Utility and Safety of 5-ALA Guided Surgery in Pediatric Brain Tumors: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction and Risk of Bias Assessment
2.3. Data Analysis
3. Results
3.1. Summary of Search Results
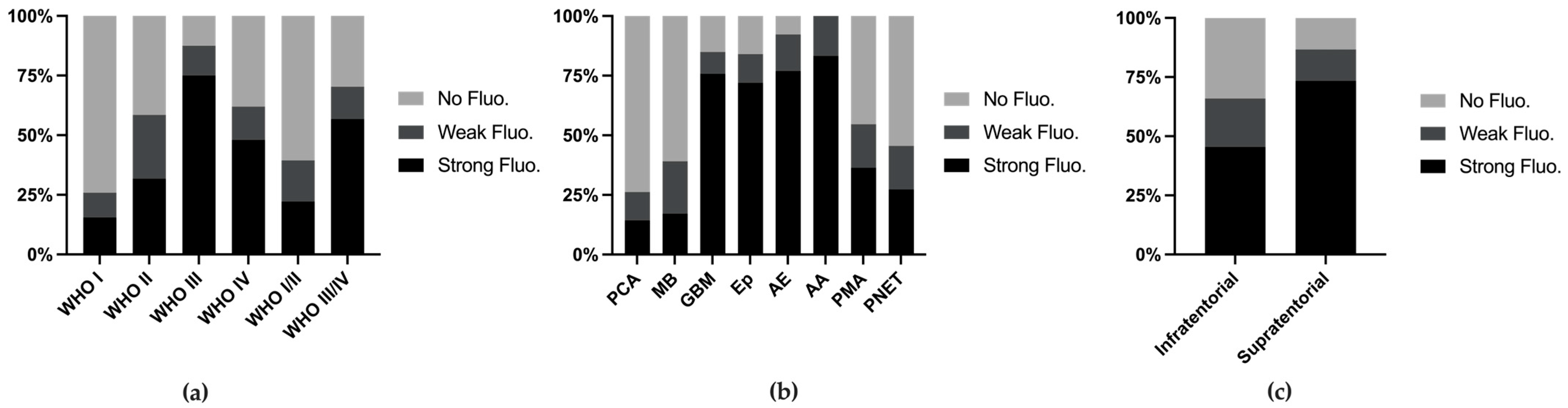
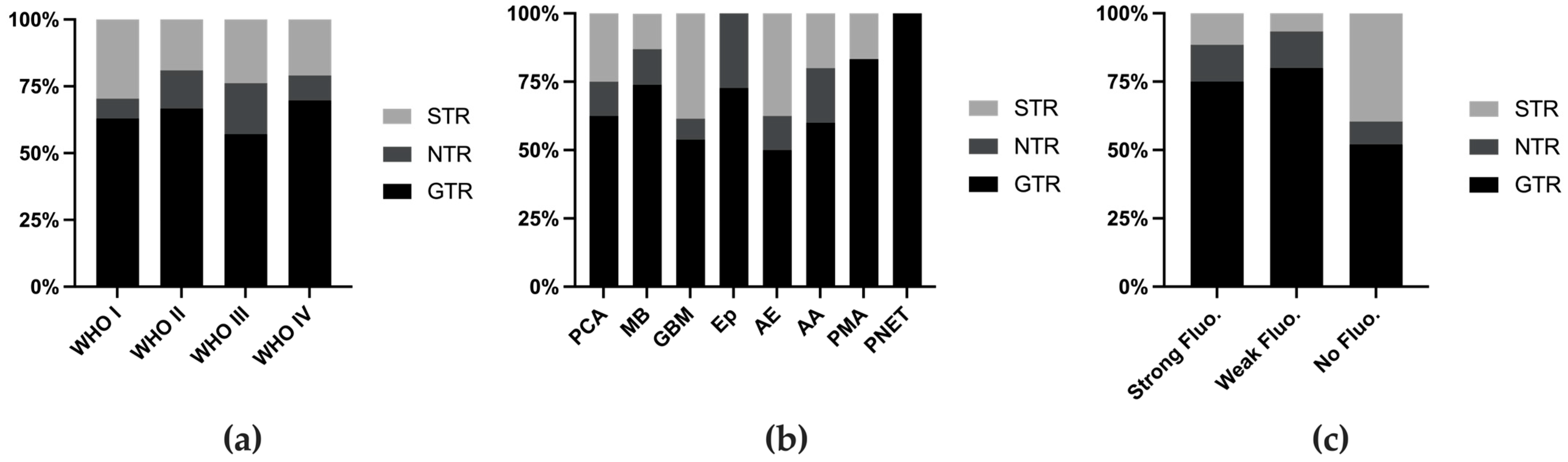
3.2. Fluorescence Rate and Utility
3.3. Side Effects
4. Discussion
4.1. Variation in the Fluorescence Rate and Utility Across Tumor Types
4.1.1. Medulloblastoma and Pilocytic Astrocytoma
4.1.2. Glioblastoma and Anaplastic Astrocytoma
4.1.3. Ependymoma
4.1.4. PNET
4.1.5. Other Rare Tumors
4.2. Mechanism and Determinants of 5-ALA-Induced Fluorescence
4.3. Limitations
4.4. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gajjar, A.; Mahajan, A.; Abdelbaki, M.; Anderson, C.; Antony, R.; Bale, T.; Bindra, R.; Bowers, D.C.; Cohen, K.; Cole, B.; et al. Pediatric Central Nervous System Cancers, Version 2.2023. J. Natl. Compr. Cancer Netw. 2022, 20, 1339–1362. [Google Scholar] [PubMed]
- Milos, P.; Haj-Hosseini, N.; Hillman, J.; Wårdell, K. 5-ALA Fluorescence in Randomly Selected Pediatric Brain Tumors Assessed by Spectroscopy and Surgical Microscope. Acta Neurochir. 2022, 165, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.D.; Barone, D.G.; Bryant, A.; Vale, L.; Bulbeck, H.; Lawrie, T.A.; Hart, M.G.; Watts, C. Intraoperative Imaging Technology to Maximise Extent of Resection for Glioma. Cochrane Database Syst. Rev. 2018, 2018, CD012788. [Google Scholar] [CrossRef]
- Teixidor, P.; Arráez, M.Á.; Villalba, G.; Garcia, R.; Tardáguila, M.; González, J.J.; Rimbau, J.; Vidal, X.; Montané, E. Safety and Efficacy of 5-Aminolevulinic Acid for High Grade Glioma in Usual Clinical Practice: A Prospective Cohort Study. PLoS ONE 2016, 11, e0149244. [Google Scholar] [CrossRef]
- Ferraro, N.; Barbarite, E.; Albert, T.R.; Berchmans, E.; Shah, A.H.; Bregy, A.; Ivan, M.E.; Brown, T.; Komotar, R.J. The Role of 5-Aminolevulinic Acid in Brain Tumor Surgery: A Systematic Review. Neurosurg. Rev. 2016, 39, 545–555. [Google Scholar] [CrossRef]
- Barbagallo, G.M.V.; Certo, F.; Heiss, K.; Albanese, V. 5-ALA Fluorescence-Assisted Surgery in Pediatric Brain Tumors: Report of Three Cases and Review of the Literature. Br. J. Neurosurg. 2014, 28, 750–754. [Google Scholar] [CrossRef]
- Schwake, M.; Schipmann, S.; Müther, M.; Köchling, M.; Brentrup, A.; Stummer, W. 5-ALA Fluorescence–Guided Surgery in Pediatric Brain Tumors—A Systematic Review. Acta Neurochir. 2019, 161, 1099–1108. [Google Scholar] [CrossRef]
- Labuschagne, J. 5-Aminolevulinic Acid-Guided Surgery for Focal Pediatric Brainstem Gliomas: A Preliminary Study. Surg. Neurol. Int. 2020, 11, 334. [Google Scholar] [CrossRef]
- Eicker, S.; Sarikaya-Seiwert, S.; Borkhardt, A.; Gierga, K.; Turowski, B.; Heiroth, H.-J.; Steiger, H.-J.; Stummer, W. ALA-Induced Porphyrin Accumulation in Medulloblastoma and Its Use for Fluorescence-Guided Surgery. Cent. Eur. Neurosurg. 2011, 72, 101–104. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Okhlopkov, V.A.; Golbin, D.A.; Chernyshov, K.A.; Svistov, D.V.; Martynov, B.V.; Kim, A.V.; Byvaltsev, V.A.; Pavlova, G.V.; Batalov, A.; et al. Fluorescence Diagnosis in Neurooncology: Retrospective Analysis of 653 Cases. Front. Oncol. 2019, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Bernal García, L.M.; Cabezudo Artero, J.M.; Royano Sánchez, M.; Marcelo Zamorano, M.B.; López Macías, M. Fluorescence-Guided Resection with 5-Aminolevulinic Acid of Meningeal Sarcoma in a Child. Childs Nerv. Syst. 2015, 31, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.; Maione, M.; Peschillo, S.; Signorelli, F.; Visocchi, M.; Sortino, G.; Fiumanò, G.; Certo, F. Intraoperative Computed Tomography, Navigated Ultrasound, 5-Amino-Levulinic Acid Fluorescence and Neuromonitoring in Brain Tumor Surgery: Overtreatment or Useful Tool Combination? J. Neurosurg. Sci. 2019, 68, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Burford, M.C.; Kalyal, M.N.; Pandit, D.A.; Tailor, M.J.; Lavrador, M.J.P.; Bravo, M.A.; Kugisaki, M.A.; Ashkan, P.K.; Bassi, M.S.; Chandler, M.C.; et al. PP39. 5-Aminolevulinic acid aided resection of paediatric brain tumours: The UK’s first case series. Neuro-Oncology 2017, 19, i11. [Google Scholar] [CrossRef]
- Zhang, C.; Boop, F.A.; Ruge, J. The Use of 5-Aminolevulinic Acid in Resection of Pediatric Brain Tumors: A Critical Review. J. Neurooncol. 2019, 141, 567–573. [Google Scholar] [CrossRef]
- Coburger, J.; Wirtz, C.R. Fluorescence Guided Surgery by 5-ALA and Intraoperative MRI in High Grade Glioma: A Systematic Review. J. Neurooncol. 2019, 141, 533–546. [Google Scholar] [CrossRef]
- Gautheron, A.; Bernstock, J.D.; Picart, T.; Guyotat, J.; Valdés, P.A.; Montcel, B. 5-ALA Induced PpIX Fluorescence Spectroscopy in Neurosurgery: A Review. Front. Neurosci. 2024, 18, 1310282. [Google Scholar] [CrossRef]
- Kamp, M.A.; Knipps, J.; Neumann, L.M.; Mijderwijk, H.-J.; Dibué-Adjei, M.; Steiger, H.-J.; Slotty, P.J.; Rapp, M.; Cornelius, J.-F.; Sabel, M. Is the Intensity of 5-Aminolevulinic Acid–Derived Fluorescence Related to the Light Source? World Neurosurg. 2019, 131, e271–e276. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Suzuki, T.; Wada, S.; Eguchi, H.; Adachi, J.; Mishima, K.; Matsutani, M.; Nishikawa, R.; Nishiyama, M. Cadherin 13 Overexpression as an Important Factor Related to the Absence of Tumor Fluorescence in 5-Aminolevulinic Acid–Guided Resection of Glioma: Laboratory Investigation. J. Neurosurg. 2013, 119, 1331–1339. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 25 September 2024).
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological Quality of Case Series Studies: An Introduction to the JBI Critical Appraisal Tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Ruge, J.R.; Liu, J. Use of 5-Aminolevulinic Acid for Visualization and Resection of a Benign Pediatric Brain Tumor: Case Report. J. Neurosurg. Pediatr. 2009, 4, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Preuß, M.; Renner, C.; Krupp, W.; Christiansen, H.; Fischer, L.; Merkenschlager, A.; Kieß, W.; Müller, W.; Manzo, N.; Meixensberger, J.; et al. The Use of 5-Aminolevulinic Acid Fluorescence Guidance in Resection of Pediatric Brain Tumors. Childs Nerv. Syst. 2013, 29, 1263–1267. [Google Scholar] [CrossRef]
- Beez, T.; Sarikaya-Seiwert, S.; Steiger, H.-J.; Hänggi, D. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Brain Tumors in Children—A Technical Report. Acta Neurochir. 2014, 156, 597–604. [Google Scholar] [CrossRef] [PubMed]
- For the European ALA Pediatric Brain Tumor Study Group; Stummer, W.; Rodrigues, F.; Schucht, P.; Preuss, M.; Wiewrodt, D.; Nestler, U.; Stein, M.; Artero, J.M.C.; Platania, N.; et al. Predicting the “Usefulness” of 5-ALA-Derived Tumor Fluorescence for Fluorescence-Guided Resections in Pediatric Brain Tumors: A European Survey. Acta Neurochir. 2014, 156, 2315–2324. [Google Scholar] [CrossRef]
- Skjøth-Rasmussen, J.; Bøgeskov, L.; Sehested, A.; Klausen, C.; Broholm, H.; Nysom, K. The Use of 5-ALA to Assist Complete Removal of Residual Non-Enhancing Part of Childhood Medulloblastoma: A Case Report. Childs Nerv. Syst. 2015, 31, 2173–2177. [Google Scholar] [CrossRef]
- Sysoev, K.; Don, O.; Kim, A.; Samochernyh, C.; Khachatrian, W. Use of 5-ALA Fluorescence to Guide Resection of Cerebral Neuroepithelial Tumors in Children. Childs Nerv. Syst. 2016, 32, 953–954. [Google Scholar] [CrossRef]
- Burford, C.; Kalyal, N.; Pandit, A.; Lavrador, J.P.; Bleil, C.; Kailaya-Vasan, A.; Zebian, B. NSRG-23. 5-AMINOLEVULINIC Acid Guided Resection of Paediatric Central Nervous System Tumours: The Largest Single Centre Series in the UK. Neuro-Oncology 2018, 20, i150. [Google Scholar] [CrossRef]
- Kim, A.V.; Khachatryan, V.A. Intraoperative fluorescence diagnosis using 5-aminolevulinic acid in surgical treatment of children with recurrent neuroepithelial tumors. Vopr. Neirokhirurgii Im. NN Burdenko 2017, 81, 51. [Google Scholar] [CrossRef]
- Roth, J.; Constantini, S. 5ALA in Pediatric Brain Tumors Is Not Routinely Beneficial. Childs Nerv. Syst. 2017, 33, 787–792. [Google Scholar] [CrossRef]
- Wataya, T. SURG-34. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Pediatric Brain Tumors. Neuro-Oncology 2017, 19, vi242. [Google Scholar] [CrossRef]
- Agawa, Y.; Wataya, T. The Use of 5-Aminolevulinic Acid to Assist Gross Total Resection of Pediatric Astroblastoma. Childs Nerv. Syst. 2018, 34, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M.; Kaneko, S.; Suero Molina, E.; Müther, M.; Schipmann, S.; Köchling, M.; Brentrup, A.; Stummer, W. Spectroscopic Measurement of 5-ALA-Induced Intracellular Protoporphyrin IX in Pediatric Brain Tumors. Acta Neurochir. 2019, 161, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Fudaba, H.; Momii, Y.; Kawasaki, Y.; Goto, H.; Nobusawa, S.; Fujiki, M. Well-Differentiated Astroblastoma with Both Focal Anaplastic Features and a Meningioma 1 Gene Alteration. NMC Case Rep. J. 2020, 7, 205–210. [Google Scholar] [CrossRef]
- Labuschagne, J.J. The Use of 5-Aminolevulinic Acid to Assist Gross Total Resection of Paediatric Posterior Fossa Tumours. Pediatr. Neurosurg. 2020, 55, 268–279. [Google Scholar] [CrossRef]
- Labuschagne, J.J. 5-Aminolevulinic Acid–Guided Surgery for Recurrent Supratentorial Pediatric Neoplasms. World Neurosurg. 2020, 141, e763–e769. [Google Scholar] [CrossRef]
- Beauchamp, L.H.; Bercu, M.M.; Avellino, A.M. 5-Aminolevulinic Acid–Assisted Resection of Pediatric Dysembryoplastic Neuroepithelial Tumor: Illustrative Case. J. Neurosurg. Case Lessons 2021, 2, CASE20153. [Google Scholar] [CrossRef]
- Maeda, M.; Nonaka, M.; Naito, N.; Ueno, K.; Kamei, T.; Asai, A. 5-ALA Fluorescence-Guided Resection of Pediatric Low-Grade Glioma Using the ORBEYE 3D Digital Exoscope: A Technical Report. Childs Nerv. Syst. 2023, 39, 1061–1064. [Google Scholar] [CrossRef]
- Mui, O.O.T.; Murray, D.B.; Walsh, B.; Crimmins, D.W.; Caird, J.D. Spontaneous Intracerebral Haemorrhage Secondary to 5-ALA-Induced Thrombocytopaenia in a Paediatric Patient: Case Report and Literature Review. Childs Nerv. Syst. 2023, 39, 1051–1058. [Google Scholar] [CrossRef]
- Nizolin, D.V.; Kim, A.V.; Zueva, Y.A.; Shmeleva, O.O.; Maslov, N.E.; Efimtsev, A.Y.; Nazaralieva, E.T.; Samochernykh, K.A. Surgical Treatment of Gangliogliomas in Functional Areas of the Brain in Child: A Literature Review and Clinical Cases. Russ. J. Neurosurg. 2024, 26, 61–69. [Google Scholar] [CrossRef]
- Finsterer, J.; Mehri, S. Rule out All Differential Causes before Attributing Cerebral Bleeding to 5-Aminolevulinic Acid. Childs Nerv. Syst. 2023, 39, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Khachatryan, W. Use of the Intraoperative Fluorescent Diagnostics (5-ALA) in Surgical Treatment of Pediatric Patients with Cerebral Tumors. Childs Nerv. Syst. 2017, 33, 1841. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M.; Kaneko, S.; Morina, E.S.; Stummer, W. Del: Surg-10. Spectroscopic measurement of 5-ala-induced intracellular protoporphyrin ix in pediatric brain tumors. Neuro-Oncology 2020, 22, iii462–iii463. [Google Scholar] [CrossRef]
- Schwake, M.; Bruns, A.-K.; Müther, M.; Stummer, W. SURG-08. 5-Aminolevulinic Acid (5-ALA)-Guided Resection of Pediatric Brain Tumors. Neuro-Oncology 2022, 24, i143. [Google Scholar] [CrossRef]
- Briel-Pump, A.; Beez, T.; Ebbert, L.; Remke, M.; Weinhold, S.; Sabel, M.C.; Sorg, R.V. Accumulation of Protoporphyrin IX in Medulloblastoma Cell Lines and Sensitivity to Subsequent Photodynamic Treatment. J. Photochem. Photobiol. B 2018, 189, 298–305. [Google Scholar] [CrossRef]
- Price, A.; O’Leary, S.; Malkova, K.; D’Souza, P.; Ogasawara, C.; Felicella, M.M.; Karas, P.J. Awake Resection of Recurrent Astroblastoma with Intraoperative 5-ALA–Induced Fluorescence: Illustrative Case. J. Neurosurg. Case Lessons 2023, 6, CASE23526. [Google Scholar] [CrossRef]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- Molina, E.S.; Black, D.; Kaneko, S.; Müther, M.; Stummer, W. Double Dose of 5-Aminolevulinic Acid and Its Effect on Protoporphyrin IX Accumulation in Low-Grade Glioma. J. Neurosurg. 2022, 137, 943–952. [Google Scholar] [CrossRef]
- Black, D.; Byrne, D.; Walke, A.; Liu, S.; Di Ieva, A.; Kaneko, S.; Stummer, W.; Salcudean, T.; Suero Molina, E. Towards Machine Learning-Based Quantitative Hyperspectral Image Guidance for Brain Tumor Resection. Commun. Med. 2024, 4, 131. [Google Scholar] [CrossRef]
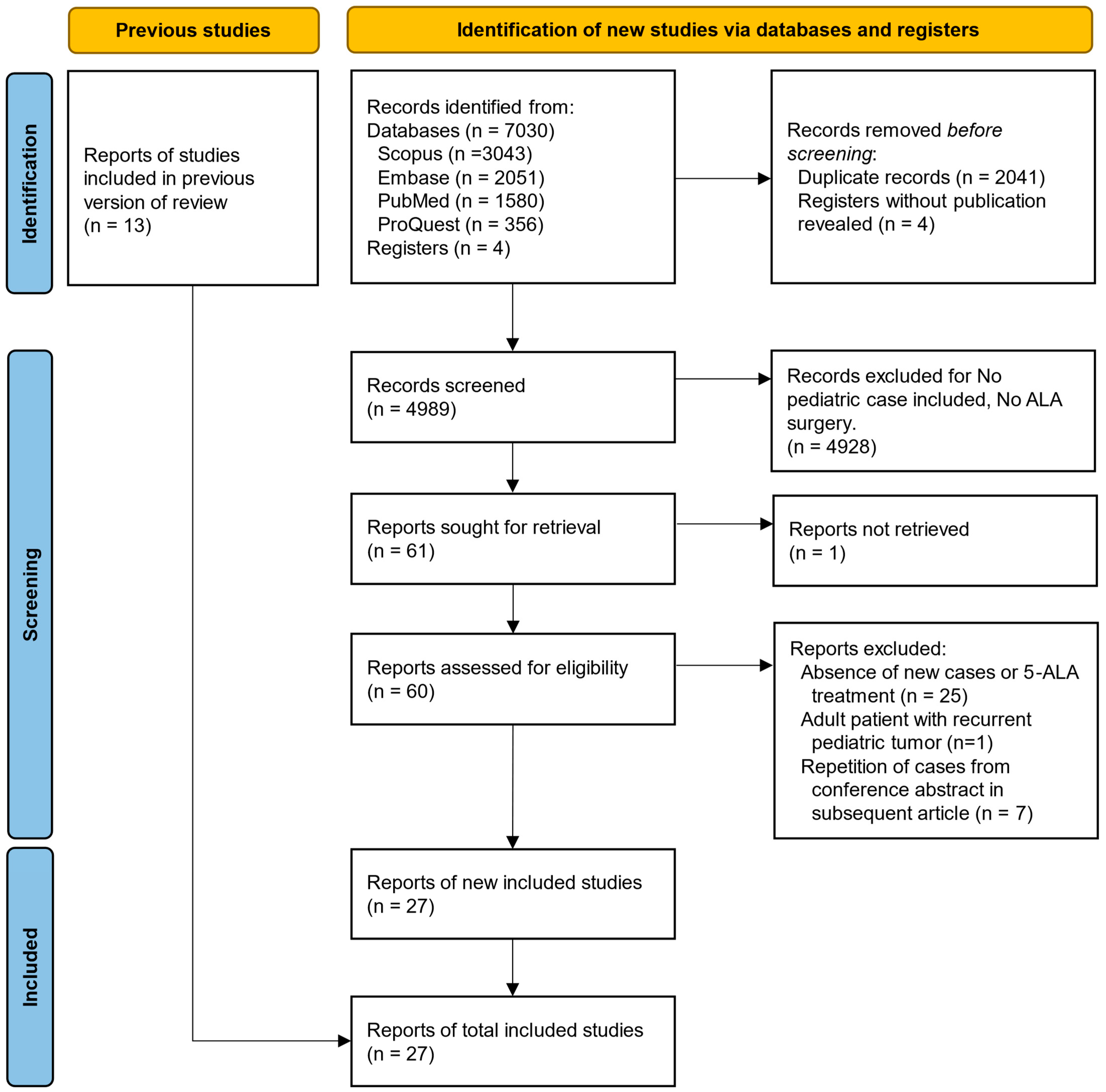
| Author | Year | Country/Region | Design | No. of Patients | No. of Newly Reported Patients * | Age (Years) | Gender | DOI |
|---|---|---|---|---|---|---|---|---|
| Ruge et al. [23] | 2009 | USA | case report | 1 | 1 | 9 | F | 10.3171/2009.6.PEDS08428 |
| Eicker et al. [10] | 2011 | Germany | case report | 1 | 1 | 15 | F | 10.1055/s-0030-1252010 |
| Preuß et al. [24] | 2013 | Germany, France | case series | 18 | 18 | range 3–18; average age = 10.94 ± 4.67 | 13M; 5F | 10.1007/s00381-013-2159-8 |
| Barbagallo et al. [7] | 2014 | Italy | case series | 3 | 3 | range 8–18; average age = 12.67 ± 5.03 | 1F; 2M | 10.3109/02688697.2014.913779 |
| Beez et al. [25] | 2014 | Germany | case series | 16 | 16 | range 1–16; average age = 9 | 10F; 6M | 10.1007/s00701-014-1997-9 |
| Stummer et al. [26] | 2014 | Germany, Spain, Denmark, Ireland, Italy, Switzerland | case series | 78 | 60 | range 1–17; median age = 13 | NA | 10.1007/s00701-014-2234-2 |
| Bernal García et al. [12] | 2015 | Spain | case report | 1 | 1 | 7 | M | 10.1007/s00381-015-2703-9 |
| Skjøth-Rasmussen et al. [27] | 2015 | Denmark | case report | 1 | 1 | 9 | M | 10.1007/s00381-015-2762-y |
| Sysoev et al. [28] | 2016 | Russia | case series ** | 20 | 20 | range 3–18 | NA | 10.1007/s00381-016-3044-z |
| Burford et al. *** [14,29] | 2017 | UK | case series ** | 6 | 6 | NA | NA | 10.1093/neuonc/now293.039 |
| 2018 | UK | case series ** | 10 | 4 | range 1.6–15; median age = 6.5 | NA | 10.1093/neuonc/noy059.545 | |
| Kim et al. [30] | 2017 | Russia | case series | 13 | 14 # | range 3–17; average 9.23 ± 4.79 | 8F; 5M | 10.17116/neiro201780751-57 |
| Roth et al. [31] | 2017 | Israel | case series | 14 | 14 | range 4–19; average 11 ± 5 | NA | 10.1007/s00381-017-3371-8 |
| Wataya et al. [32] | 2017 | Japan | case series ** | 11 ## | 11 | range 1–18 | NA | 10.1093/neuonc/nox168.988 |
| Agawa et al. [33] | 2018 | Japan | case report | 1 | 0 | 13 | 1F | 10.1007/s00381-017-3714-5 |
| Goryaynov et al. [11] | 2019 | Russia | case series | 42 | 8 | NA | NA | 10.3389/fonc.2019.00830 |
| Schwake et al. [34] | 2019 | Germany | case series | 11 | 11 | range 1–16; median = 10 | 6F; 5M | 10.1007/s00701-019-04039-4 |
| Zhang et al.(J Ruge’s personal communication) [15] | 2019 | USA | case report | 3 | 3 | range 8–15; median = 13 | 3F | 10.1007/s11060-018-03004-y |
| Fudaba et al. [35] | 2020 | Japan | case report | 1 | 1 | 6 | F | 10.2176/nmccrj.cr.2020-0028 |
| Labuschagne et al. [36] | 2020 | South Africa | case series | 19 | 19 | range 2–12; average = 5 | 8F; 11M | 10.1159/000511289 |
| Labuschagne et al. [9] | 2020 | South Africa | case series | 8 | 8 | range 1–13; average = 6.1 | 2F; 6M | 10.25259/SNI_246_2020 |
| Labuschagne et al. [37] | 2020 | South Africa | case series | 11 | 11 | range 2–12; median = 4 | 7F; 4M | 10.1016/j.wneu.2020.06.019 |
| Beauchamp et al. [38] | 2021 | USA | case report | 1 | 1 | 10 | 1M | 10.3171/CASE20153 |
| Maeda et al. [39] | 2023 | Japan | case report | 1 | 1 | 14 | 1F | 10.1007/s00381-022-05612-6 |
| Milos et al. [2] | 2023 | Sweden | case series | 14 | 14 | range 4–17; median = 9 | 6F; 8M | 10.1007/s00701-022-05360-1 |
| Mui et al. [40] | 2023 | Ireland | case report | 1 | 1 | 4 | 1M | 10.1007/s00381-023-05846-y |
| Nizolin et al. [41] | 2024 | Russia | case report | 1 | 1 | 9 | NA | 10.17650/1683-3295-2024-26-2-61-69 |
| Histology | No. of Cases | Fluorescence Grade | Extent of Resection | Utility of Fluorescence | Location | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strong | Weak | No | Total Fluorescence Reported | GTR | NTR | STR | Total EOR Reported | Useful | Total Utility Reported | Supratentorial | Infratentorial | Total Location Reported | ||
| PCA | 42 | 6 | 5 | 31 | 42 | 10 | 2 | 4 | 16 | 3 | 9 | 7 | 14 | 21 |
| MB | 41 | 7 | 9 | 25 | 41 | 17 | 3 | 3 | 23 | 10 | 24 | 0 | 29 | 29 |
| GBM | 33 | 25 | 3 | 5 | 33 | 7 | 1 | 5 | 13 | 21 | 24 | 24 | 3 | 27 |
| Ep | 25 | 18 | 3 | 4 | 25 | 8 | 3 | 0 | 11 | 14 | 21 | 6 | 14 | 20 |
| AE | 13 | 10 | 2 | 1 | 13 | 4 | 1 | 3 | 8 | 4 | 4 | 6 | 2 | 8 |
| AA | 13 | 10 | 2 | 0 | 12 | 3 | 1 | 1 | 5 | 4 | 7 | 6 | 3 | 9 |
| PMA | 11 | 4 | 2 | 5 | 11 | 5 | 0 | 1 | 6 | 0 | 2 | 1 | 4 | 5 |
| PNET | 12 | 3 | 2 | 6 | 11 | 2 | 0 | 0 | 2 | 4 | 9 | 8 | 0 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yu, Y.; Wang, Y.; Yu, J.; Zhang, C. Utility and Safety of 5-ALA Guided Surgery in Pediatric Brain Tumors: A Systematic Review. Cancers 2024, 16, 3677. https://doi.org/10.3390/cancers16213677
Wang C, Yu Y, Wang Y, Yu J, Zhang C. Utility and Safety of 5-ALA Guided Surgery in Pediatric Brain Tumors: A Systematic Review. Cancers. 2024; 16(21):3677. https://doi.org/10.3390/cancers16213677
Chicago/Turabian StyleWang, Cheng, Ying Yu, Yafei Wang, Jiahua Yu, and Chenran Zhang. 2024. "Utility and Safety of 5-ALA Guided Surgery in Pediatric Brain Tumors: A Systematic Review" Cancers 16, no. 21: 3677. https://doi.org/10.3390/cancers16213677
APA StyleWang, C., Yu, Y., Wang, Y., Yu, J., & Zhang, C. (2024). Utility and Safety of 5-ALA Guided Surgery in Pediatric Brain Tumors: A Systematic Review. Cancers, 16(21), 3677. https://doi.org/10.3390/cancers16213677






