Multi-Omic Characterization of Single Cells and Cell-Free Components Detected in the Cerebrospinal Fluid of Patients with Leptomeningeal Disease
Simple Summary
Abstract
1. Introduction
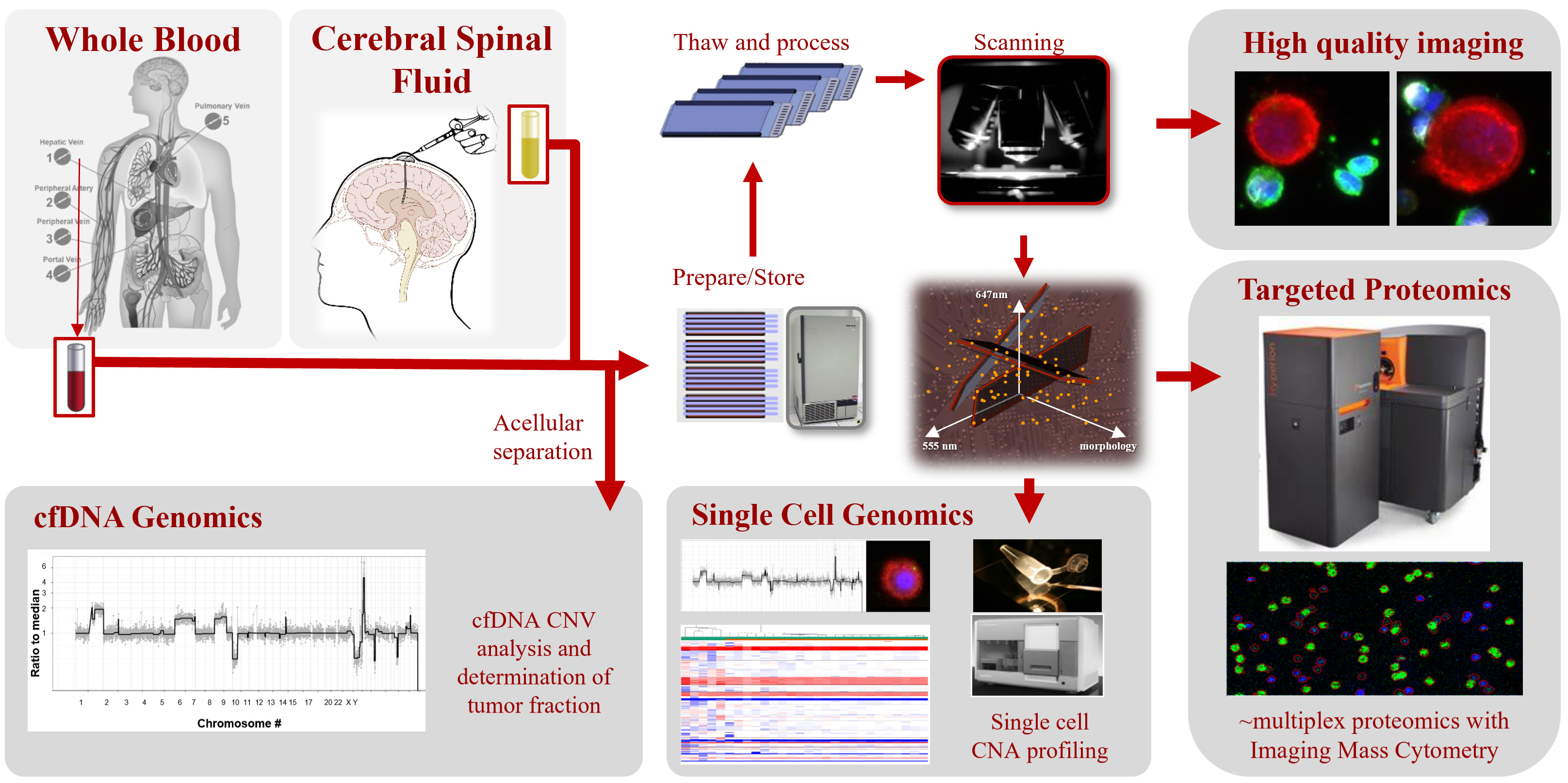
2. Materials and Methods
2.1. Study Design
2.2. Liquid Biopsy Processing
2.3. Immunofluorescent Staining and Automated Scanning
2.4. Liquid Biopsy Analyte Detection and Classification
2.5. Single Cell Genomic Analysis
2.6. Targeted Multiplexed Proteomics Using IMC
2.7. MACSPlex Exosome Assay and Flow Cytometry Analysis
3. Results
3.1. Patient Information
3.2. Correlation of PB and CSF Liquid Biopsies
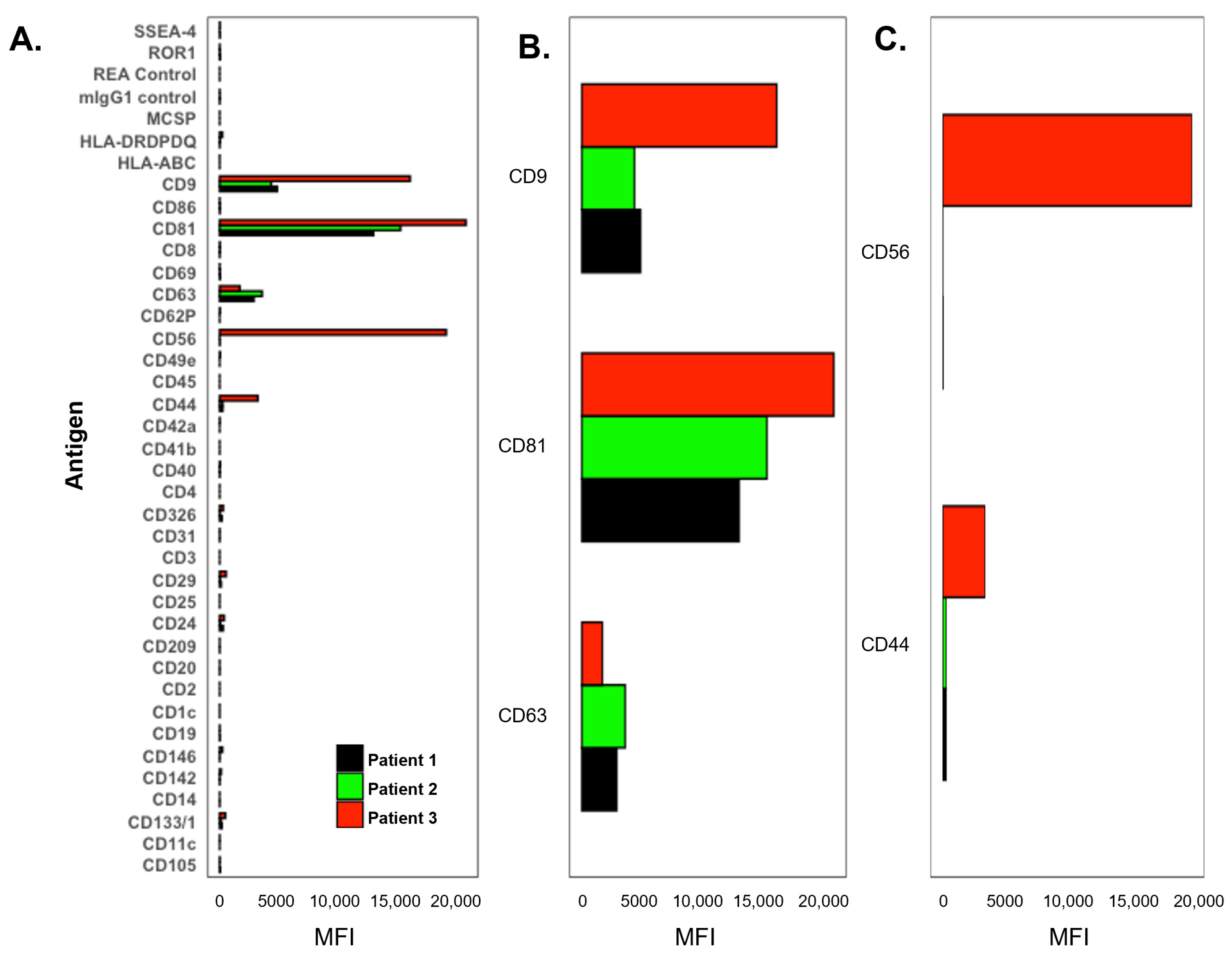
3.3. CSF Exosome Analysis
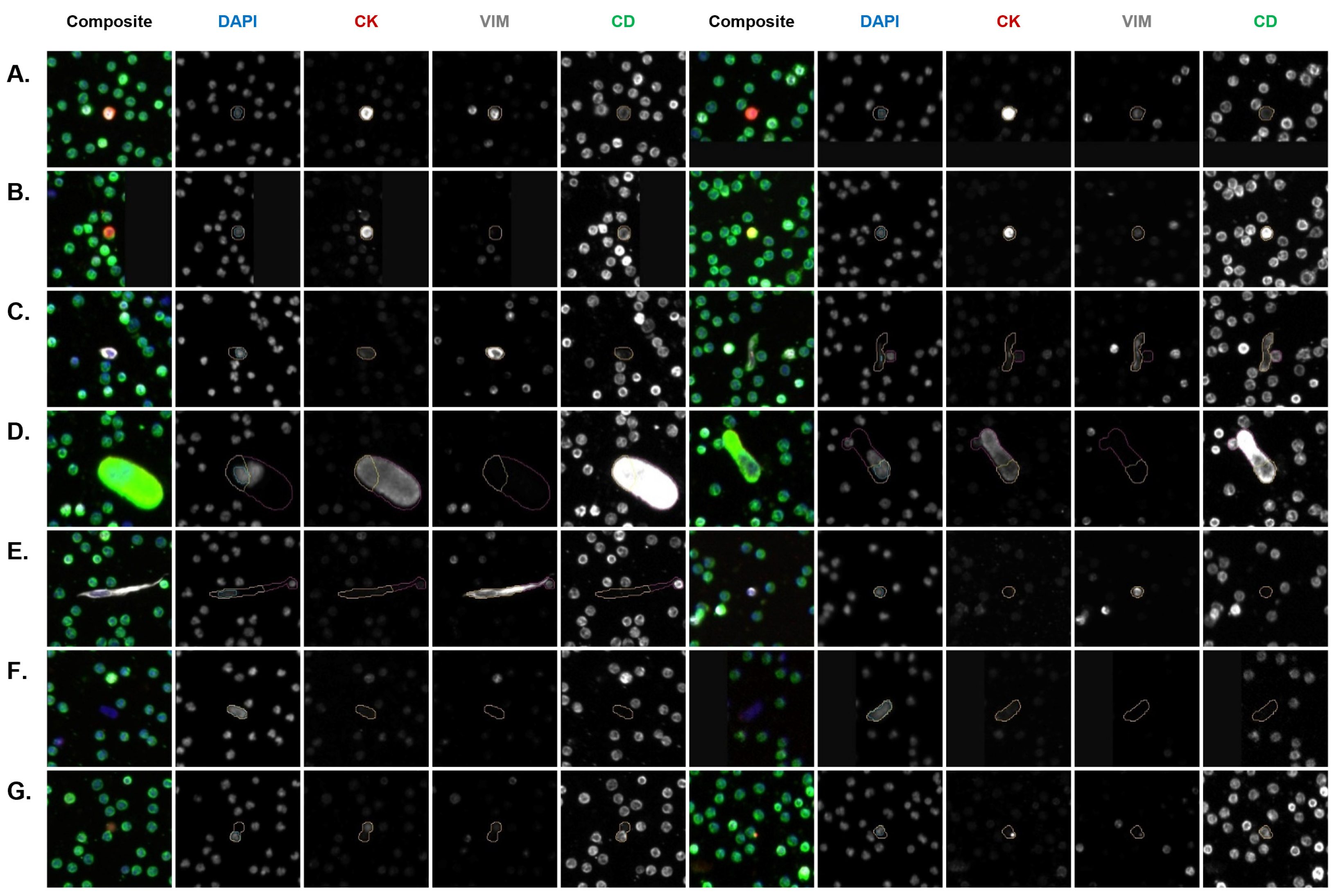
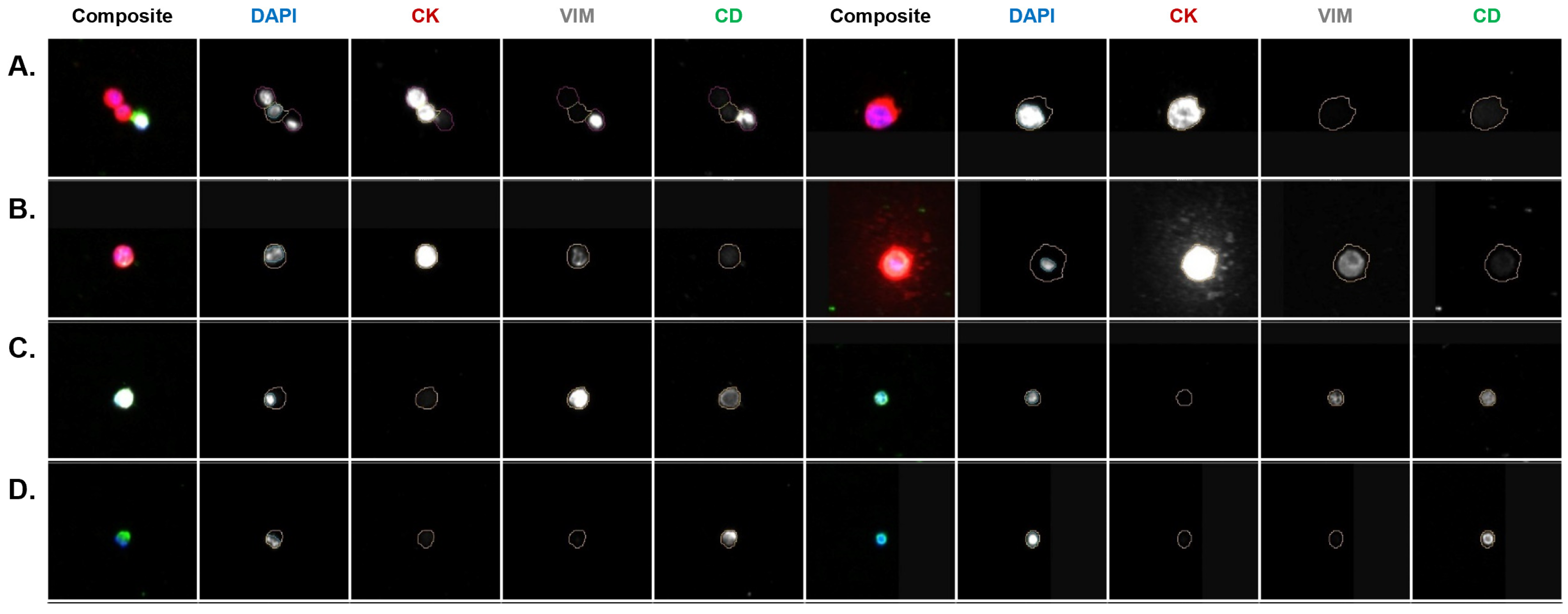
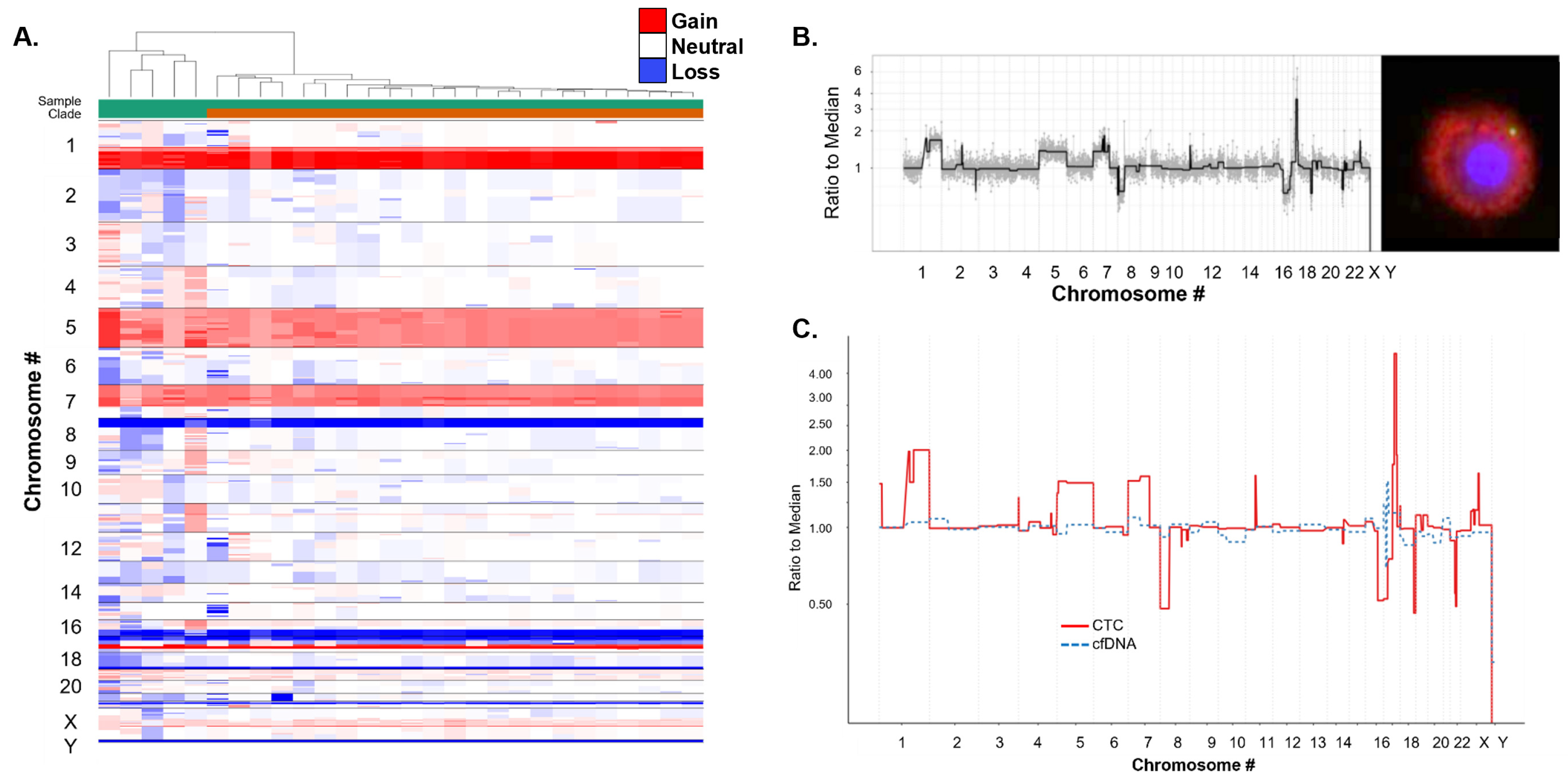
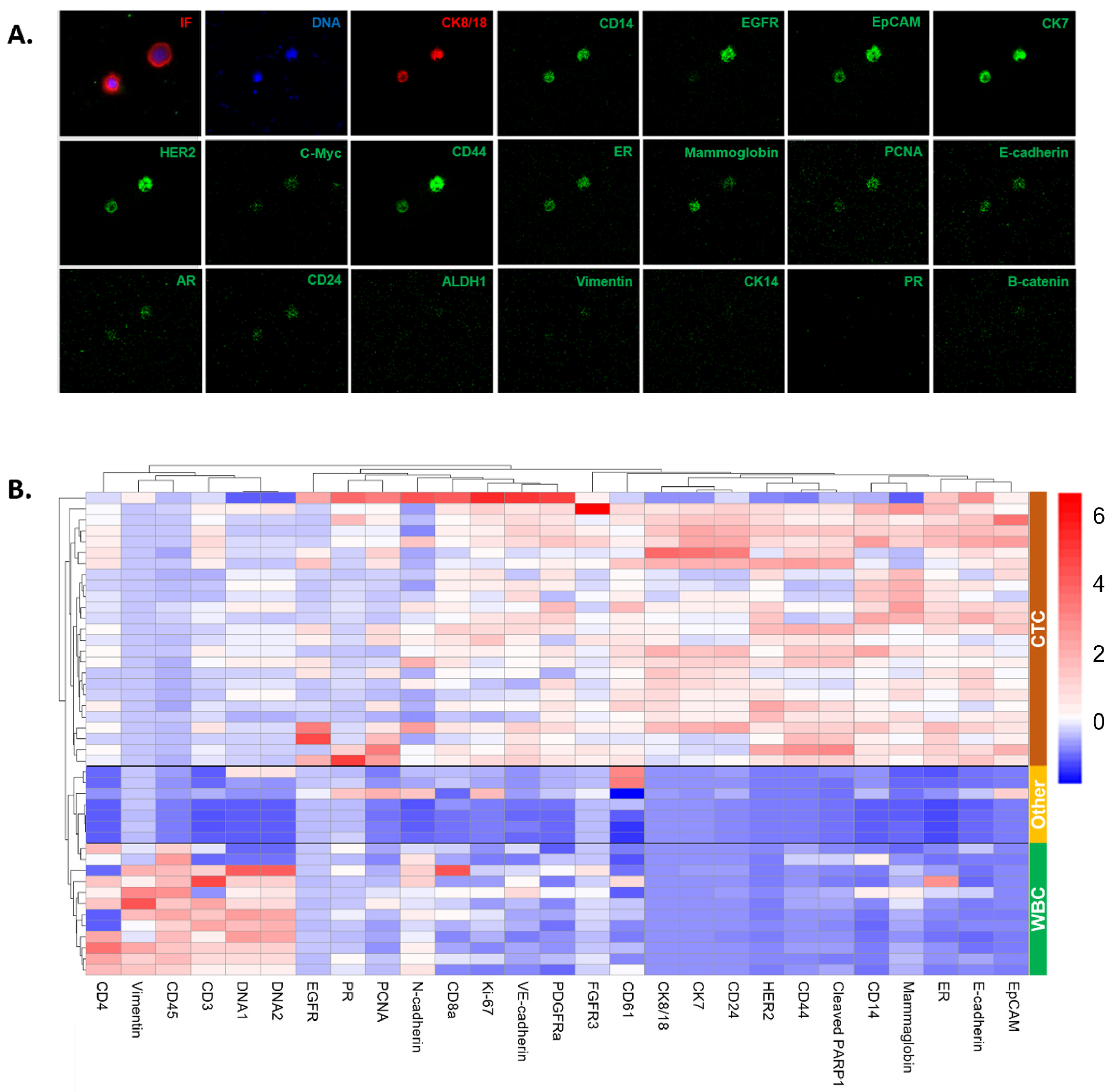
3.4. Molecular Characterization of CSF CTCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, N.U. Breast cancer brain metastases: New directions in systemic therapy. Ecancermedicalscience 2013, 7, 307. [Google Scholar] [CrossRef]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Brown, P.D.; Alexander, B.M.; Lin, N.U.; Aizer, A.A. Implications of Screening for Brain Metastases in Patients With Breast Cancer and Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Arslan, U.Y.; Oksuzoglu, B.; Aksoy, S.; Harputluoglu, H.; Turker, I.; Ozisik, Y.; Dizdar, O.; Altundag, K.; Alkis, N.; Zengin, N. Breast cancer subtypes and outcomes of central nervous system metastases. Breast 2011, 20, 562–567. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Kępka, L.; Tyc-Szczepaniak, D.; Bujko, K.; Olszyna-Serementa, M.; Michalski, W.; Sprawka, A.; Trąbska-Kluch, B.; Komosińska, K.; Wasilewska-Teśluk, E.; Czeremszyńska, B. Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: Results from a randomized trial. Radiother. Oncol. 2016, 121, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Le Rhun, E.; Rudà, R.; Devos, P.; Hoang-Xuan, K.; Brandsma, D.; Pérez Segura, P.; Soffietti, R.; Weller, M. Diagnosis and treatment patterns for patients with leptomeningeal metastasis from solid tumors across Europe. J. Neurooncol. 2017, 133, 419–427. [Google Scholar] [CrossRef]
- Oechsle, K.; Lange-Brock, V.; Kruell, A.; Bokemeyer, C.; Wit, M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: A Retrospective Analysis. J. Cancer Res. Clin. Oncol. 2010, 136, 1729–1735. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.J.; Lee, J.O.; Lee, K.W.; Kim, J.H.; Bang, S.M.; Chung, J.H.; Kim, J.S.; Lee, J.S. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer 2012, 76, 387–392. [Google Scholar] [CrossRef]
- Wallace, G.; Kundalia, R.; Vallebuona, E.; Cao, B.; Kim, Y.; Forsyth, P.; Soyano, A.; Smalley, I.; Pina, Y. Factors associated with overall survival in breast cancer patients with leptomeningeal disease (LMD): A single institutional retrospective review. Breast Cancer Res. 2024, 26, 55. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Cortese, I.C.; Vuolo, L.; Nair, G.; de Alwis, M.P.; Ohayon, J.; Meani, A.; Martinelli, V.; Scotti, R.; Falini, A.; et al. Leptomeningeal gadolinium enhancement across the spectrum of chronic neuroinflammatory diseases. Neurology 2017, 88, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Nayar, G.; Ejikeme, T.; Chongsathidkiet, P.; Elsamadicy, A.A.; Blackwell, K.L.; Clarke, J.M.; Lad, S.P.; Fecci, P.E. Leptomeningeal disease: Current diagnostic and therapeutic strategies. Oncotarget 2017, 8, 73312–73328. [Google Scholar] [CrossRef]
- Batool, A.; Kasi, A. Leptomeningeal Carcinomatosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wang, H.; Wang, L.; Fang, C.; Li, C.; Zhang, L. Comparison of the diagnostic value of liquid biopsy in leptomeningeal metastases: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 1079796. [Google Scholar] [CrossRef]
- Wijaya, J.H.; Patel, U.D.; Quintero-Consuegra, M.D.; Aguilera-Peña, M.P.; Madriñán-Navia, H.J.; Putra, A.W.; July, J.; Kataria, S. Liquid biopsy in the setting of leptomeningeal metastases: A systematic review and meta-analysis. J. Neuro-Oncol. 2023, 165, 431–438. [Google Scholar] [CrossRef]
- Chai, S.; Ruiz-Velasco, C.; Naghdloo, A.; Pore, M.; Singh, M.; Matsumoto, N.; Kolatkar, A.; Xu, L.; Shishido, S.; Aparicio, A.; et al. Identification of epithelial and mesenchymal circulating tumor cells in clonal lineage of an aggressive prostate cancer case. NPJ Precis. Oncol. 2022, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Welter, L.; Xu, L.; McKinley, D.; Dago, A.E.; Prabakar, R.K.; Restrepo-Vassalli, S.; Xu, K.; Rodriguez-Lee, M.; Kolatkar, A.; Nevarez, R.; et al. Treatment response and tumor evolution: Lessons from an extended series of multianalyte liquid biopsies in a metastatic breast cancer patient. Cold Spring Harb. Mol. Case Stud. 2020, 6, a005819. [Google Scholar] [CrossRef]
- Carlsson, A.; Kuhn, P.; Luttgen, M.S.; Keomanee-Dizon, K.; Troncoso, P.; Corn, P.G.; Kolatkar, A.; Hicks, J.B.; Logothetis, C.J.; Zurita, A.J. Paired High-Content Analysis of Prostate Cancer Cells in Bone Marrow and Blood Characterizes Increased Androgen Receptor Expression in Tumor Cell Clusters. Clin. Cancer Res. 2017, 23, 1722–1732. [Google Scholar] [CrossRef]
- Chai, S.; Matsumoto, N.; Storgard, R.; Peng, C.-C.; Aparicio, A.; Ormseth, B.; Rappard, K.; Cunningham, K.; Kolatkar, A.; Nevarez, R.; et al. Platelet-coated circulating tumor cells are a predictive biomarker in patients with metastatic castrate resistant prostate cancer. Mol. Cancer Res. 2021, 19, 2036–2045. [Google Scholar] [CrossRef]
- Gerdtsson, A.S.; Thiele, J.A.; Shishido, S.N.; Zheng, S.; Schaffer, R.; Bethel, K.; Curley, S.; Lenz, H.J.; Hanna, D.L.; Nieva, J.; et al. Single cell correlation analysis of liquid and solid biopsies in metastatic colorectal cancer. Oncotarget 2019, 10, 7016–7030. [Google Scholar] [CrossRef][Green Version]
- Malihi, P.D.; Graf, R.P.; Rodriguez, A.; Ramesh, N.; Lee, J.; Sutton, R.; Jiles, R.; Ruiz Velasco, C.; Sei, E.; Kolatkar, A.; et al. Single-Cell Circulating Tumor Cell Analysis Reveals Genomic Instability as a Distinctive Feature of Aggressive Prostate Cancer. Clin. Cancer Res. 2020, 26, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Malihi, P.D.; Morikado, M.; Welter, L.; Liu, S.T.; Miller, E.T.; Cadaneanu, R.M.; Knudsen, B.S.; Lewis, M.S.; Carlsson, A.; Velasco, C.R.; et al. Clonal diversity revealed by morphoproteomic and copy number profiles of single prostate cancer cells at diagnosis. Converg. Sci. Phys. Oncol. 2018, 4, 015003. [Google Scholar] [CrossRef] [PubMed]
- Marrinucci, D.; Bethel, K.; Kolatkar, A.; Luttgen, M.S.; Malchiodi, M.; Baehring, F.; Voigt, K.; Lazar, D.; Nieva, J.; Bazhenova, L.; et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys. Biol. 2012, 9, 016003. [Google Scholar] [CrossRef]
- Narayan, S.; Courcoubetis, G.; Mason, J.; Naghdloo, A.; Kolencik, D.; Patterson, S.D.; Kuhn, P.; Shishido, S.N. Defining A Liquid Biopsy Profile of Circulating Tumor Cells and Oncosomes in Metastatic Colorectal Cancer for Clinical Utility. Cancers 2022, 14, 4891. [Google Scholar] [CrossRef]
- Rodriguez-Lee, M.; Kolatkar, A.; McCormick, M.; Dago, A.D.; Kendall, J.; Carlsson, N.A.; Bethel, K.; Greenspan, E.J.; Hwang, S.E.; Waitman, K.R.; et al. Effect of Blood Collection Tube Type and Time to Processing on the Enumeration and High-Content Characterization of Circulating Tumor Cells Using the High-Definition Single-Cell Assay. Arch. Pathol. Lab. Med. 2018, 142, 198–207. [Google Scholar] [CrossRef]
- Setayesh, S.M.; Hart, O.; Naghdloo, A.; Higa, N.; Nieva, J.; Lu, J.; Hwang, S.; Wilkinson, K.; Kidd, M.; Anderson, A.; et al. Multianalyte liquid biopsy to aid the diagnostic workup of breast cancer. NPJ Breast Cancer 2022, 8, 112. [Google Scholar] [CrossRef]
- Shishido, S.N.; Welter, L.; Rodriguez-Lee, M.; Kolatkar, A.; Xu, L.; Ruiz, C.; Gerdtsson, A.S.; Restrepo-Vassalli, S.; Carlsson, A.; Larsen, J.; et al. Preanalytical Variables for the Genomic Assessment of the Cellular and Acellular Fractions of the Liquid Biopsy in a Cohort of Breast Cancer Patients. J. Mol. Diagn. 2020, 22, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Lu, D.; Schreiber, N.A.; Louw, J.; Graf, R.P.; Vargas, H.A.; Johnson, A.; Jendrisak, A.; Bambury, R.; Danila, D.; et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016, 2, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Graf, R.P.; Schreiber, N.A.; Jayaram, A.; Winquist, E.; McLaughlin, B.; Lu, D.; Fleisher, M.; Orr, S.; Lowes, L.; et al. Assessment of the Validity of Nuclear-Localized Androgen Receptor Splice Variant 7 in Circulating Tumor Cells as a Predictive Biomarker for Castration-Resistant Prostate Cancer. JAMA Oncol. 2018, 4, 1179–1186. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Halabi, S.; Luo, J.; Nanus, D.M.; Giannakakou, P.; Szmulewitz, R.Z.; Danila, D.C.; Healy, P.; Anand, M.; Rothwell, C.J.; et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J. Clin. Oncol. 2019, 37, 1120–1129. [Google Scholar] [CrossRef]
- Shishido, S.N.; Masson, R.; Xu, L.; Welter, L.; Prabakar, R.K.; D’Souza, A.; Spicer, D.; Kang, I.; Jayachandran, P.; Hicks, J.; et al. Disease characterization in liquid biopsy from HER2-mutated, non-amplified metastatic breast cancer patients treated with neratinib. NPJ Breast Cancer 2022, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Shishido, S.N.; Sayeed, S.; Courcoubetis, G.; Djaladat, H.; Miranda, G.; Pienta, K.J.; Nieva, J.; Hansel, D.E.; Desai, M.; Gill, I.S.; et al. Characterization of Cellular and Acellular Analytes from Pre-Cystectomy Liquid Biopsies in Patients Newly Diagnosed with Primary Bladder Cancer. Cancers 2022, 14, 758. [Google Scholar] [CrossRef]
- Gerdtsson, A.S.; Setayesh, S.M.; Malihi, P.D.; Ruiz, C.; Carlsson, A.; Nevarez, R.; Matsumoto, N.; Gerdtsson, E.; Zurita, A.; Logothetis, C.; et al. Large Extracellular Vesicle Characterization and Association with Circulating Tumor Cells in Metastatic Castrate Resistant Prostate Cancer. Cancers 2021, 13, 1056. [Google Scholar] [CrossRef]
- Welter, L.; Zheng, S.; Setayesh, S.M.; Morikado, M.; Agrawal, A.; Nevarez, R.; Naghdloo, A.; Pore, M.; Higa, N.; Kolatkar, A.; et al. Cell State and Cell Type: Deconvoluting Circulating Tumor Cell Populations in Liquid Biopsies by Multi-Omics. Cancers 2023, 15, 3949. [Google Scholar] [CrossRef] [PubMed]
- Gerdtsson, E.; Pore, M.; Thiele, J.A.; Gerdtsson, A.S.; Malihi, P.D.; Nevarez, R.; Kolatkar, A.; Velasco, C.R.; Wix, S.; Singh, M.; et al. Multiplex protein detection on circulating tumor cells from liquid biopsies using imaging mass cytometry. Converg. Sci. Phys. Oncol. 2018, 4, 015002. [Google Scholar] [CrossRef]
- Setayesh, S.M.; Ndacayisaba, L.J.; Rappard, K.E.; Hennes, V.; Rueda, L.Y.M.; Tang, G.; Lin, P.; Orlowski, R.Z.; Symer, D.E.; Manasanch, E.E.; et al. Targeted single-cell proteomic analysis identifies new liquid biopsy biomarkers associated with multiple myeloma. NPJ Precis. Oncol. 2023, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef]
- Catena, R.; Montuenga, L.M.; Bodenmiller, B. Ruthenium counterstaining for imaging mass cytometry. J. Pathol. 2018, 244, 479–484. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Vacchi, E.; Burrello, J.; Di Silvestre, D.; Burrello, A.; Bolis, S.; Mauri, P.; Vassalli, G.; Cereda, C.W.; Farina, C.; Barile, L.; et al. Immune profiling of plasma-derived extracellular vesicles identifies Parkinson disease. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e866. [Google Scholar] [CrossRef]
- Chin, A.R.; Wang, S.E. Cancer-derived extracellular vesicles: The ‘soil conditioner’ in breast cancer metastasis? Cancer Metastasis Rev. 2016, 35, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, C.; Zhou, T.; Liu, X.; Liu, X.; Li, X.; Chen, D. Role of exosomal proteins in cancer diagnosis. Mol. Cancer 2017, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Görgens, A.; Wiklander, O.; Bostancıoğlu, R.B.; Zickler, A.; Murke, F.; Heider, U.; Wild, S.; Giebel, B.; Andaloussi, S. A robust and semi-quantitative method for analyzing exosomes by flow cytometry. Trillium Extracell. Vesicles 2021, 3, 41–45. [Google Scholar]
- Vacchi, E.; Burrello, J.; Burrello, A.; Bolis, S.; Monticone, S.; Barile, L.; Kaelin-Lang, A.; Melli, G. Profiling Inflammatory Extracellular Vesicles in Plasma and Cerebrospinal Fluid: An Optimized Diagnostic Model for Parkinson’s Disease. Biomedicines 2021, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Agouni, A.; Parray, A.S.; Akhtar, N.; Mir, F.A.; Bourke, P.J.; Joseph, S.; Morgan, D.M.; Santos, M.D.; Wadiwala, M.F.; Kamran, S.; et al. There Is Selective Increase in Pro-thrombotic Circulating Extracellular Vesicles in Acute Ischemic Stroke and Transient Ischemic Attack: A Study of Patients From the Middle East and Southeast Asia. Front. Neurol. 2019, 10, 251. [Google Scholar] [CrossRef]
- Li, P.; Qin, C. Elevated circulating VE-cadherin+CD144+endothelial microparticles in ischemic cerebrovascular disease. Thromb. Res. 2015, 135, 375–381. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Feng, J. The potential role of exosomes in the diagnosis and therapy of ischemic diseases. Cytotherapy 2018, 20, 1204–1219. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martinez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 2015, 6, 8839. [Google Scholar] [CrossRef]
- Miller, A.M.; Shah, R.H.; Pentsova, E.I.; Pourmaleki, M.; Briggs, S.; Distefano, N.; Zheng, Y.; Skakodub, A.; Mehta, S.A.; Campos, C.; et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature 2019, 565, 654–658. [Google Scholar] [CrossRef]
- Pan, C.; Diplas, B.H.; Chen, X.; Wu, Y.; Xiao, X.; Jiang, L.; Geng, Y.; Xu, C.; Sun, Y.; Zhang, P.; et al. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. 2019, 137, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Gu, W.; Nagpal, S.; Gephart, M.H.; Quake, S.R. Brain tumor mutations detected in cerebral spinal fluid. Clin. Chem. 2015, 61, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Panditharatna, E.; Kilburn, L.B.; Aboian, M.S.; Kambhampati, M.; Gordish-Dressman, H.; Magge, S.N.; Gupta, N.; Myseros, J.S.; Hwang, E.I.; Kline, C.; et al. Clinically Relevant and Minimally Invasive Tumor Surveillance of Pediatric Diffuse Midline Gliomas Using Patient-Derived Liquid Biopsy. Clin. Cancer Res. 2018, 24, 5850–5859. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishido, S.N.; Marvit, A.; Pham, D.; Luo, T.; Xu, L.; Mason, J.; Priceman, S.J.; Portnow, J.; Kuhn, P. Multi-Omic Characterization of Single Cells and Cell-Free Components Detected in the Cerebrospinal Fluid of Patients with Leptomeningeal Disease. Cancers 2024, 16, 3746. https://doi.org/10.3390/cancers16223746
Shishido SN, Marvit A, Pham D, Luo T, Xu L, Mason J, Priceman SJ, Portnow J, Kuhn P. Multi-Omic Characterization of Single Cells and Cell-Free Components Detected in the Cerebrospinal Fluid of Patients with Leptomeningeal Disease. Cancers. 2024; 16(22):3746. https://doi.org/10.3390/cancers16223746
Chicago/Turabian StyleShishido, Stephanie N., Amelia Marvit, Doanna Pham, Theresa Luo, Liya Xu, Jeremy Mason, Saul J. Priceman, Jana Portnow, and Peter Kuhn. 2024. "Multi-Omic Characterization of Single Cells and Cell-Free Components Detected in the Cerebrospinal Fluid of Patients with Leptomeningeal Disease" Cancers 16, no. 22: 3746. https://doi.org/10.3390/cancers16223746
APA StyleShishido, S. N., Marvit, A., Pham, D., Luo, T., Xu, L., Mason, J., Priceman, S. J., Portnow, J., & Kuhn, P. (2024). Multi-Omic Characterization of Single Cells and Cell-Free Components Detected in the Cerebrospinal Fluid of Patients with Leptomeningeal Disease. Cancers, 16(22), 3746. https://doi.org/10.3390/cancers16223746








