can Help Predict Cervical Nodal Status in Patients with Head and Neck Cancer. Cancers, 16(22), 3759. https://doi.org/10.3390/cancers16223759









