Biphenotypic Sinonasal Sarcoma: Literature Review of a Peculiar Pathological Entity—The Neurosurgical Point of View
Simple Summary
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
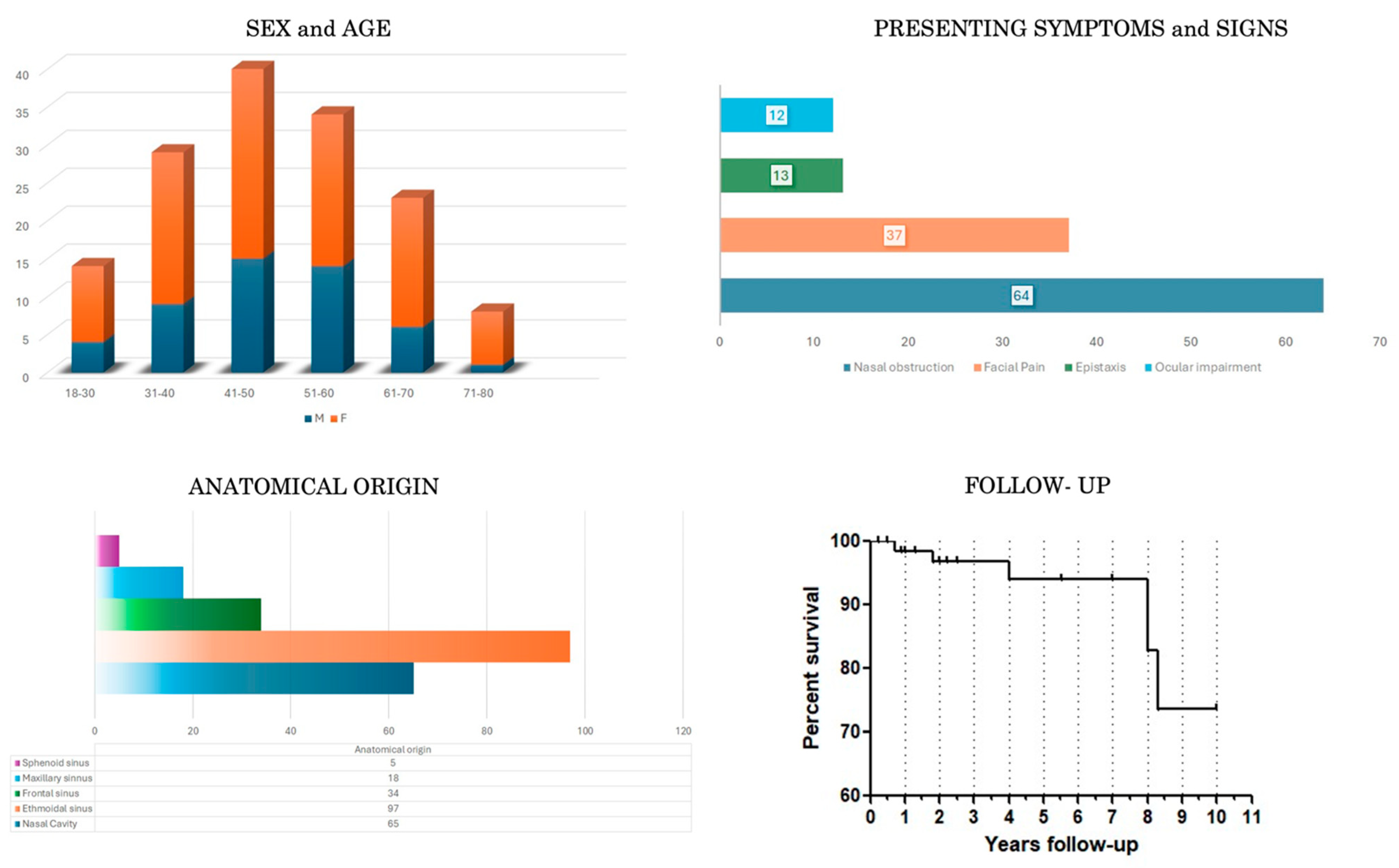
3.1. Demographic, Clinical, Neuroradiological and Pathological Data (Table 1 and Table 3, Figure 2)
3.2. Treatment and Outcome Data (Table 2 and Table 4, Figure 2)
4. Discussion
- Slow growth: mean time from clinical onset to treatment is 12 months (even if cases up to 3 years are described (Table 2)); nevertheless, considering the small sizes and the function of the common sites of origin of the tumor—such as nasal cavity and paranasal sinuses—as well as of the adjacent structures usually involved—such as the orbit—it is easy to understand that a tumor becomes symptomatic quite early;
- Local aggressiveness: the tumor invades and destructs adjacent structures, both bony and soft tissues, including the medial wall, floor and roof of the orbit, cribriform plate, and orbital fat; therefore, a prompt and proper diagnosis and treatment are mandatory to prevent neuro-ophthalmological complications, such as CSF leak, meningitis, meningocele, seizures, pneumocephalus, anosmia, proptosis, and diplopia;
- Infiltrative pattern of growth: this makes it hard to achieve clear margins after surgical excision despite the high rates of gross total resection and low rate of peri- and post-operative complications;
- Long time to and very low frequency of malignant transformation;
- Tendency to locally recur: Recurrence was observed in patients regardless of the extent of tumor resection and the administration of adjuvant radiotherapy; there is no significant evidence to support the need for concomitant radiotherapy or surgical excision alone. Post-operative RT is mainly adopted when the examination of the surgical margins is found to be positive or inconclusive [6]. Therefore, it is important to collect further studies with large case series and long follow-up to analyze the main risk factors for recurrence.
- No distant metastasis.
Limitations and Advantages of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, J.T.; Oliveira, A.M.; Nascimento, A.G.; Schembri-Wismayer, D.; Moore, E.A.; Olsen, K.D.; Garcia, J.G.; Lonzo, M.L.; Lewis, J.E. Low-grade sinonasal sarcoma with neural and myogenic features: A clinicopathologic analysis of 28 cases. Am. J. Surg. Pathol. 2012, 36, 517–525. [Google Scholar] [CrossRef]
- Stelow, E.B.; Bishop, J.A. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Nasal Cavity, Paranasal Sinuses and Skull Base. Head. Neck Pathol. 2017, 11, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; East, E.G.; McHugh, J.B. Biphenotypic Sinonasal Sarcoma: A Review and Update. Arch. Pathol. Lab. Med. 2018, 142, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Triki, M.; Ayadi, L. Low-Grade Sinonasal Sarcoma with Neural and Myogenic Features: A Recently Discovered Entity with Unique Features and Diagnostic Challenge. Arch. Pathol. Lab. Med. 2017, 141, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Chitguppi, C.; Koszewski, I.; Collura, K.; Curtis, M.; Nyquist, G.; Rabinowitz, M.; Rosen, M. Biphenotypic Sinonasal Sarcoma-Case Report and Review of Clinicopathological Features and Diagnostic Modalities. J. Neurol. Surg. B Skull Base 2019, 80, 51–58. [Google Scholar] [CrossRef]
- Powers, K.A.; Han, L.M.; Chiu, A.G.; Aly, F.Z. Low-grade sinonasal sarcoma with neural and myogenic features—Diagnostic challenge and pathogenic insight. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2015, 119, e265–e269. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Huang, S.C.; Antonescu, C.R.; Westra, W.H.; Bishop, J.A. Biphenotypic sinonasal sarcoma: An expanded immunoprofile including consistent nuclear β-catenin positivity and absence of SOX10 expression. Hum. Pathol. 2016, 55, 44–50. [Google Scholar] [CrossRef]
- Wong, W.J.; Lauria, A.; Hornick, J.L.; Xiao, S.; Fletcher, J.A.; Marino-Enriquez, A. Alternate PAX3-FOXO1 oncogenic fusion in biphenotypic sinonasal sarcoma. Genes. Chromosomes Cancer 2016, 55, 25–29. [Google Scholar] [CrossRef]
- Huang, S.C.; Ghossein, R.A.; Bishop, J.A.; Zhang, L.; Chen, T.C.; Huang, H.Y.; Antonescu, C.R. Novel PAX3-NCOA1 Fusions in Biphenotypic Sinonasal Sarcoma With Focal Rhabdomyoblastic Differentiation. Am. J. Surg. Pathol. 2016, 40, 51–59. [Google Scholar] [CrossRef]
- Cannon, R.B.; Wiggins, R.H.; Witt, B.L.; Dundar, Y.; Johnston, T.M.; Hunt, J.P. Imaging and Outcomes for a New Entity: Low-Grade Sinonasal Sarcoma with Neural and Myogenic Features. J. Neurol. Surg. Rep. 2017, 78, e15–e19. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, B.; Han, A. Biphenotypic sinonasal sarcoma with diffuse infiltration and intracranial extension: A case report. Int. J. Clin. Exp. Pathol. 2017, 10, 11743–11746. [Google Scholar] [PubMed]
- Andreasen, S.; Bishop, J.A.; Hellquist, H.; Hunt, J.; Kiss, K.; Rinaldo, A.; Skálová, A.; Willems, S.M.; Williams, M.; Ferlito, A. Biphenotypic sinonasal sarcoma: Demographics, clinicopathological characteristics, molecular features, and prognosis of a recently described entity. Virchows Arch. 2018, 473, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.; Rajeshwari, M.; Sakthivel, P.; Sharma, M.C.; Sharma, S.C. Biphenotypic sinonasal sarcoma: A series of six cases with evaluation of role of β-catenin immunohistochemistry in differential diagnosis. Ann. Diagn. Pathol. 2018, 33, 6–10. [Google Scholar] [CrossRef]
- Fudaba, H.; Momii, Y.; Hirano, T.; Yamamoto, H.; Fujiki, M. Recurrence of Biphenotypic Sinonasal Sarcoma With Cerebral Hemorrhaging. J. Craniofac Surg. 2019, 30, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Alkhudher, S.M.; Al Zamel, H.; Bhat, I.N. A rare case of nasal biphenotypic sino-nasal sarcoma in a young female. Ann. Med. Surg. 2019, 37, 4–6. [Google Scholar] [CrossRef]
- Le Loarer, F.; Laffont, S.; Lesluyes, T.; Tirode, F.; Antonescu, C.; Baglin, A.C.; Delespaul, L.; Soubeyran, I.; Hostein, I.; Pérot, G.; et al. Clinicopathologic and Molecular Features of a Series of 41 Biphenotypic Sinonasal Sarcomas Expanding Their Molecular Spectrum. Am. J. Surg. Pathol. 2019, 43, 747–754. [Google Scholar] [CrossRef]
- Sethi, S.; Cody, B.; Farhat, N.A.; Pool, M.D.; Katabi, N. Biphenotypic sinonasal sarcoma: Report of 3 cases with a review of literature. Hum. Pathol. 2021, 24, 200491. [Google Scholar] [CrossRef]
- Hanbazazh, M.; Jakobiec, F.A.; Curtin, H.D.; Lefebvre, D.R. Orbital Involvement by Biphenotypic Sinonasal Sarcoma with a Literature Review. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Phan, J.; DeMonte, F.; Hanna, E.Y. High-grade transformation of low-grade biphenotypic sinonasal sarcoma: Radiological, morphophenotypic variation and confirmatory molecular analysis. Ann. Diagn. Pathol. 2022, 57, 151889. [Google Scholar] [CrossRef] [PubMed]
- Hasnie, S.; Glenn, C.; Peterson, J.E.G.; El Rassi, E.T.; McKinney, K.A. High-Grade Biphenotypic Sinonasal Sarcoma: A Case Report. J. Neurol. Surg. Rep. 2022, 83, e105–e109. [Google Scholar] [CrossRef] [PubMed]
- Turri-Zanoni, M.; Dalfino, G.; Lechner, M.; Dallan, I.; Battaglia, P.; Facco, C.; Franzi, F.; Gravante, G.; Ferrari, M.; Terzakis, D.; et al. Biphenotypic sinonasal sarcoma: European multicentre case-series and systematic literature review. Acta Otorhinolaryngol. Ital. 2022, 42, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.M.; Alruwaii, F.; Chaaban, M.; Cheng, Y.W.; Griffith, C.C. Biphenotypic Sinonasal Sarcoma with a Novel PAX3::FOXO6 Fusion: A Case Report and Review of the Literature. Head. Neck Pathol. 2023, 17, 259–264. [Google Scholar] [CrossRef]
- Ingle, A.; Mahendra, N.; Gopal Reddy, G.V. Biphenotypic sinonasal sarcoma-A recently described entity with many mimics: A case report. Indian. J. Pathol. Microbiol. 2023, 66, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Klubíčková, N.; Mosaieby, E.; Grossmann, P.; Kalmykova, A.; Koshyk, O.; Michal, M. Biphenotypic sinonasal sarcoma with PAX3::MAML3 fusion transforming into high-grade rhabdomyosarcoma: Report of an emerging rare phenomenon. Virchows Arch. 2023, 482, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, E.; Boyke, A.E.; Madani, D.; Kamat, A.; Schiff, B.A.; Agarwal, V. Biphenotypic Sinonasal Sarcoma: A Case Report and Review of Literature. Ear Nose Throat J. 2023, 102, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Bhele, S.; Chrisinger, J.S.A.; Farrell, N.F.; Van Tine, B.A.; Raptis, C.A.; Chernock, R.D. Biphenotypic Sinonasal Sarcoma with a Novel PAX7::PPARGC1 Fusion: Expanding the Spectrum of Gene Fusions Beyond the PAX3 Gene. Head. Neck Pathol. 2023, 17, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Viramontes, A.; Mueller, N.; Gocke, C.D.; Deklotz, T.R.; Ozdemirli, M. Novel PAX3::INO80D Fusion in Biphenotypic Sinonasal Sarcoma in an Adult. JAMA Otolaryngol. Head. Neck Surg. 2023, 149, 849–850. [Google Scholar] [CrossRef]
- Muraoka, E.; Kato, I.; Matsumura, M.; Arai, Y.; Suenaga, J.; Yamanaka, S.; Fujii, S. Biphenotypic Sinonasal Sarcoma: A Genetically Confirmed Case Showing Bone Invasion Accompanying a Non-neoplastic Respiratory Epithelium. Int. J. Surg. Pathol. 2023, 31, 1414–1419. [Google Scholar] [CrossRef]
- Anastasiadou, S.; Karkos, P.; Constantinidis, J. Biphenotypic Sinonasal Sarcoma with Orbital and Skull Base Involvement Report of 3 Cases and Systematic Review of the Literature. Indian. J. Otolaryngol. Head. Neck Surg. 2023, 75, 3353–3363. [Google Scholar] [CrossRef]
- Okuda, H.; Kuze, B.; Shibata, H.; Hayashi, H.; Nishihori, T.; Mizuta, K.; Kohyama, K.; Yasue, Y.; Kato, H.; Aoki, M. Biphenotypic siononasal sarcoma with acute exacerbation: A case report. Otolaryngol. Case Rep. 2020, 16, 100190. [Google Scholar]
- Okafor, S.; Halderman, A.; Bishop, J.; Ryan, M.; Marple, B. Biphenotypic Sinonasal Sarcoma a Newly Recognized Sinonasal Neoplasm: Case Report and Review of the Literature. J. Neurol. Surg. Part B Skull Base 2020, 81, S1–S272. [Google Scholar]
- Kühn, A.; Jalisi, S.; Nishino, M.; Ivanovic, V. Biphenotypic sinonasal sarcoma—Description of radiologic, intraoperative and pathologic findings. Otolaryngol. Case Rep. 2019, 11, 100113. [Google Scholar] [CrossRef]
- Miglani, A.; Lal, D.; Weindling, S.M.; Wood, C.P.; Hoxworth, J.M. Imaging characteristics and clinical outcomes of biphenotypic sinonasal sarcoma. Laryngoscope Investig. Otolaryngol. 2019, 4, 484–488. [Google Scholar] [CrossRef]
- Koszewski, I.J.; Garcia, H.G.; Rabinowitz, M.R.; Nyquist, G.G.; Evans, J.J.; Rosen, M.R. Biphenotypic Sinonasal Sarcoma with Focal Rhabdomyoblastic Differentiation: Case Report of a Newly Described Malignancy with a Review of the Literature. J. Neurol. Surg. Part B Skull Base 2018, 79, S1–S188. [Google Scholar] [CrossRef]
- Hockstein, N.G.; Dross, P.E.; Farooqui, S.; Wilhelm, I.N. Low-grade sinonasal sarcoma with neural and myogenic features. Ear Nose Throat J. 2018, 97, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Corvino, S.; de Divitiis, O.; Iuliano, A.; Russo, F.; Corazzelli, G.; Cohen, D.; Di Crescenzo, R.M.; Palmiero, C.; Pontillo, G.; Staibano, S.; et al. Biphenotypic Sinonasal Sarcoma with Orbital Invasion: A Literature Review and Modular System of Surgical Approaches. Cancers 2024, 16, 3316. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Policeni, B. Sinonasal Neoplasms. Semin. Roentgenol. 2019, 54, 244–257. [Google Scholar] [CrossRef]
- Kassam, A.; Snyderman, C.H.; Mintz, A.; Gardner, P.; Carrau, R.L. Expanded endonasal approach: The rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg. Focus. 2005, 19, E4. [Google Scholar] [CrossRef]
- Kassam, A.; Snyderman, C.H.; Mintz, A.; Gardner, P.; Carrau, R.L. Expanded endonasal approach: The rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg. Focus 2005, 19, E3. [Google Scholar] [CrossRef]
- Özer, M.; Kutlay, A.M.; Durmaz, M.O.; Kirik, A.; Yaşar, S.; Tehli, Ö.; Kural, C.; Temiz, N.; Durmaz, A.; Ezgu, M.C.; et al. Extended endonasal endoscopic approach for anterior midline skull base lesions. Clin. Neurol. Neurosurg. 2020, 196, 106024. [Google Scholar] [CrossRef]
- Gellner, V.; Tomazic, P.V. Limits of the endoscopic transnasal transtubercular approach. J. Neurosurg. Sci. 2018, 62, 297–300. [Google Scholar] [CrossRef]
- Ceylan, S.; Koc, K.; Anik, I. Extended endoscopic approaches for midline skull-base lesions. Neurosurg. Rev. 2009, 32, 309–319, discussion 318–309. [Google Scholar] [CrossRef]
- Ceylan, S.; Anik, I.; Koc, K.; Cabuk, B. Extended endoscopic transsphenoidal approach infrachiasmatic corridor. Neurosurg. Rev. 2015, 38, 137–147, discussion 147. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.S.; Bergeron, C.M.; Ellenbogen, R.G. Transorbital neuroendoscopic surgery. Neurosurgery 2010, 67, ons16–ons28. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Kong, D.S.; de Notaris, M.; Moe, K.S.; Sánchez España, J.C.; Schwartz, T.H.; Enseñat, J. Endoscopic transorbital surgery levels of difficulty. J. Neurosurg. 2022, 137, 1187–1190. [Google Scholar] [CrossRef]
- de Notaris, M.; Kong, D.S.; Di Somma, A.; Enseñat, J.; Hong, C.K.; Moe, K.; Schwartz, T.H. Superior eyelid transorbital approaches: A modular classification system. J. Neurosurg. 2024, 1, 1–6. [Google Scholar] [CrossRef]
- Corvino, S.; Sacco, M.; Somma, T.; Berardinelli, J.; Ugga, L.; Colamaria, A.; Corrivetti, F.; Iaconetta, G.; Kong, D.-S.; de Notaris, M. Functional and clinical outcomes after superior eyelid transorbital endoscopic approach for spheno-orbital meningiomas: Illustrative case and literature review. Neurosurg. Rev. 2023, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Corvino, S.; Armocida, D.; Offi, M.; Pennisi, G.; Burattini, B.; Mondragon, A.V.; Esposito, F.; Cavallo, L.M.; de Notaris, M. The anterolateral triangle as window on the foramen lacerum from transorbital corridor: Anatomical study and technical nuances. Acta Neurochir. 2023, 165, 2407–2419. [Google Scholar] [CrossRef]
- Corvino, S.; Guizzardi, G.; Sacco, M.; Corrivetti, F.; Bove, I.; Enseñat, J.; Colamaria, A.; Prats-Galino, A.; Solari, D.; Cavallo, L.M.; et al. The feasibility of three port endonasal, transorbital, and sublabial approach to the petroclival region: Neurosurgical audit and multiportal anatomic quantitative investigation. Acta Neurochir. 2023, 165, 1–11. [Google Scholar] [CrossRef]
- Corvino, S.; Villanueva-Solórzano, P.; Offi, M.; Armocida, D.; Nonaka, M.; Iaconetta, G.; Esposito, F.; Cavallo, L.; de Notaris, M. A New Perspective on the Cavernous Sinus as Seen through Multiple Surgical Corridors: Anatomical Study Comparing the Transorbital, Endonasal, and Transcranial Routes and the Relative Coterminous Spatial Regions. Brain Sci. 2023, 13, 1215. [Google Scholar] [CrossRef]
- de Notaris, M.; Sacco, M.; Corrivetti, F.; Grasso, M.; Corvino, S.; Piazza, A.; Kong, D.S.; Iaconetta, G. The Transorbital Approach, A Game-Changer in Neurosurgery: A Guide to Safe and Reliable Surgery Based on Anatomical Principles. J. Clin. Med. 2023, 12, 6484. [Google Scholar] [CrossRef] [PubMed]
- Corvino, S.; Kassam, A.; Piazza, A.; Corrivetti, F.; Spiriev, T.; Colamaria, A.; Cirrottola, G.; Cavaliere, C.; Esposito, F.; Cavallo, L.M.; et al. Open-door extended endoscopic transorbital technique to the paramedian anterior and middle cranial fossae: Technical notes, anatomomorphometric quantitative analysis, and illustrative case. Neurosurg. Focus 2024, 56, E7. [Google Scholar] [CrossRef] [PubMed]
- Vural, A.; Carobbio, A.L.C.; Ferrari, M.; Rampinelli, V.; Schreiber, A.; Mattavelli, D.; Doglietto, F.; Buffoli, B.; Rodella, L.F.; Taboni, S.; et al. Transorbital endoscopic approaches to the skull base: A systematic literature review and anatomical description. Neurosurg. Rev. 2021, 44, 2857–2878. [Google Scholar] [CrossRef] [PubMed]
- Paluzzi, A.; Gardner, P.A.; Fernandez-Miranda, J.C.; Tormenti, M.J.; Stefko, S.T.; Snyderman, C.H.; Maroon, J.C. "Round-the-Clock" Surgical Access to the Orbit. J. Neurol. Surg. B Skull Base 2015, 76, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Corvino, S.; de Notaris, M.; Sommer, D.; Kassam, A.; Kong, D.S.; Piazza, A.; Corrivetti, F.; Cavallo, L.M.; Iaconetta, G.; Reddy, K. Assessing the Feasibility of Selective Piezoelectric Osteotomy in Transorbital Approach to the Middle Cranial Fossa: Anatomical and Quantitative Study and Surgical Implications. World Neurosurg. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Corvino, S.; Kassam, A.; Piazza, A.; Corrivetti, F.; Esposito, F.; Iaconetta, G.; de Notaris, M. Navigating the Intersection Between the Orbit and the Skull Base: The “Mirror” McCarty Keyhole During Transorbital Approach: An Anatomic Study with Surgical Implications. Oper. Neurosurg. 2024. [Google Scholar] [CrossRef]
- Mariniello, G.; Corvino, S.; Iuliano, A.; Maiuri, F. Spheno-orbital Meningiomas. In Cranio-Orbital Mass Lesions; Bonavolontà, G., Maiuri, F., Mariniello, G., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Kong, D.S.; Kim, Y.H.; Hong, C.K. Optimal indications and limitations of endoscopic transorbital superior eyelid surgery for spheno-orbital meningiomas. J. Neurosurg. 2020, 134, 1472–1479. [Google Scholar] [CrossRef]
- Mariniello, G.; Corvino, S.; Corazzelli, G.; de Divitiis, O.; Fusco, G.; Iuliano, A.; Strianese, D.; Briganti, F.; Elefante, A. Spheno-Orbital Meningiomas: The Rationale behind the Decision-Making Process of Treatment Strategy. Cancers 2024, 16, 2148. [Google Scholar] [CrossRef]
- Mariniello, G.; de Divitiis, O.; Corvino, S.; Strianese, D.; Iuliano, A.; Bonavolontà, G.; Maiuri, F. Recurrences of spheno-orbital meningiomas: Risk factors and management. World Neurosurg. 2022, 161, e514–e522. [Google Scholar] [CrossRef]
- Makary, C.A.; Limjuco, A.; Nguyen, J.; Ramadan, H.H. Combined Lid Crease and Endoscopic Approach to Lateral Frontal Sinus Disease With Orbital Extension. Ann. Otol. Rhinol. Laryngol. 2018, 127, 637–642. [Google Scholar] [CrossRef]
- Miller, C.; Berens, A.; Patel, S.A.; Humphreys, I.M.; Moe, K.S. Transorbital Approach for Improved Access in the Management of Paranasal Sinus Mucoceles. J. Neurol. Surg. B Skull Base 2019, 80, 593–598. [Google Scholar] [CrossRef]
- Arosio, A.D.; Coden, E.; Valentini, M.; Czaczkes, C.; Battaglia, P.; Bignami, M.; Castelnuovo, P.; Karligkiotis, A. Combined Endonasal-Transorbital Approach to Manage the Far Lateral Frontal Sinus: Surgical Technique. World Neurosurg. 2021, 151, 5. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, D.A.; Montaser, A.; Kreatsoulas, D.; Shah, V.S.; VanKoevering, K.K.; Otto, B.A.; Carrau, R.L.; Prevedello, D.M. Complications after 1002 endoscopic endonasal approach procedures at a single center: Lessons learned, 2010–2018. J. Neurosurg. 2022, 136, 393–404. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Carrau, R.L.; Ditzel Filho, L.F.; Prevedello, D.M.; Otto, B.A.; Old, M.; de Lara, D.; Kassam, A.B. Advantages and limitations of endoscopic endonasal approaches to the skull base. World Neurosurg. 2014, 82, S12–S21. [Google Scholar] [CrossRef]
- Consortium, C. CSF Rhinorrhoea After Endonasal Intervention to the Skull Base (CRANIAL)—Part 1: Multicenter Pilot Study. World Neurosurg. 2021, 149, e1077–e1089. [Google Scholar] [CrossRef]
- Porras, J.L.; Rowan, N.R.; Mukherjee, D. Endoscopic Endonasal Skull Base Surgery Complication Avoidance: A Contemporary Review. Brain Sci. 2022, 12, 1685. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.T.; Yeoh, D.; Fastenberg, J.H.; Chaskes, M.B.; Pollack, A.Z.; Boockvar, J.A.; Langer, D.J.; D’Amico, R.S.; Ellis, J.A.; Miles, B.A.; et al. Reconstruction of the Anterior Skull Base Using the Nasoseptal Flap: A Review. Cancers 2023, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Snyderman, C.H.; Wang, E.W.; Zenonos, G.A.; Gardner, P.A. Reconstruction after endoscopic surgery for skull base malignancies. J. Neurooncol. 2020, 150, 463–468. [Google Scholar] [CrossRef]

| Studies | Demographic and Clinical Data | Radiological Data | Diagnosis | |||||
|---|---|---|---|---|---|---|---|---|
| Authors/Year | Num of Cases | Sex, Mean Age (Years) | Presenting Symptoms | Anatomical Origin | Skull Base Involvement | Orbit Involvement | ||
| 1 | Lewis et al. [1], 2012 | 28 | 21 F 7 M (52 years) | Breath difficulty, congestion, facial pressure | 19 ES, 8 NC, 1 SS. | 3 YES (ACF) | 7 YES | Immunohistochemical |
| 2 | Powers et al. [7] 2015 | 1 | M, 59 | Sinusitis, congestion, facial pressure, anosmia, dysgeusia | ES-NC | YES (ACF) | None | Immunohistochemical |
| 3 | Rooper et al. [8] 2016 | 11 | 8 F 3 M (44 years) | n.a. | 4 ES 3 FS 3 NC 1 ES-NC | None | 2 YES | Immunohistochemical molecular |
| 4 | Wong et al. [9] 2016 | 1 | M, 33 | Recurrent brisk epistaxis | NC-SS | None | None | Immunohistochemical molecular |
| 5 | Huang et al. [10] 2016 | 7 | 4 M 3 F (52 years) | n.a. | 2 FS 2 ES-NC 2 NC 1 ES | None | None | Immunohistochemical molecular |
| 6 | Cannon et al. [11] 2017 | 3 | 3 F (67.6 years) | Diplopia, facial discomfort, supraorbital swelling nasal obstruction, facial pressure | 3 FS-ES | 3 YES | 3 Lamina papiracea | Immunohistochemical molecular |
| 7 | Lin et al. [12] 2017 | 1 | F, 67 | Nasal obstruction | ES-FS-SS-MS | YES | None | Immunohistochemical |
| 8 | Hockstein et al. [36] 2018 | 1 | F, 79 | Asymptomatic | FS | YES | Roof | Immunohistochemical |
| 9 | Andreasen et al. [13] 2018 | 3 | 2 F, 1 M (59.6 years) | Nasal obstruction and midfacial pressure | 2 ES 1 ES-NC | None | None | Immunohistochemical |
| 10 | Koszewski et al. [35] 2018 | 1 | M, 53 | Unilateral nasal obstruction and epiphora | NC | YES (ACF) | Lamina papiracea | Immunohistochemical |
| 11 | Kakkar et al. [14] 2018 | 6 | 5 F, 1 M (51 years) | Nasal obstruction | 1 NC 1 NC, MS 1 NC, MS, ES 1 NC, MS, ES 1 NC, MS, ES, FS 1 NC, ES | 1 YES | None | Immunohistochemical |
| 12 | Quadros et al. 2019 | 1 | F, 55 | Obstruction of the left nasal cavity | NC | None | None | Immunohistochemical |
| 13 | Chitguppi et al. [6] 2019 | 1 | M, 53 | n.a. | ES-NC | YES | YES | Immunohistochemical molecular |
| 14 | Alkhudher et al. [16] 2019 | 1 | F, 35 | Nasal obstruction, epistaxis | NC, MS, ES | None | Lamina papiracea | Immunohistochemical |
| 15 | Miglani et al. [34] 2019 | 5 | 4 F, 1 M (56 years) | n.a. | 5 NC-ES | 5 YES (ACF) | 5 Lamina Papiracea | Immunohistochemical |
| 16 | Fudaba et al. [15] 2019 | 1 | M, 70 | Loss of consciousness and vomiting | ES | YES | None | Immunohistochemical molecular |
| 17 | Le Loarer et al. [17] 2019 | 41 | 16 M, 25 F (51 years) | n.a. | 14 NC 11 ES 10 ES-FS 6 n.a. | 4 YES | 4 YES | Immunohistochemical molecular |
| 18 | Kuhn et al. [33] 2019 | 1 | n.a. | Worsening nasal obstruction, rhinorrhea, left orbital pain, proptosis and blurry vision | NC-ES | YES (ACF) | Lamina papiracea | Immunohistochemical molecular |
| 19 | Okafor et al. [32] 2020 | 1 | M, 54 | Left-sided nasal airway obstruction and anosmia | NC-MS-ES-FS | YES (ACF) | Lamina papiracea | Immunohistochemical |
| 20 | Okuda et al. [31] 2020 | 1 | F, 64 | Nasal obstruction | NC-MS-ES pterygopalatine fossa | YES (MCF) | YES | Immunohistochemical |
| 21 | Sethi et al. [18] 2021 | 3 | 3 F (56 years) | Left-sided nasal congestion and headaches/right nasal obstruction/rhinorrhea and left-sided nasal congestion | 3 ES-MS-FS-NC | 1 YES (ACF) | 2 YES | Immunohistochemical |
| 22 | Hanbazazh et al. [19] 2021 | 1 | M, 50 | Orbital pain and pressure, diplopia, blurred vision, lateral gaze restriction | ES | YES | Lamina papiracea | Immunohistochemical molecular |
| 23 | Bell et al. [20] 2022 | 1 | M, 66 | Swelling of left eyelid, vertical diplopia and purulent nasal discharge | NC | YES (ACF) | YES | Immunohistochemical molecular |
| 24 | Hasnie et al. [21] 2022 | 1 | F, 72 | Nasal obstruction, episodic epistaxis and facial pressure/headaches, decreased sense of smell | MS-ES-Bilateral FS-NC | YES (ACF) | Lamina papiracea | Immunohistochemical molecular |
| 25 | Turri-Zanoni et al. [22] 2022 | 15 | 3 M, 12 F (54 years) | 14 nasal airway obstruction 9 epistaxis, 6 olfactory disfunction3 facial pain | 13 ES 2 FS | None | None | Immunohistochemical molecular |
| 26 | Nichols et al. [23] 2023 | 1 | M, 54 | Persistent headaches, postnasal drip, thickened nasal secretions, and epistaxis after sneezing | ES-SS | None | None | Immunohistochemical molecular |
| 27 | Ingle et al. [24] 2023 | 1 | F, 47 | Swelling eyelid, proptosis | NC, FS, ES, MS | None | Lamina papiracea | Immunohistochemical |
| 28 | Meyer et al. [25] 2023 | 1 | M, 67 | Nasal congestion and epiphora, right-sided ocular proptosis | ES-MS-FS | None | YES | Immunohistochemical molecular |
| 29 | Kominsky et al. [26] 2023 | 2 | 2 M (65 years) | Bilateral nasal congestion and blurry vision | ES-NC-FS | 2 YES | 2 Lamina papiracea | Immunohistochemical molecular |
| 30 | Bhele et al. [27] 2023 | 1 | F,22 | Vision loss, headache, hyposmia, facial pressure | NC-ES-SS-MS | YES (ACF) | Lamina papiracea | Immunohistochemical |
| 31 | Viramontes et al. [28] 2023 | 1 | F, 40 | Progressive obstruction of the right nasal cavity, | NC | None | None | Immunohistochemical molecular |
| 32 | Muraoka et al. [29] 2023 | 1 | F, 73 | Purulent nasal discharge and dull pain in the left cheek area | NC-ES-FS | YES (ACF) | None | Immunohistochemical molecular |
| 33 | Anastasiadou et al. [30] 2023 | 3 | 3 F (43 years) | Exophthalmos, headaches | NC-MS | 1 YES | 2 YES | Immunohistochemical molecular |
| 34 | Corvino et al. [37] 2024 | 1 | M, 46 | l. proptosis, upward gaze restriction | FS-ES | YES (ACF) | Roof | Immunohistochemical |
| Studies | Treatment Data | Outcome Data at Last Follow Up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors/Year | Num of Cases | Time to Treatment | Type of Treatment | Type of Surgical Approach | EOR | Peri-Post Operative Complications | Clinics | Recurrence | Status | |
| 1 | Lewis et al. [1] 2012 | 28 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 7/16 (range 12–118 mo.) | (mean 8.3 years) 14 alive 2 dead due to other causes |
| 2 | Powers et al. [7] 2015 | 1 | n.a. | S | EEA | GTR | CSF leak | n.a. | None | Alive 10 mo. |
| 3 | Rooper et al. [8] 2016 | 11 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2/7 (range 1–26 mo.) | (mean 4 years) 1/7 dead due to tumor |
| 4 | Wong et al. [9] 2016 | 1 | n.a. | S Ad-CHT-RT | EEA | GTR | n.a. | n.a. | None | Alive 5 mo. |
| 5 | Huang et al. [10] 2016 | 7 | n.a. | 6 S 1 S + Ad-CHT-RT | n.a. | 4 GTR | n.a. | n.a. | 1/4 (36 mo) | (mean 8 years) 4 alive |
| 6 | Cannon et al. [11] 2017 | 3 | n.a | 2 S 1 Biopsy | 1 EEA − 1 EEA + TCA1 EEA Biopsy | 2 GTR 1 STR | n.a. | n.a. | 1/3 (17 mo.) | (mean 25 mo.) 3 alive |
| 7 | Lin et al. [12] 2017 | 1 | n.a | S | EEA | GTR | Subarachnoid hemorrhage; brain herniation | n.a. | n.a. | Dead due to surgery |
| 8 | Hockstein et al. [36] 2018 | 1 | 12 mo. | S | EEA + TCA | GTR | n.a. | n.a. | None | Alive |
| 9 | Andreasen et al. [13] 2018 | 3 | n.a | 1S 2 S + Ad.RT | n.a. | 3 GTR | None | n.a. | 1/3 (11, 21 and 24 mo) | (mean 67.3 mo.) 3 alive |
| 10 | Koszewski et al. [35] 2018 | 1 | 4 mo. | S + Ad.RT | n.a. | STR | n.a. | n.a. | None | Alive |
| 11 | Kakkar et al. [14] 2018 | 6 | n.a. | 3 S 3 Biopsy | 3 EEA | 4 STR | n.a. | n.a. | None | 1/6 dead due to other causes |
| 12 | Quadros et al. 2019 | 1 | n.a | S | EEA | n.a. | n.a. | n.a. | n.a. | n.a. |
| 13 | Chitguppi et al. [6] 2019 | 1 | n.a. | S + Ad-RT | TCA + ETOA | STR | n.a. | n.a. | None | Alive |
| 14 | Alkhudher et al. [16] 2019 | 1 | 2 mo. | S | EEA | GTR | n.a. | Improved | None | Alive 2 years |
| 15 | Miglani et al. [34] 2019 | 5 | n.a | 4 S 1 S + Ad-RT | 3 TCA 2 EEA | 4 GTR 1 STR | n.a. | n.a. | 2/5 (mean 31.4 mo.) | (mean 31.4 mo.) 5 alive |
| 16 | Fudaba et al. [15] 2019 | 1 | REC after 11 years | S | EEA + TCA | GTR | n.a. | n.a. | No further | Alive |
| 17 | Le Loarer et al. [17] 2019 | 41 | n.a. | 20 S 8 S + RT 2 S + RT + CHT 1 RT+ CHT 2 S + CHT | n.a. | n.a. | n.a. | n.a. | 8/25 (range 9–95 mo.) | (mean 45 mo.) |
| 18 | Kuhn et al. [33] 2019 | 1 | n.a | S | TCA | GTR | None | n.a. | n.a. | n.a. |
| 19 | Okafor et al. [32] 2020 | 1 | 5 mo. | 2 S | EEA | 1 STR 1 GTR | None | n.a. | n.a. | n.a. |
| 20 | Okuda et al. [31] 2020 | 1 | REC after 2 mo. | S + Ad.CHT | TCA | GTR | None | n.a. | YES (after 2 mo) | Dead 8 mo., death due to tumor progression |
| 21 | Sethi et al. [18] 2021 | 3 | n.a | 1 S + Ad.RT 2 S | 3 EEA | 3 GTR | None | n.a. | None | 2 alive (mean 22 mo) |
| 22 | Hanbazazh et al. [19] 2021 | 1 | 36 mo | 1 Biopsy 1 S 1 S + Ad.RT | Biopsy EEA TO TCA | STR | None | Improved | None | Alive |
| 23 | Bell et al. [20] 2022 | 1 | REC after 15 years | 1 S + Ad.RT | TCA | GTR | None | Stable | No further | Alive 10 mo. |
| 24 | Hasnie et al. [21] 2022 | 1 | 24 mo. | S | EEA + TCA | GTR | Infection, pneumocephal | n.a. | None | Death due to other causes |
| 25 | Turri-Zanoni et al. [22] 2022 | 15 | n.a | 13 S 2S + RT | 7 EEA 8 EEA + TCA | 13 GTR2 STR | n.a. | n.a. | 1/15 (after 35 and 47 mo) | (27.3 months) 15 alive |
| 26 | Nichols et al. [23] 2023 | 1 | n.a | S | EEA | n.a. | n.a. | Improved | None | Alive 3 mo. |
| 27 | Ingle et al. [24] 2023 | 1 | 2 mo. | S | EEA + TCA | GTR | n.a. | n.a. | None | Alive 3 mo. |
| 28 | Meyer et al. [25] 2023 | 1 | 36 mo. | Biopsy, RT, CHT | EEA | Biopsy | n.a. | n.a. | Progression | Dead 15 mo., death due to tumor progression |
| 29 | Kominsky et al. [26], 2023 | 2 | 3 weeks (1) | 2 S | 2 EEA | 2 GTR | n.a. | n.a. | None | 2 alive (mean 13 mo.) |
| 30 | Bhele et al. [27] 2023 | 1 | 8 mo. | Biopsy, Neo-CHT, S, Ad-PB | TCA + EEA | STR | n.a. | n.a. | None | Alive, 10 mo. |
| 31 | Viramontes et al. [28], 2023 | 1 | n.a | S | EEA | GTR | n.a. | n.a. | None | Alive, 16 mo. |
| 32 | Muraoka et al. [29] 2023 | 1 | n.a | S | TCA + EEA | GTR | n.a. | n.a. | None | Alive |
| 33 | Anastasiadou et al. [30], 2023 | 3 | n.a | 1 S, 2 S + Ad.RT | 3 EEA | 3 GTR | 1 CSF leak | n.a. | None | Alive 7 years |
| 34 | Corvino et al. [37] 2024 | 1 | 2 mo. | S | TCA + EEA | GTR | None | Improved | None | Alive, 10 mo. |
| Covariates | Overall Sample 149 (%) | Statistical Analysis (p Value) |
|---|---|---|
| Demographic and clinical data | ||
| Sex -F -M | 148/149 * (99.3%) 99/148 (66.9%) 49/148 (33.1%) | p = 0.6 |
| Age range (median) | 22–79 years (54.88 y.o.) | p = 0.04 |
| Main presenting symptoms -Nasal obstruction -Facial pressure/pain/discomfort -Epistaxis -Ocular impairment | 84/149 * (56.3%) 68/84 (81%) 37/84 (44%) 13/84 (15.5%) 12/84 (14.3%) | p = 0.46 |
| Radiological data | ||
| Anatomical Origin -NC -ES -FS -MS -SS | 143/149 * (96%) 65/143 (45.4%) 97/143 (67.8%) 34/143 (23.7%) 18/143 (12.6%) 5/143 (3.5%) | p = 0.32 |
| Skull Base involvement -Yes -Not | 143/149 * (96%) 35/143 (24.5%) 108/143 (75.5%) | p = 0.22 |
| Orbit involvement -Yes -Not | 143/149 * (96%) 41/143 (28.7%) 102/143 (71.3%) | p = 0.26 |
| Pathological Diagnosis | ||
| Diagnostic method -immunohistochemical alone -immunohistochemical and molecular | 149/149 * (100%) 56/149 (37.6%) 93/149 (62.4%) | p = 0.55 |
| Covariates | Overall Sample 149 (%) | Statistical Analysis (p Value) |
|---|---|---|
| Treatment Data | ||
| Time to treatment (mean in months) | 11/149 (7.3%) 12 months | p = 0.11 |
| Type of treatment -S -S + RT -Biopsy alone -S + CHT -S + RT + CHT -RT + CHT | 104/149 * (69.8%) 69/104 (66.3%) 20/104 (19.2%) 5/104 (4.8%) 3/104 (2.9%) 5/104 (4.8%) 2/104 (1.9%) | p = 0.43 |
| Type of surgical approach -EEA -TCA -TOA -Combined | 58/149 * (39%) 33/58 (56.9%) 7/58 (12%) 1/58 (1.7%) 17/58 (29.3%) | p = 0.1 |
| EOR -GTR -STR | 62/149 * (41.6%) 49/62 (79%) 13/62 (21%) | p = 0.45 |
| Peri- and post-operative complications -Yes -None | 12/149 * (8%) 4/12 (33.3%) 8/12 (66.7%) | |
| Outcome | ||
| Clinical -Improved -Stable -Worsened | 5/149 * (3.3%) 4/5 (80%) 1/5 (20%) --- | |
| Recurrence -Yes -Not | 84/149 * (56.3%) 22/84 (26.2%) 62/84 (73.8%) | p = 0.6 |
| Status -Alive -Dead | 85/149 * (57%) 77/85 (91.8%) 8/85 (8.2%) | p = 0.87 |
| Follow-up | Mean 4.6 years | St. Dev = 3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corvino, S.; Corazzelli, G.; Mariniello, G.; Iuliano, A.; Altieri, R.; Pontillo, G.; Strianese, D.; Barbarisi, M.; Elefante, A.; de Divitiis, O. Biphenotypic Sinonasal Sarcoma: Literature Review of a Peculiar Pathological Entity—The Neurosurgical Point of View. Cancers 2024, 16, 3747. https://doi.org/10.3390/cancers16223747
Corvino S, Corazzelli G, Mariniello G, Iuliano A, Altieri R, Pontillo G, Strianese D, Barbarisi M, Elefante A, de Divitiis O. Biphenotypic Sinonasal Sarcoma: Literature Review of a Peculiar Pathological Entity—The Neurosurgical Point of View. Cancers. 2024; 16(22):3747. https://doi.org/10.3390/cancers16223747
Chicago/Turabian StyleCorvino, Sergio, Giuseppe Corazzelli, Giuseppe Mariniello, Adriana Iuliano, Roberto Altieri, Giuseppe Pontillo, Diego Strianese, Manlio Barbarisi, Andrea Elefante, and Oreste de Divitiis. 2024. "Biphenotypic Sinonasal Sarcoma: Literature Review of a Peculiar Pathological Entity—The Neurosurgical Point of View" Cancers 16, no. 22: 3747. https://doi.org/10.3390/cancers16223747
APA StyleCorvino, S., Corazzelli, G., Mariniello, G., Iuliano, A., Altieri, R., Pontillo, G., Strianese, D., Barbarisi, M., Elefante, A., & de Divitiis, O. (2024). Biphenotypic Sinonasal Sarcoma: Literature Review of a Peculiar Pathological Entity—The Neurosurgical Point of View. Cancers, 16(22), 3747. https://doi.org/10.3390/cancers16223747








