Implications of GPIIB-IIIA Integrin and Liver X Receptor in Platelet-Induced Compression of Ovarian Cancer Multi-Cellular Spheroids
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Platelets
2.2. Cell Lines
2.3. Metabolic Viability Assays
2.4. Generation of In Vitro Spheroid Cultures
2.5. Protein Isolation
2.6. Mass Spectrometry
2.7. Generation of LXRα Inducible Luciferase Reporter ES-2 Cells
2.8. Statistical and Bioinformatic Analysis
3. Results
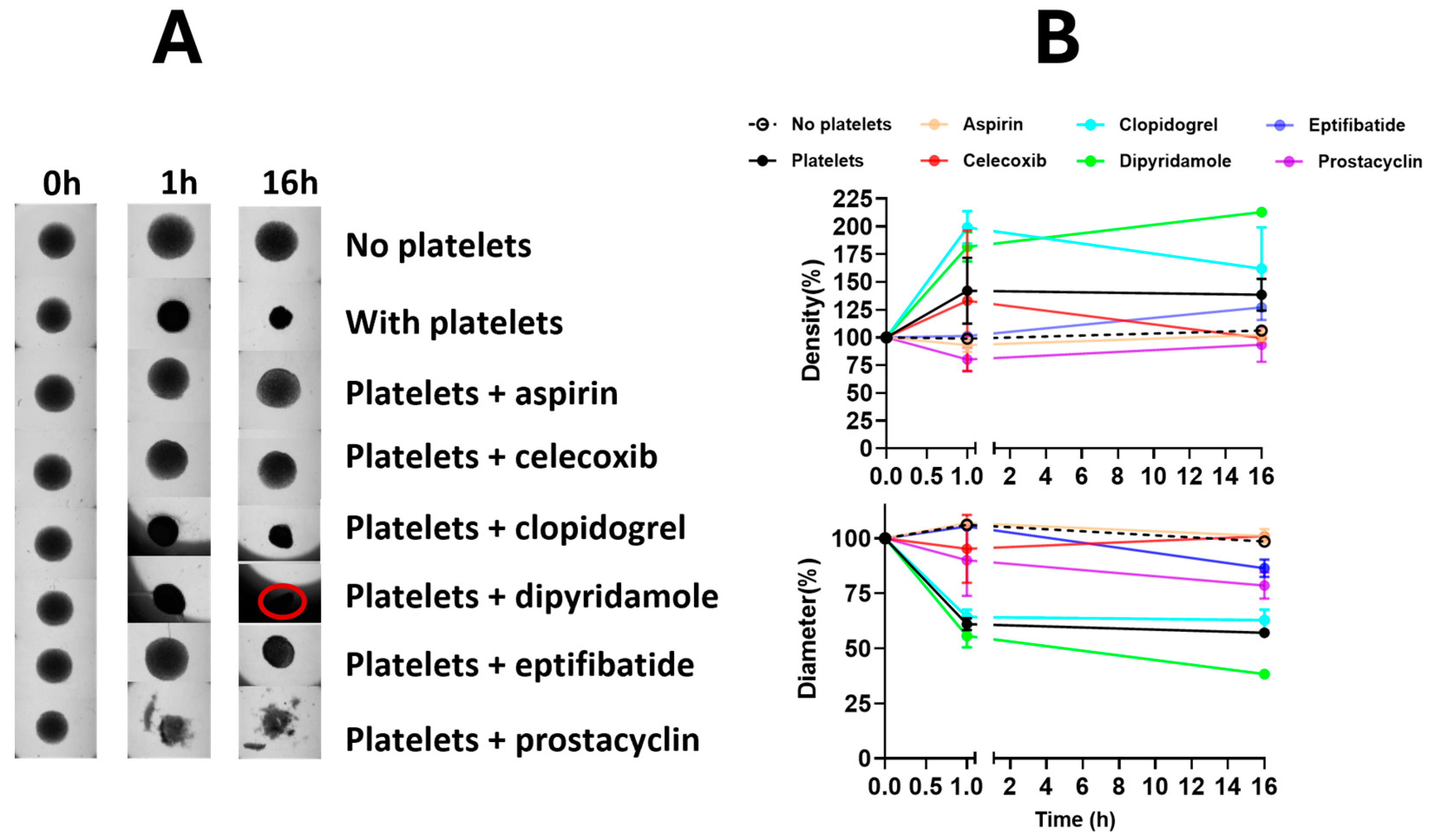
3.1. Platelets, but Not Platelet Inhibitors, Altered Ovarian Cancer Spheroids Properties
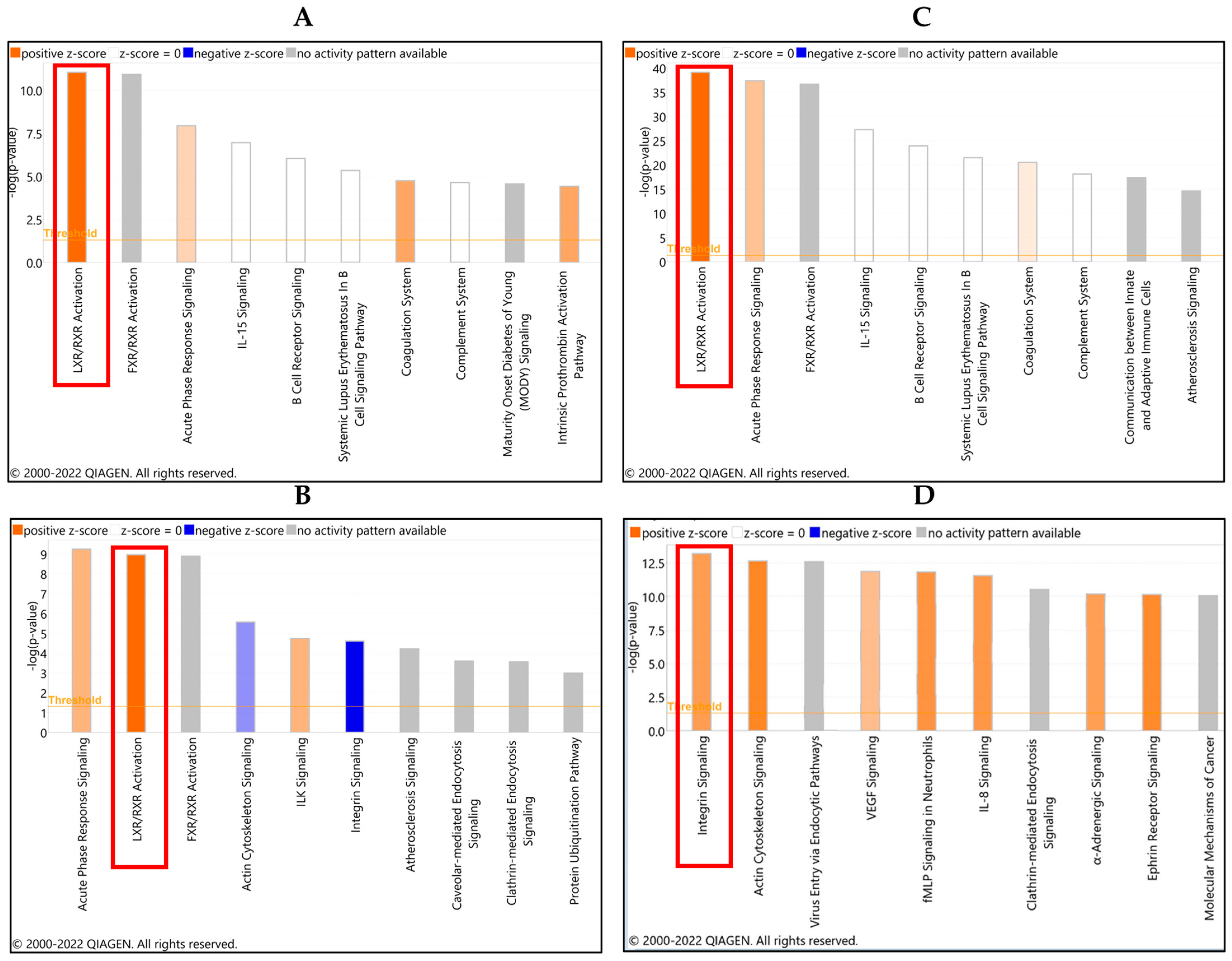
3.2. Ovarian Cancer Spheroids Co-Incubation with Platelets Affected the LXR and Integrin Signaling Pathways
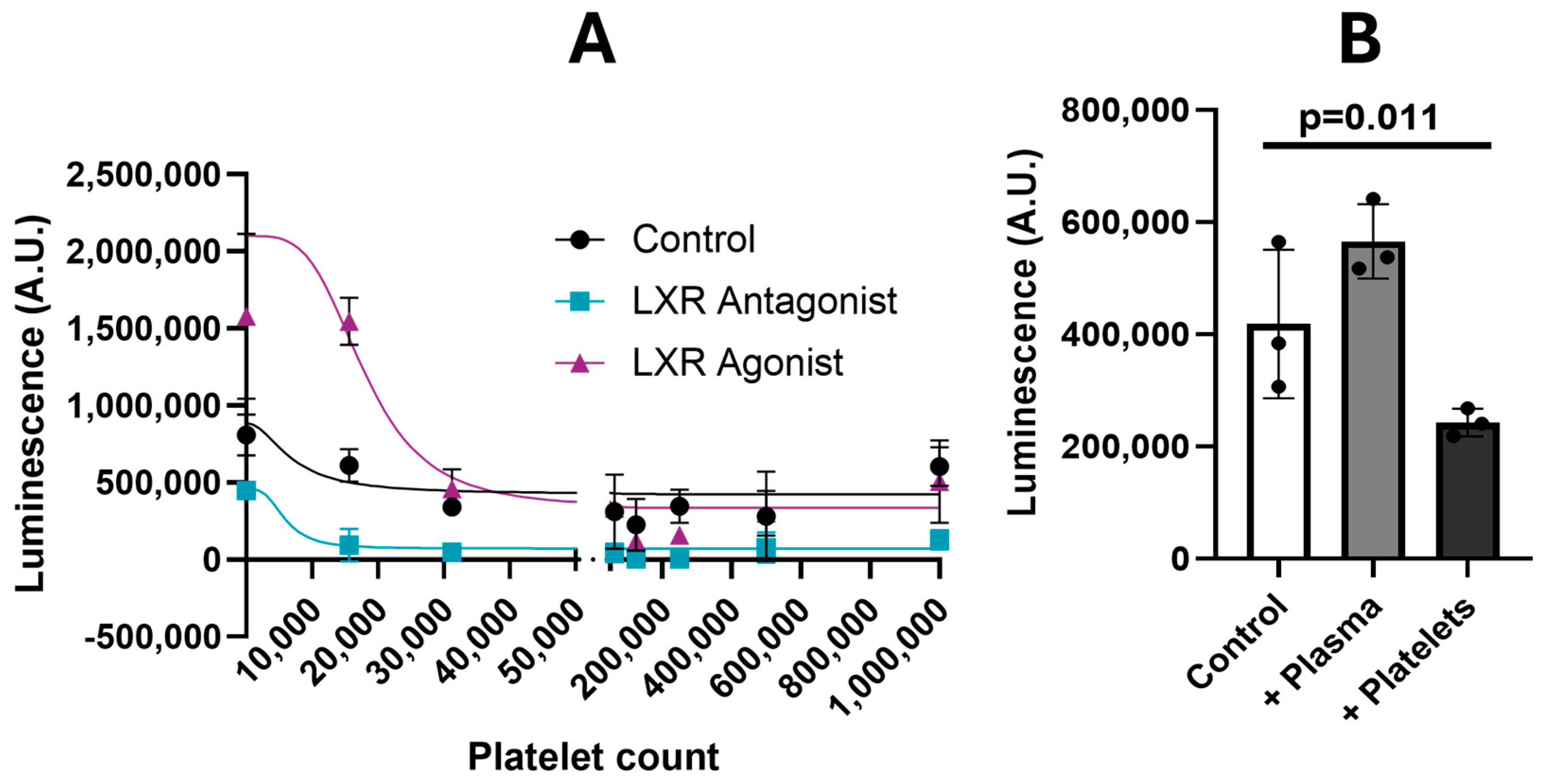
3.3. Validation of LXR/RXR Signaling Pathways in the Platelet-Ovarian Cancer Spheroids Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, H.; Zhang, Y.; Zhang, Y.; Li, X.; Zhao, Q.; Meng, F.; Huang, Q.; Wang, Y. High-grade serous ovarian and fallopian tube carcinomas with similar clinicopathological characteristics might originate from serous tubal intraepithelial carcinoma in Chinese women. Int. J. Clin. Exp. Pathol. 2017, 10, 8222–8232. [Google Scholar] [PubMed]
- Prat, J.; Oncology, F.C.o.G. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Haemmerle, M.; Taylor, M.L.; Gutschner, T.; Pradeep, S.; Cho, M.S.; Sheng, J.; Lyons, Y.M.; Nagaraja, A.S.; Dood, R.L.; Wen, Y.; et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat. Commun. 2017, 8, 310. [Google Scholar] [CrossRef]
- Bottsford-Miller, J.; Choi, H.J.; Dalton, H.J.; Stone, R.L.; Cho, M.S.; Haemmerle, M.; Nick, A.M.; Pradeep, S.; Zand, B.; Previs, R.A.; et al. Differential platelet levels affect response to taxane-based therapy in ovarian cancer. Clin. Cancer Res. 2015, 21, 602–610. [Google Scholar] [CrossRef]
- Nakao, S.; Minaguchi, T.; Itagaki, H.; Hosokawa, Y.; Shikama, A.; Tasaka, N.; Akiyama, A.; Ochi, H.; Matsumoto, K.; Satoh, T. Pretreatment thrombocytosis as an independent predictive factor for chemoresistance and poor survival in epithelial ovarian cancer. J. Ovarian Res. 2020, 13, 55. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef]
- Cho, M.S.; Bottsford-Miller, J.; Vasquez, H.G.; Stone, R.; Zand, B.; Kroll, M.H.; Sood, A.K.; Afshar-Kharghan, V. Platelets increase the proliferation of ovarian cancer cells. Blood 2012, 120, 4869–4872. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Harmon, S.; Inkielewicz, I.; Santos-Martinez, M.J.; Jones, M.; Cantwell, P.; Bazou, D.; Ledwidge, M.; Radomski, M.W.; Gilmer, J.F. Differential inhibition of tumour cell-induced platelet aggregation by the nicotinate aspirin prodrug (ST0702) and aspirin. Br. J. Pharmacol. 2012, 166, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Llobat, P.; Dovizio, M.; Bruno, A.; Ricciotti, E.; Cufino, V.; Sacco, A.; Grande, R.; Alberti, S.; Arena, V.; Cirillo, M.; et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget 2016, 7, 32462–32477. [Google Scholar] [CrossRef] [PubMed]
- Isingizwe, Z.R.; Mortan, L.F.; Benbrook, D.M. Platelet and epithelial cell interations can be modeled in cell culture, and are not affected by dihomo-gamma-linolenic acid. PLoS ONE 2024, 19, e0309125. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, L.M.; Townsend, M.K.; Jordan, S.J.; Patel, A.V.; Teras, L.R.; Lacey, J.V., Jr.; Doherty, J.A.; Harris, H.R.; Goodman, M.T.; Shvetsov, Y.B.; et al. Modification of the Association between Frequent Aspirin Use and Ovarian Cancer Risk: A Meta-Analysis Using Individual-Level Data from Two Ovarian Cancer Consortia. J. Clin. Oncol. 2022, 40, 4207–4217. [Google Scholar] [CrossRef] [PubMed]
- Moisan, F.; Francisco, E.B.; Brozovic, A.; Duran, G.E.; Wang, Y.C.; Chaturvedi, S.; Seetharam, S.; Snyder, L.A.; Doshi, P.; Sikic, B.I. Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol. Oncol. 2014, 8, 1231–1239. [Google Scholar] [CrossRef]
- Elayapillai, S.; Ramraj, S.; Benbrook, D.M.; Bieniasz, M.; Wang, L.; Pathuri, G.; Isingizwe, Z.R.; Kennedy, A.L.; Zhao, Y.D.; Lightfoot, S.; et al. Potential and mechanism of mebendazole for treatment and maintenance of ovarian cancer. Gynecol. Oncol. 2021, 160, 302–311. [Google Scholar] [CrossRef]
- Javadian, P.; Xu, C.; Sjoelund, V.; Borden, L.E.; Garland, J.; Benbrook, D.M. Identification of Candidate Biomarker and Drug Targets for Improving Endometrial Cancer Racial Disparities. Int. J. Mol. Sci. 2022, 23, 7779. [Google Scholar] [CrossRef]
- Wiśniewski, J.R. Filter-Aided Sample Preparation for Proteome Analysis. In Microbial Proteomics: Methods and Protocols; Becher, D., Ed.; Springer: New York, NY, USA, 2018; pp. 3–10. [Google Scholar] [CrossRef]
- Arif, H.; Aggarwal, S. Salicylic Acid (Aspirin); StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rader, J.S.; Sill, M.W.; Beumer, J.H.; Lankes, H.A.; Benbrook, D.M.; Garcia, F.; Trimble, C.; Tate Thigpen, J.; Lieberman, R.; Zuna, R.E.; et al. A stratified randomized double-blind phase II trial of celecoxib for treating patients with cervical intraepithelial neoplasia: The potential predictive value of VEGF serum levels: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 145, 291–297. [Google Scholar] [CrossRef]
- Peer, C.J.; Spencer, S.D.; VanDenBerg, D.A.; Pacanowski, M.A.; Horenstein, R.B.; Figg, W.D. A sensitive and rapid ultra HPLC-MS/MS method for the simultaneous detection of clopidogrel and its derivatized active thiol metabolite in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 880, 132–139. [Google Scholar] [CrossRef]
- Bjornsson, T.D.; Mahony, C. Clinical pharmacokinetics of dipyridamole. Thromb. Res. Suppl. 1983, 4, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, I.C.; O’Shea, J.C.; Kosoglou, T.; Jennings, L.K.; Lorenz, T.J.; Kitt, M.M.; Kleiman, N.S.; Talley, D.; Aguirre, F.; Davidson, C.; et al. Pharmacodynamics and pharmacokinetics of higher-dose, double-bolus eptifibatide in percutaneous coronary intervention. Circulation 2001, 104, 406–411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicolas, L.B.; Krause, A.; Gutierrez, M.M.; Dingemanse, J. Integrated pharmacokinetics and pharmacodynamics of epoprostenol in healthy subjects. Br. J. Clin. Pharmacol. 2012, 74, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Pergialiotis, V.; Vogiatzi Vokotopoulou, L.; Vlachos, D.E.; Liontos, M.; Kontomanolis, E.; Thomakos, N. Pre-treatment thrombocytosis and ovarian cancer survival: A meta-analysis. Eur. J. Obs. Gynecol. Reprod. Biol. X 2024, 22, 100312. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, D.H.; Cozzi, G.D.; Crispens, M.A.; Beeghly-Fadiel, A. Platelets, Thrombocytosis, and Ovarian Cancer Prognosis: Surveying the Landscape of the Literature. Int. J. Mol. Sci. 2020, 21, 8169. [Google Scholar] [CrossRef]
- Tardiff, B.E.; Jennings, L.K.; Harrington, R.A.; Gretler, D.; Potthoff, R.F.; Vorchheimer, D.A.; Eisenberg, P.R.; Lincoff, A.M.; Labinaz, M.; Joseph, D.M.; et al. Pharmacodynamics and pharmacokinetics of eptifibatide in patients with acute coronary syndromes: Prospective analysis from PURSUIT. Circulation 2001, 104, 399–405. [Google Scholar] [CrossRef]
- Bansal, A.B.; Sattar, Y.; Patel, P.; Jamil, R.T. Eptifibatide; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bartolomé, R.A.; Robles, J.; Martin-Regalado, Á.; Pintado-Berninches, L.; Burdiel, M.; Jaén, M.; Aizpurúa, C.; Imbaud, J.I.; Casal, J.I. CDH6-activated αIIbβ3 crosstalks with α2β1 to trigger cellular adhesion and invasion in metastatic ovarian and renal cancers. Mol. Oncol. 2021, 15, 1849–1865. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, F.; Gu, W.; Yang, H.; Meng, Q.; Zhang, Y.; Yang, H.; Duan, Q. The roles of platelet GPIIb/IIIa and alphavbeta3 integrins during HeLa cells adhesion, migration, and invasion to monolayer endothelium under static and dynamic shear flow. J. Biomed. Biotechnol. 2009, 2009, 829243. [Google Scholar] [CrossRef]
- Kononczuk, J.; Surazynski, A.; Czyzewska, U.; Prokop, I.; Tomczyk, M.; Palka, J.; Miltyk, W. alphaIIbbeta3-integrin Ligands: Abciximab and Eptifibatide as Proapoptotic Factors in MCF-7 Human Breast Cancer Cells. Curr. Drug Targets 2015, 16, 1429–1437. [Google Scholar] [CrossRef]
- Zhao, F.; Li, L.; Guan, L.; Yang, H.; Wu, C.; Liu, Y. Roles for GP IIb/IIIa and αvβ3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett. 2014, 344, 62–73. [Google Scholar] [CrossRef]
- Crippa, M.; Bersini, S.; Gilardi, M.; Arrigoni, C.; Gamba, S.; Falanga, A.; Candrian, C.; Dubini, G.; Vanoni, M.; Moretti, M. A microphysiological early metastatic niche on a chip reveals how heterotypic cell interactions and inhibition of integrin subunit β(3) impact breast cancer cell extravasation. Lab Chip 2021, 21, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Nishikawa, J.; Semma, M.; Ichikawa, A. Induction of integrin β3 in PGE₂-stimulated adhesion of mastocytoma P-815 cells to the Arg-Gly-Asp-enriched fragment of fibronectin. Biochem. Pharmacol. 2011, 81, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Asgari, A.; Lesyk, G.; Poitras, E.; Govindasamy, N.; Terry, K.; To, R.; Back, V.; Rudzinski, J.K.; Lewis, J.D.; Jurasz, P. Platelets stimulate programmed death-ligand 1 expression by cancer cells: Inhibition by anti-platelet drugs. J. Thromb. Haemost. 2021, 19, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Bortot, B.; Mangogna, A.; Peacock, B.; Lees, R.; Valle, F.; Brucale, M.; Tassinari, S.; Romano, F.; Ricci, G.; Biffi, S. Platelet Activation in Ovarian Cancer Ascites: Assessment of GPIIb/IIIa and PF4 in Small Extracellular Vesicles by Nano-Flow Cytometry Analysis. Cancers 2022, 14, 4100. [Google Scholar] [CrossRef] [PubMed]
- Parikka, M.; Nissinen, L.; Kainulainen, T.; Bruckner-Tuderman, L.; Salo, T.; Heino, J.; Tasanen, K. Collagen XVII promotes integrin-mediated squamous cell carcinoma transmigration--a novel role for alphaIIb integrin and tirofiban. Exp. Cell Res. 2006, 312, 1431–1438. [Google Scholar] [CrossRef]
- Wu, Z.; Li, B.; Qie, Y.; Wu, S.; Qi, F.; Chu, T.; Nie, G.; Hu, H. Targeted Inhibition of Lymphovascular Invasion Formation with CREKA Peptide-Modified Silicasomes to Boost Chemotherapy in Bladder Cancer. Nano Lett. 2024, 24, 10186–10195. [Google Scholar] [CrossRef]
- Kerndt, C.C.; Nagalli, S. Dipyridamole; StatPear: Treasure Island, FL, USA, 2024. [Google Scholar]
- Suzuki, N.; Oiwa, Y.; Sugano, I.; Inaba, N.; Sekiya, S.; Fukazawa, I.; Yoshida, J.; Takakubo, Y.; Isogai, E.; Saito-Ebihara, M. Dipyridamole enhances an anti-proliferative effect of interferon in various types of human tumor cells. Int. J. Cancer 1992, 51, 627–633. [Google Scholar] [CrossRef]
- Suzuki, N.; Sekiya, S.; Sugano, I.; Kojima, T.; Yamamori, H.; Takakubo, Y. Dipyridamole combined with tumor necrosis factor-alpha enhances inhibition of proliferation in human tumor cell lines. Jpn. J. Cancer Res. 1995, 86, 761–769. [Google Scholar] [CrossRef]
- Sureechatchaiyan, P.; Hamacher, A.; Brockmann, N.; Stork, B.; Kassack, M.U. Adenosine enhances cisplatin sensitivity in human ovarian cancer cells. Purinergic Signal 2018, 14, 395–408. [Google Scholar] [CrossRef]
- Isonishi, S.; Kirmani, S.; Kim, S.; Plaxe, S.C.; Braly, P.S.; McClay, E.F.; Howell, S.B. Phase I and pharmacokinetic trial of intraperitoneal etoposide in combination with the multidrug-resistance-modulating agent dipyridamole. J. Natl. Cancer Inst. 1991, 83, 621–626. [Google Scholar] [CrossRef]
- Weeks, K.S.; Herbach, E.; McDonald, M.; Charlton, M.; Schweizer, M.L. Meta-Analysis of VTE Risk: Ovarian Cancer Patients by Stage, Histology, Cytoreduction, and Ascites at Diagnosis. Obs. Gynecol. Int. 2020, 2020, 2374716. [Google Scholar] [CrossRef] [PubMed]
- Ebina, Y.; Uchiyama, M.; Imafuku, H.; Suzuki, K.; Miyahara, Y.; Yamada, H. Risk factors for deep venous thrombosis in women with ovarian cancer. Medicine 2018, 97, e11009. [Google Scholar] [CrossRef] [PubMed]
- Basaran, D.; Boerner, T.; Suhner, J.; Sassine, D.; Liu, Y.; Grisham, R.N.; Tew, W.P.; Gardner, G.J.; Zivanovic, O.; Sonoda, Y.; et al. Risk of venous thromboembolism in ovarian cancer patients receiving neoadjuvant chemotherapy. Gynecol. Oncol. 2021, 163, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Willy, P.J.; Umesono, K.; Ong, E.S.; Evans, R.M.; Heyman, R.A.; Mangelsdorf, D.J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995, 9, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, T.; Wang, F.; Tian, H.; Samuels, H.H. Functional evidence for retinoid X receptor (RXR) as a nonsilent partner in the thyroid hormone receptor/RXR heterodimer. Mol. Cell. Biol. 2002, 22, 5782–5792. [Google Scholar] [CrossRef]
- Bovenga, F.; Sabba, C.; Moschetta, A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 2015, 21, 517–526. [Google Scholar] [CrossRef]
- Ju, X.; Huang, P.; Chen, M.; Wang, Q. Liver X receptors as potential targets for cancer therapeutics. Oncol. Lett. 2017, 14, 7676–7680. [Google Scholar] [CrossRef]
- Candelaria, N.R.; Addanki, S.; Zheng, J.; Nguyen-Vu, T.; Karaboga, H.; Dey, P.; Gabbi, C.; Vedin, L.L.; Liu, K.; Wu, W.; et al. Antiproliferative effects and mechanisms of liver X receptor ligands in pancreatic ductal adenocarcinoma cells. PLoS ONE 2014, 9, e106289. [Google Scholar] [CrossRef]
- Wan, W.; Hou, Y.; Wang, K.; Cheng, Y.; Pu, X.; Ye, X. The LXR-623-induced long non-coding RNA LINC01125 suppresses the proliferation of breast cancer cells via PTEN/AKT/p53 signaling pathway. Cell Death Dis. 2019, 10, 248. [Google Scholar] [CrossRef]
- Rough, J.J.; Monroy, M.A.; Yerrum, S.; Daly, J.M. Anti-proliferative effect of LXR agonist T0901317 in ovarian carcinoma cells. J. Ovarian Res. 2010, 3, 13. [Google Scholar] [CrossRef]
- Vedin, L.L.; Gustafsson, J.; Steffensen, K.R. The oxysterol receptors LXRα and LXRβ suppress proliferation in the colon. Mol. Carcinog. 2013, 52, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.P.; Kokontis, J.M.; Hiipakka, R.A.; Liao, S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J. Biomed. Sci. 2007, 14, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.P.; Lin, H.P. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010, 30, 3643–3648. [Google Scholar] [PubMed]
- Chuu, C.P.; Hiipakka, R.A.; Kokontis, J.M.; Fukuchi, J.; Chen, R.Y.; Liao, S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006, 66, 6482–6486. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wei, Z.; Zhang, Y.; Liu, Y.; Zhang, S.; Li, Q.; Feng, K.; Yang, X.; Liu, G.; Chen, Y.; et al. Activation of CTU2 expression by LXR promotes the development of hepatocellular carcinoma. Cell Biol. Toxicol. 2024, 40, 23. [Google Scholar] [CrossRef]
- Lou, R.; Cao, H.; Dong, S.; Shi, C.; Xu, X.; Ma, R.; Wu, J.; Feng, J. Liver X receptor agonist T0901317 inhibits the migration and invasion of non-small-cell lung cancer cells in vivo and in vitro. Anticancer Drugs 2019, 30, 495–500. [Google Scholar] [CrossRef]
- Noghero, A.; Perino, A.; Seano, G.; Saglio, E.; Lo Sasso, G.; Veglio, F.; Primo, L.; Hirsch, E.; Bussolino, F.; Morello, F. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arter. Thromb. Vasc. Biol. 2012, 32, 2280–2288. [Google Scholar] [CrossRef]
- Filippelli, A.; Del Gaudio, C.; Simonis, V.; Ciccone, V.; Spini, A.; Donnini, S. Scoping Review on Platelets and Tumor Angiogenesis: Do We Need More Evidence or Better Analysis? Int. J. Mol. Sci. 2022, 23, 13401. [Google Scholar] [CrossRef]
- Kolluri, S.K.; Corr, M.; James, S.Y.; Bernasconi, M.; Lu, D.; Liu, W.; Cottam, H.B.; Leoni, L.M.; Carson, D.A.; Zhang, X.K. The R-enantiomer of the nonsteroidal antiinflammatory drug etodolac binds retinoid X receptor and induces tumor-selective apoptosis. Proc. Natl. Acad. Sci. USA 2005, 102, 2525–2530. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Ning, H.; Yuan, Z.; Zhong, Y.; Wu, S.; Zeng, J.Z. α-Mangostin Induces Apoptosis and Inhibits Metastasis of Breast Cancer Cells via Regulating RXRα-AKT Signaling Pathway. Front. Pharmacol. 2021, 12, 739658. [Google Scholar] [CrossRef]
- Wang, P.-y.; Zeng, W.-j.; Liu, J.; Wu, Y.-L.; Ma, Y.; Zeng, Z.; Pang, J.-y.; Zhang, X.-k.; Yan, X.; Wong, A.S.T.; et al. TRC4, an improved triptolide derivative, specifically targets to truncated form of retinoid X receptor-alpha in cancer cells. Biochem. Pharmacol. 2017, 124, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, J.; Lin, J.; Cheltsov, A.V.; Xu, L.; Chen, Y.; Zeng, Z.; Chen, L.; Huang, M.; Hu, M.; et al. NSC-640358 acts as RXRα ligand to promote TNFα-mediated apoptosis of cancer cell. Protein Cell 2015, 6, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-H.; Jiang, F.-Q.; Duan, Y.-H.; Zeng, Z.-P.; Chen, F.; Dai, Y.; Chen, J.-B.; Liu, J.-X.; Liu, J.; Zhou, H. Targeting truncated retinoid X receptor-α by CF31 induces TNF-α–dependent apoptosis. Cancer Res. 2013, 73, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wu, H.; Sheng, L.; Liu, Y.-X.; Ye, F.; Wang, M.; Zhou, H.; Su, Y.; Zhang, X.-K. Oncogenic potential of truncated RXRα during colitis-associated colorectal tumorigenesis by promoting IL-6-STAT3 signaling. Nat. Commun. 2019, 10, 1463. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, W.; Su, Y.; Wei, Z.; Liu, J.; Kolluri, S.K.; Wu, H.; Cao, Y.; Chen, J.; Wu, Y.; et al. NSAID Sulindac and Its Analog Bind RXRα and Inhibit RXRα-Dependent AKT Signaling. Cancer Cell 2010, 17, 560–573. [Google Scholar] [CrossRef]
- Chen, L.; Aleshin, A.E.; Alitongbieke, G.; Zhou, Y.; Zhang, X.; Ye, X.; Hu, M.; Ren, G.; Chen, Z.; Ma, Y.; et al. Modulation of nongenomic activation of PI3K signalling by tetramerization of N-terminally-cleaved RXRα. Nat. Commun. 2017, 8, 16066. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, Y.; Tu, X.; Ye, X.; Xu, L.; Xiao, Z.; Wang, Q.; Wang, X.; Du, M.; Chen, Z.; et al. Centrosomal Localization of RXRα Promotes PLK1 Activation and Mitotic Progression and Constitutes a Tumor Vulnerability. Dev. Cell 2020, 55, 707–722.e709. [Google Scholar] [CrossRef]
- Oyarce, C.; Vizcaino-Castro, A.; Chen, S.; Boerma, A.; Daemen, T. Re-polarization of immunosuppressive macrophages to tumor-cytotoxic macrophages by repurposed metabolic drugs. OncoImmunology 2021, 10, 1898753. [Google Scholar] [CrossRef]
- Rambow, F.; Rogiers, A.; Marin-Bejar, O.; Aibar, S.; Femel, J.; Dewaele, M.; Karras, P.; Brown, D.; Chang, Y.H.; Debiec-Rychter, M.; et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell 2018, 174, 843–855.e819. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Chen, R.; Ni, J.; Huang, X.; Li, S.; Lu, X.; Cao, X. RXR signaling targeted cancer therapy. Innov. Life 2023, 1, 100014. [Google Scholar] [CrossRef]
- Jurutka, P.W.; di Martino, O.; Reshi, S.; Mallick, S.; Sausedo, M.A.; Moen, G.A.; Lee, I.J.; Ivan, D.J.; Krall, T.D.; Peoples, S.J.; et al. An Isochroman Analog of CD3254 and Allyl-, Isochroman-Analogs of NEt-TMN Prove to Be More Potent Retinoid-X-Receptor (RXR) Selective Agonists Than Bexarotene. Int. J. Mol. Sci. 2022, 23, 16213. [Google Scholar] [CrossRef] [PubMed]
- Nehdi, A.; Ali, R.; Alhallaj, A.; Alzahrani, H.; Samman, N.; Mashhour, A.; Baz, O.; Barhoumi, T.; Alghanem, B.; Khan, A.; et al. Nuclear Receptors Are Differentially Expressed and Activated in KAIMRC1 Compared to MCF7 and MDA-MB231 Breast Cancer Cells. Molecules 2019, 24, 2028. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Mitsuhashi, A.; Hongying, P.; Shioya, M.; Kojima, K.; Nishikimi, K.; Yahiro, K.; Shozu, M. Bexarotene-induced cell death in ovarian cancer cells through Caspase-4-gasdermin E mediated pyroptosis. Sci. Rep. 2022, 12, 11123. [Google Scholar] [CrossRef] [PubMed]
- Marquez, C.B.; Smithberger, E.E.; Bair, S.M.; Wenham, R.M.; Fenske, N.A.; Glass, L.F.; Cherpelis, B.S. Multiple keratoacanthomas arising in the setting of sorafenib therapy: Novel chemoprophylaxis with bexarotene. Cancer Control 2009, 16, 66–69. [Google Scholar] [CrossRef]
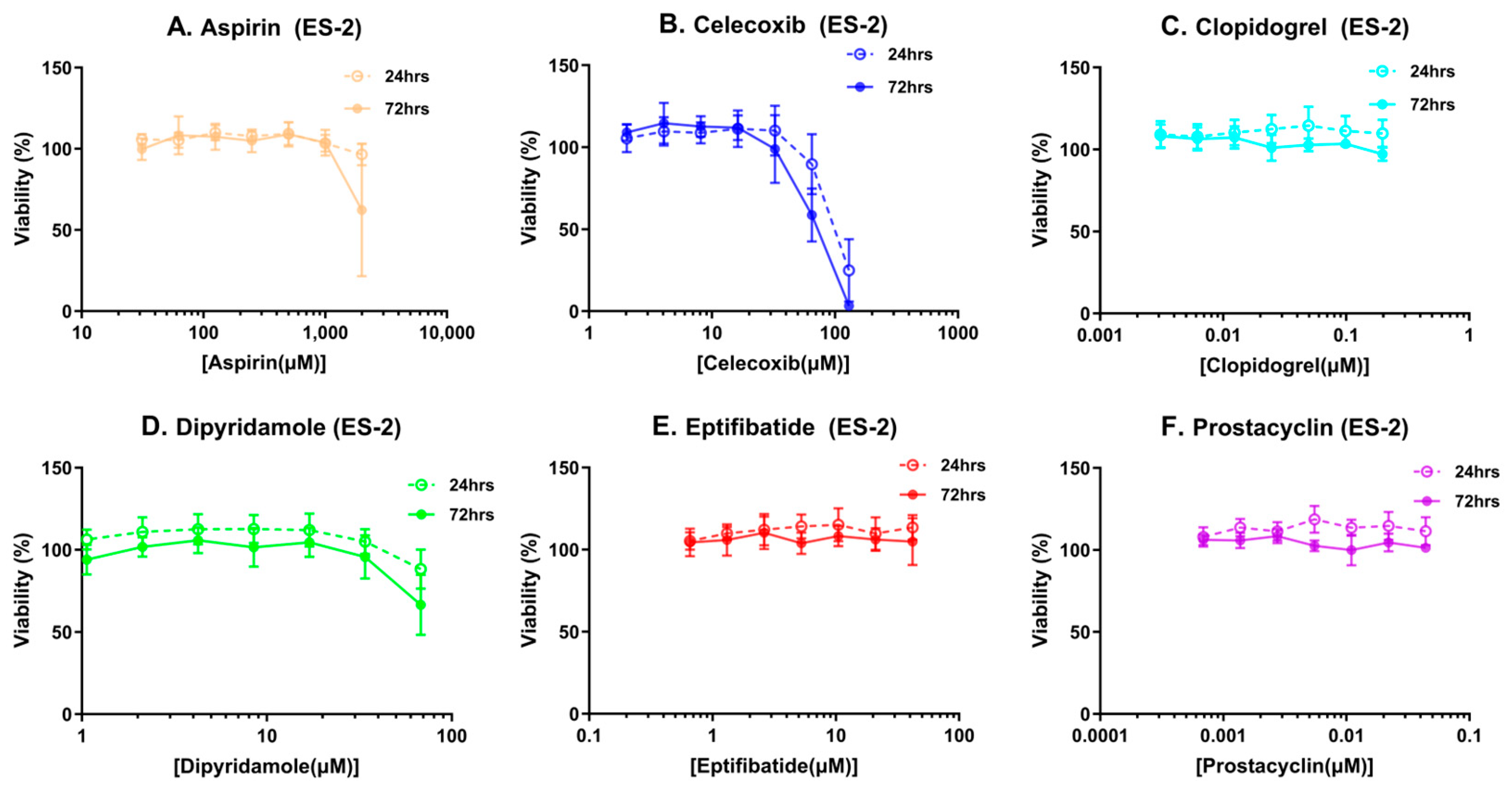


| Compound Name | Mechanism of Action | Achievable Plasma Concentrations (µM) | Reference |
|---|---|---|---|
| Aspirin | Cyclooxygenase 1 inhibitor | 555 | [20] |
| Celecoxib | Cyclooxygenase 2 inhibitor | 64.37 | [21] |
| Clopidogrel | Inhibits adenosine disphosphate binding to its purinergic receptor P2Y12 | 0.099 | [22] |
| Dipyridamole | Inhibits the reuptake of adenosine into platelets | 33.69 | [23] |
| Eptifibatide | Reversible binding of glycoprotein IIB/IIIA | 26.44 | [24] |
| Prostacyclin * | Platelet aggregation inhibitor | 0.11 | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isingizwe, Z.R.; Sjoelund, V.; Benbrook, D.M. Implications of GPIIB-IIIA Integrin and Liver X Receptor in Platelet-Induced Compression of Ovarian Cancer Multi-Cellular Spheroids. Cancers 2024, 16, 3533. https://doi.org/10.3390/cancers16203533
Isingizwe ZR, Sjoelund V, Benbrook DM. Implications of GPIIB-IIIA Integrin and Liver X Receptor in Platelet-Induced Compression of Ovarian Cancer Multi-Cellular Spheroids. Cancers. 2024; 16(20):3533. https://doi.org/10.3390/cancers16203533
Chicago/Turabian StyleIsingizwe, Zitha Redempta, Virginie Sjoelund, and Doris Mangiaracina Benbrook. 2024. "Implications of GPIIB-IIIA Integrin and Liver X Receptor in Platelet-Induced Compression of Ovarian Cancer Multi-Cellular Spheroids" Cancers 16, no. 20: 3533. https://doi.org/10.3390/cancers16203533
APA StyleIsingizwe, Z. R., Sjoelund, V., & Benbrook, D. M. (2024). Implications of GPIIB-IIIA Integrin and Liver X Receptor in Platelet-Induced Compression of Ovarian Cancer Multi-Cellular Spheroids. Cancers, 16(20), 3533. https://doi.org/10.3390/cancers16203533







