Simple Summary
Following technological advancements, there has been a rise in the early detection of pulmonary nodules, with more patients found to have multiple lesions. These lesions may indicate benign or malignant stages, each requiring careful evaluation and management. Multiple treatment strategies can be followed while ensuring patient comfort and minimizing complications. Thus, our study aims to provide an example of single-stage management for multiple lung lesions, using percutaneous ablation and thoracoscopic resection in a hybrid operating room (HOR). These combined procedures in a single stage and setting allows for a minimally invasive experience for patients, with minimal complications and a shorter operation time. In addition to personalized therapy, there is more flexibility for managing complications in the HOR. Thus, these initial results indicate a feasible and safe single-stage workflow and an alternative way to manage multiple pulmonary lesions in patients.
Abstract
Background: Different approaches are required in treating patients with multiple pulmonary lesions. A multistage procedure may increase the risk of complications and patient discomfort. This study reports an initial experience with single-stage management of multiple lung lesions using percutaneous ablation with thoracoscopic resection in a hybrid operating room (HOR). Methods: We retrospectively evaluated patients who underwent combined ablation and resection in an HOR between May 2022 and July 2024. All patients received a single anesthesia via endotracheal tube intubation. The clinical data, operative findings, and pathological characteristics of the lung nodules were recorded. Results: A total of 22 patients were enrolled in this study. Twenty patients underwent unilateral procedures, while the other two patients underwent bilateral procedures. Ablations were performed before lung resection in 21 patients; only 1 patient underwent surgery first. The median global operating room time was 227.0 min. The median total radiation dose (dose area product) was 14,076 μGym2. The median hospital postoperative length of stay was 2 days. Conclusions: The single-stage procedure of percutaneous ablation with thoracoscopic resection under general anesthesia in an HOR is feasible and safe. This procedure is an alternative method for managing multiple pulmonary lesions.
1. Introduction
Lung cancer is a leading cause of death worldwide. Recently, pulmonary nodules have been frequently detected during low-dose computed tomography (CT) screenings [1]. With increased early detection, the early management of lung lesions allows for better survival of certain patient groups [2,3]. This demands a more efficient curative treatment, especially for suspected malignant cases [4]. Despite the numerous methods available for the management of pulmonary nodules [5,6,7,8,9], surgical resection is the most often used method [10,11]; however, only approximately 30% of patients are potential surgical candidates because of cardiopulmonary limitations, advancing age, and the presence of other comorbidities [12,13].
Recently, an increasing number of patients have been diagnosed with multiple lesions [14]. Multiple scattered pulmonary nodules may indicate early-stage multiple lung cancers or several stages of lung cancer, from benign to atypical to malignant [5,14]. Regardless of the presence of isolated or multiple lung lesions, the evaluation and management of benign and malignant lesions are common concerns that need to be addressed, as they significantly impact clinical treatment strategies and could lead to unnecessary surgeries for noncancerous lesions [14]. For instance, surgical excision may involve sacrificing a large lung volume when the lesion is located in the central zone. These aspects have led to the development of alternative nonsurgical interventions, such as stereotactic body radiation therapy (SBRT) or proton therapy. However, these therapies involve high radiation doses, with potential damage to the surrounding tissue, chest wall pain, or skin damage [15]. Further advancements have led to the emergence of percutaneous ablative therapies, such as radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, and laser ablation [16,17,18,19,20]. Clinically, thermal ablation has several advantages, including procedural safety and preservation of lung function [16,17,18,21,22,23].
There are several existing clinical guidelines for thermal ablation, including those published by the American College of Chest Physicians [24], the National Comprehensive Cancer Network [5], and the Cardiovascular and Interventional Radiology Society of Europe [25,26]. It is essential to adhere to these guidelines for the optimal management of lung lesions. MWA is a heat-based ablation technique involving a lower heat-sink effect and related pain than RFA [27]. Various factors must be considered when using MWA, including the method of monitoring the ablation zone during the procedure, as well as the choice of MWA needle brands for different shapes and ablation zones [28,29,30,31,32]. Similarly, cryoablation is another emerging minimally invasive technique for malignant lung nodules and an alternative surgical treatment method [33]. A cross-sectional assessment of the post-ablation zones following cryoablation is easy to perform. Moreover, it is an option for lung nodules with ground glass opacities [34]. MWA is a more time-efficient method that helps preserve the lung parenchyma, shape, and function; however, it may result in significant pain when managing lesions adjacent to the pleura. As mentioned in one guideline, thermal ablation does not preclude subsequent treatment options, such as surgical resection [35,36]. In these cases, wedge resection is easier to perform than ablation. Management of such multiple existing lesions can be performed simultaneously and independently.
The development of a hybrid operating room (HOR) is an important advancement, especially in supporting the management of pulmonary nodules [37]. Real-time and high-definition imaging guidance during thoracic surgical procedures has improved existing techniques [38]. Two-dimensional fluoroscopy and three-dimensional cone-beam computed tomography (CBCT) in the HOR can aid appropriate device navigation and positioning [39]. Furthermore, invasive thoracic surgical procedures involving ablation and resection can be efficiently performed under general anesthesia (GA) with single-lung ventilation. The HOR provides the necessary infrastructure for combined procedures in the same suite and is suitable for the appropriate treatment of different lesions, along with a tailored approach [40]. Here, we present our initial experience of using the HOR for the management of multiple lung lesions with a single-stage procedure combining percutaneous ablation and thoracoscopic resection.
2. Materials and Methods
2.1. Study Design and Patients
We retrospectively evaluated consecutive patients who underwent combined image-guided percutaneous lung ablation and thoracoscopic lung resection in an HOR at the National Taiwan University Hospital, Hsinchu Branch, between May 2022 and July 2024. This study was approved by the Institutional Review Board of the National Taiwan University Hospital Hsinchu Branch (approval number: 202408097RINA). The indications for curative treatment of multiple lung lesions were as follows: (1) pathologically confirmed primary or metastatic lung cancers or (2) persistence of a subsolid nodule on follow-up CT with highly suspicious malignancy or precancerous lesions. Centrally located lesions were considered for ablation, and peripheral lesions were considered for resection. The decision was made by a multidisciplinary team comprising a thoracic oncologist, a chest surgeon, and an interventional pulmonologist.
2.2. Anesthesia and Surgical Preparation
The entire single-stage procedure of combined image-guided ablation and thoracoscopic resection was performed in an HOR equipped with a robotic C-arm CBCT system (ARTIS pheno; Siemens Healthcare GmbH). All patients underwent GA via double-lumen endotracheal tube intubation or a single-lumen tube with an endobronchial blocker. The fraction of inspired oxygen (FiO2) was maintained under 40% to prevent lung collapse. According to the location of the lung lesion, the patients were positioned in the supine, prone, or lateral decubitus position according to the optimal access route for insertion of the ablation needle. In this study cohort, we used the Emprint™ ablation system (Medtronic, Minneapolis, MN, USA) or Hi-Sphere 16 G/20 cm (ECO Medical Instruments Co., Ltd., Nanjing, China) for microwave ablation and the CryoCare System (Endocare, Inc., Irvine, CA, USA) for cryoablation.
2.3. Image-Guided Lung Ablation
Under end-inspiratory breath-hold, an initial CBCT scan with a 4 s acquisition protocol (4s DynaCT Body) was obtained. The needle path was defined by marking the entry and target points of the needle, which was subsequently projected with a laser beam onto the skin of the patient (Figure 1A). Multi-joint arm-supporting systems (Unitrac® Pneumatic Holding Arm, B. Braun) were used for precise control of the needle insertion, and a real-time fluoroscope with a progressive view was used to confirm needle advancement (Figure 1B). After the ablation needle was inserted, a confirmation scan was performed to check the final position of the ablation needle and the target lesion before initiating the ablation process (Figure 1C). Ablation was performed under contralateral lung ventilation, and the treated lung side was kept in apnea [41,42]. After completing the ablation based on the established protocol, CBCT was performed to check the ablation zone.
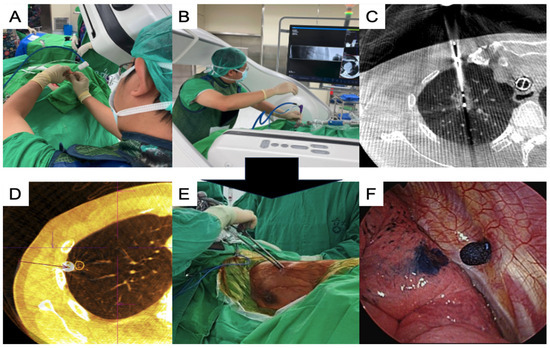
Figure 1.
Single-stage synchronized procedures of ablation and VATS in an HOR. (A) The C-arm projects the laser cross to insert the coaxial needle for the ablation procedure; (B) insertion of the ablation needle using arm support under a progressive view augmented fluoroscopy; (C) post-ablation CT showing the ablation zone while the needle was still inside the lesion; (D) confirmation CT scan for checking the stamped area alignment with the actual lesion location; (E) thoracoscopic surgery using a uniportal approach; (F) the dye-stamped area was identified to guide thoracoscopic resection. CT, computed tomography; VATS, video-assisted thoracic surgery; HOR, hybrid operating room.
2.4. Image-Guided VATS
Thoracoscopic surgery was performed after ablation, except in one patient, who underwent surgery first. Localization using the Artis Pheno system with different methods, including transbronchial [38] and transthoracic approaches [43,44], was performed before or during the thoracoscopic surgery, if necessary (Figure 1D). Uniportal video-assisted thoracic surgery (VATS) was routinely performed for simple wedge and anatomical lung resections (Figure 1E). Based on the thoracoscopic findings, the wedge resection was performed with the inclusion of a dye-containing area (Figure 1F) and/or centrally placed microcoils, which served as fiducial markers, as detected by intraoperative C-arm fluoroscopy. After wedge resection, the presence of the lesions was confirmed, and lymphadenectomy with nodal dissection or sampling was performed for suspected primary lung cancer. Additional pulmonary resection was performed if the section margin was inadequate (<tumor size), and a chest drainage tube was routinely placed. The patients were allowed to recover in the recovery room for observation. Figure 2 shows the images of the CT scans and resected lesions.
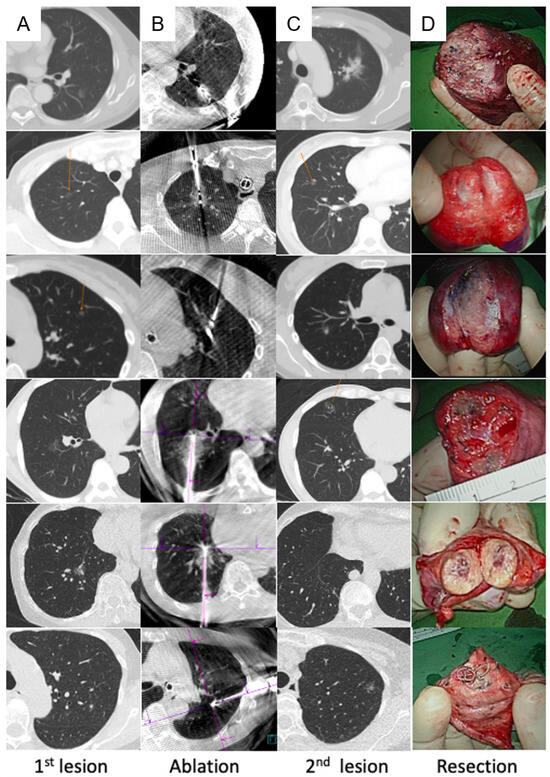
Figure 2.
Demonstration of six cases from pre-ablation to resection. (A) Pre-ablation CT scan to indicate the location of the lesion to undergo ablation; (B) post-ablation CT showing the ablation zone and ablation needle in the zone; (C) CT to indicate the location of the lesion to undergo resection; (D) resected part of the lung showing that the lesion was successfully removed. CT, computed tomography.
2.5. Postoperative Care
Following the completion of the procedure, all patients were kept in the recovery room for 1–2 h before returning to the general ward, and oral nonsteroidal analgesic agents and acetaminophen were administered once the patients resumed oral intake 2–4 h after the procedure. Roentgenograms of the chest were obtained at 6 h postoperatively and the following morning. All patients were examined in the outpatient department 7 days and 1 month after the procedure, and a chest roentgenogram was routinely performed on the same day.
2.6. Data Collection
Clinical data, operative findings, and pathological characteristics of the lung nodules were collected from medical records. The lesions were measured on the preoperative CT images. Lesion size was defined as the largest diameter observed in the axial view, and lesion depth was defined as the smallest distance from the center of the lesion to the pleura. The total accumulated radiation dose, expressed as the dose area product (DAP), was retrospectively calculated using data stored in the ARTIS workstation (Syngo X-Workplace; Siemens Healthcare GmbH, Erlangen, Germany). The durations of the procedures for ablation and surgical resection were recorded separately. The duration of ablation refers to the time from the initiation of the first CBCT scan to the conclusion of the last scan. The duration of surgery refers to the time from skin incision to the end of skin closure. The total anesthesia time was defined as the time between the start of anesthetic induction and extubation of the endotracheal tube.
2.7. Statistical Analysis
Descriptive statistics for continuous data are summarized as medians with interquartile ranges (IQRs) and means with standard deviations, whereas categorical data are presented as counts (percentages). All analyses were performed using SPSS version 20 software (IBM Corp., Armonk, NY, USA).
3. Results
We performed 49 procedures on 22 patients during the study period (Table 1). Patients were aged 36–68 years, and the majority (n = 18, 81.81%) were females. Each patient underwent at least one lesion ablation and lesion resection via VATS. The median lesion size and depth were 8.2 mm (IQR 7.1–11.3 mm) and 26.6 (IQR 21.3–37.4 mm), respectively, for the 24 lesions treated with MWA. The median duration of ablation was 48 min (IQR 32–68 min). Among the 24 ablated lesions, 2 were treated with cryoablation, and the remaining 22 were treated with microwave ablation. One patient underwent microwave ablation for two lesions located in the same lung.

Table 1.
Patients and lesions characteristics.
The lesions treated with VATS had a median size of 8.9 mm (IQR 6.3–14.2 mm) and a depth of 10.5 mm (IQR 4.4–13.3 mm). The median duration of the VATS was 91.5 min (IQR 72–114 min). Among the 26 lesions, 5 were resected via segmentectomy, 1 via lobectomy, and the remaining 20 via wedge resection. Two patients underwent wedge resection and segmentectomy for two lesions located in the same lung. Another two patients underwent wedge resection for two lesions located in the same lung.
The operative findings and pathological characteristics are presented in Table 2. The median fluoroscopy duration was 2.5 min (IQR, 1.6–2.9 min), and the total DAP was 14,076 μGym2 (IQR, 11,764–22,354 μGym2). Among the 22 patients, 16 underwent nine or fewer DynaCT scans, whereas 6 underwent more than nine scans. The median global operation time was 227 min (IQR 196–249 min). The hospital length of stay was 1–3 days for 19 (86.36%) patients, whereas for 3 patients, it was 4, 8, and 6 days owing to complications, such as hemothorax and air leak. The needle biopsy findings for the lesions treated with ablation indicated adenocarcinoma (n = 2), benign alveolar parenchyma (n = 2), adenocarcinoma in situ (AIS) (n = 4), and atypical adenomatous hyperplasia (AAH) (n = 1). The pathological findings of the 26 resected lesions included adenocarcinoma (n = 7), AIS (n = 11), minimally invasive adenocarcinoma (MIA) (n = 4), AAH (n = 1), sclerosing pneumocytoma (n = 1), and metastatic colon cancer (n = 2). The median follow-up interval was 10.5 months (IQR 5–19 months). No patient experienced recurrence.

Table 2.
Details of operative procedures and postoperative results.
4. Discussion
In patients with multiple nodules, multiple-stage procedures are conventionally performed for various treatments. These management options include surgical excision in most cases, such as SBRT, RFA, or a combination of the above therapies [45]. It is important to consider complications, comorbidities, and compromised lung function before making treatment decisions. Performing the procedures at different times could involve patient exposure to general anesthetics at different times, as well as the potential risk of disease progression. Moreover, in the absence of an HOR for intraoperative imaging, patient transfer from the radiology room to the operating room can also involve the risk of complications and patient discomfort [46]. In some studies, SBRT has been used for multiple lesions synchronously or consecutively at 1-month intervals [47]. However, some cases of acute toxicity have been reported. A recent study demonstrated the preliminary outcomes of MWA and VATS in an HOR with some manageable complications [40]. These indicate the need for more advanced techniques to preserve the lung volume and minimize the risk of complications while providing patient comfort. Our early experience with VATS ablation has indicated that it is safe and minimally invasive, achieves tissue preservation, and can be individualized.
An HOR allows for one-step procedures while facilitating intraoperative image guidance and surgical intervention in a room. Single-stage augmented fluoroscopic bronchoscopy was performed under general anesthesia, followed by thoracoscopy.
Surgery is safe and feasible in an HOR [38]. Moreover, pleural stamping techniques for the localization of small pulmonary nodules before resection can be performed as a one-step procedure in an HOR [43,44]. In addition, percutaneous MWA is feasible in an HOR [41], where lung separation under general anesthesia can be efficiently performed with a lowered risk of complications [42]. Thus, there are many different management approaches for nodules with different features and specific requirements for which an HOR is favorable. For instance, if multiple nodules are located at different locations, one anterior and the other posterior, changing the patient’s position several times as needed in an HOR is convenient. Specific possibilities for a specific procedure are easily achievable in an HOR, for example, during procedures, such as a lobectomy, segmentectomy, or wedge resection. Considering these factors, our study combined ablation with VATS in an HOR equipped with CBCT guidance.
Another concern during synchronous procedures is the sequence of approaches and deciding whether ablation or surgical resection should be performed first. In our study, we performed ablation for deeper lesions and surgical resection for peripheral pulmonary lesions. Ablation was performed first, because lung collapse was easily achievable before initiating VATS. Moreover, ablation demands better image quality for an efficient procedure, especially for small ground glass nodules, and in some cases, it requires a synchronous biopsy with a high accuracy for needle placement. Thus, the choice of the first procedure would depend on the physician’s experience and how the initial procedure would impact the latter, while simultaneously preparing for consequences and modifying as needed. Only one patient (no. 19) underwent surgical resection before the ablation of the same pulmonary lobe, and the deformed lobe owing to the wedge resection posed a challenge in recognizing the target area for placing the ablation needle (Figure 3A). However, tubular structures, such as pulmonary vessels and bronchi, around the target area could be traced to the division of the main trunks, and the area could be identified despite minor changes in the spatial relationship between the target and its surrounding structures (Figure 3B).
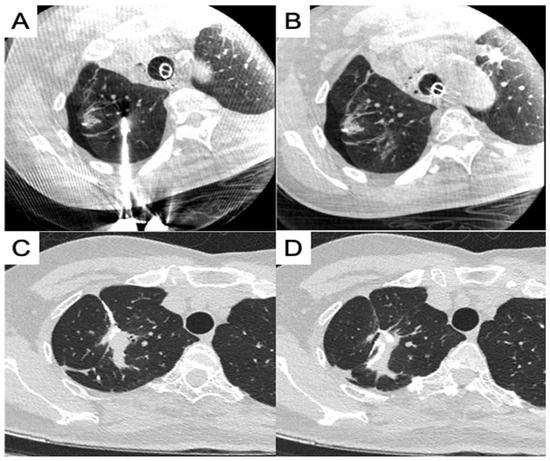
Figure 3.
Combined ablation and wedge resection of the same pulmonary lobe. (A) Ablation placement after partial resection; (B) focal ablation zone away from the staple line; (C) post-procedure CT showed a staple line near the ablation zone; (D) post-procedure CT showed a staple line in the ablation zone.
Bilateral sequential procedures for ablation and surgical resection are clinically challenging scenarios, and care should be taken for ventilation of the post-procedural lung during the procedure on the contralateral side, especially when no pleural drainage tube is placed on the post-procedural side. Once a pneumothorax exists, it can be aggravated during one-lung ventilation during the contralateral side procedure. In three cases involving bilateral procedures, we opted to perform ablation first because of the higher demand for image quality for lung ablation and because atelectasis during anesthesia could negatively impact successful ablation. Because we did not routinely perform chest drainage after ablation, we observed that for more than 5–10 min after one-lung ventilation, the lung shifted to the ablated side and no pneumothorax was detected under fluoroscopy, and the procedure was moved on to the surgical resection of the contralateral side. Although a chest drainage tube can be prophylactically placed on the ablation side, this is not mandatory if there is no evidence of a pneumothorax.
In this study, 11 patients underwent ablation and surgical resection of the same pulmonary lobe, and the resection and ablation zones were mostly completely separate. In some cases, the ablation zone for the central lesion can still be partially resected, along with another peripheral lesion that was surgically resected. The outcome was observed in the follow-up CT images (Figure 3C,D); however, no staple line leakage or prolonged air leak occurred in these cases.
This study has some limitations. First, there were very few bilateral lesions, which could have affected the results when comparing cases with multiple lesions located in one and both lungs. Most patients underwent MWA, with a few undergoing cryoablation, which may have been the reason for the varied procedure times in different patients. Future studies should consider bilateral lesions and a single form of ablation during synchronous procedures. All procedures were performed under GA in a single center, the sample size was small, and the study was retrospective. Future studies in multiple centers with larger numbers of participants are warranted.
5. Conclusions
Ablation with VATS under GA in an HOR is a minimally invasive procedure for patients with multiple pulmonary nodules. It is a safe technique with a minimal complication rate and a lower operation time, and it can be individualized. Future explorations with a larger number of patients and technical refinements are in progress and may lead to further success.
Author Contributions
Conceptualization, L.-K.C. and S.-M.Y.; methodology, L.-K.C., W.-Y.C., P.-S.C. and S.-M.Y.; software, W.-Y.C.; formal analysis, L.-K.C., S.M. and S.-M.Y.; investigation, P.-K.S. and S.-M.Y.; resources, S.-M.Y.; writing—original draft preparation, L.-K.C., S.M. and S.-M.Y.; writing—review and editing, S.-M.Y.; visualization, S.M. and S.-M.Y.; supervision, S.-M.Y.; project administration, L.-K.C., P.-K.S., W.-Y.C., P.-S.C. and S.-M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the National Taiwan University Hospital, Hsin-Chu Branch, Taiwan (Grant Number 112-T105).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the National Taiwan University Hospital Hsinchu Branch (approval number: 202408097RINA).
Informed Consent Statement
Written patient consent was waived by the institutional review board.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
Shwetambara Malwade works for Siemens Company as a research scientist. The other authors declare no conflicts of interest.
References
- Schmid-Bindert, G.; Vogel-Claussen, J.; Gütz, S.; Fink, J.; Hoffmann, H.; Eichhorn, M.E.; Herth, F.J.F. Incidental pulmonary nodules—What do we know in 2022. Respiration 2022, 101, 1024–1034. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Lin, M.W.; Chen, J.S. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J. Thorac. Dis. 2016, 8, S749–S755. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN guidelines insights: Non-small cell lung cancer, Version 2.2021. J. Natl. Compr. Canc Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef]
- Moyer, V.A.; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Donahoe, L.L.; Nguyen, E.T.; Chung, T.B.; Kha, L.C.; Cypel, M.; Darling, G.E.; de Perrot, M.; Keshavjee, S.; Pierre, A.F.; Waddell, T.K.; et al. CT-guided microcoil VATS resection of lung nodules: A single-centre experience and review of the literature. J. Thorac. Dis. 2016, 8, 1986–1994. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chen, K.C.; Chen, J.S. Ultrasound for intraoperative localization of lung nodules during thoracoscopic surgery. Ann. Transl. Med. 2019, 7, 37. [Google Scholar] [CrossRef]
- Ko, W.C.; Lee, Y.F.; Chen, Y.C.; Chien, N.; Huang, Y.S.; Tseng, Y.H.; Lee, J.M.; Hsu, H.H.; Chen, J.S.; Chang, Y.C. CT-guided percutaneous microwave ablation of pulmonary malignant tumors. J. Thorac. Dis. 2016, 8, S659–S665. [Google Scholar] [CrossRef]
- Bhatia, S.; Pereira, K.; Mohan, P.; Narayanan, G.; Wangpaichitr, M.; Savaraj, N. Radiofrequency ablation in primary non-small cell lung cancer: What a radiologist needs to know. Indian J. Radiol. Imaging 2016, 26, 81–91. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Huang, J. Clinical and pathological research status of multiple pulmonary nodules. J. Cancer Ther. 2023, 14, 170–181. [Google Scholar] [CrossRef]
- Chockalingam, A.; Konstantinidis, M.; Koo, B.; Moon, J.T.; Tran, A.; Nourouzpour, S.; Lawson, E.; Fox, K.; Habibollahi, P.; Odisio, B.; et al. Surgical resection, radiotherapy and percutaneous thermal ablation for treatment of stage 1 non-small cell lung cancer: Protocol for a systematic review and network meta-analysis. BMJ Open 2022, 12, e057638. [Google Scholar] [CrossRef]
- Palussiere, J.; Catena, V.; Buy, X. Percutaneous thermal ablation of lung tumors—Radiofrequency, microwave and cryotherapy: Where are we going? Diagn. Interv. Imaging 2017, 98, 619–625. [Google Scholar] [CrossRef]
- Li, G.; Xue, M.; Chen, W.; Yi, S. Efficacy and safety of radiofrequency ablation for lung cancers: A systematic review and meta-analysis. Eur. J. Radiol. 2018, 100, 92–98. [Google Scholar] [CrossRef]
- Healey, T.T.; March, B.T.; Baird, G.; Dupuy, D.E. Microwave ablation for lung neoplasms: A retrospective analysis of long-term results. J. Vasc. Interv. Radiol. 2017, 28, 206–211. [Google Scholar] [CrossRef]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef]
- Bailey, C.W.; Sydnor, M.K., Jr. Current state of tumor ablation therapies. Dig. Dis. Sci. 2019, 64, 951–958. [Google Scholar] [CrossRef]
- Alexander, E.S.; Dupuy, D.E. Lung cancer ablation: Technologies and techniques. Semin. Intervent Radiol. 2013, 30, 141–150. [Google Scholar] [CrossRef]
- Abtin, F.G.; Eradat, J.; Gutierrez, A.J.; Lee, C.; Fishbein, M.C.; Suh, R.D. Radiofrequency ablation of lung tumors: Imaging features of the postablation zone. Radiographics 2012, 32, 947–969. [Google Scholar] [CrossRef]
- Mouli, S.K.; Kurilova, I.; Sofocleous, C.T.; Lewandowski, R.J. The role of percutaneous image-guided thermal ablation for the treatment of pulmonary malignancies. AJR Am. J. Roentgenol. 2017, 209, 740–751. [Google Scholar] [CrossRef]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- Genshaft, S.J.; Suh, R.D.; Abtin, F.; Baerlocher, M.O.; Dariushnia, S.R.; Devane, A.M.; Himes, E.; Lisberg, A.; Padia, S.; Patel, S.; et al. Society of Interventional Radiology Quality Improvement Standards on percutaneous ablation of non-small cell lung cancer and metastatic disease to the lungs. J. Vasc. Interv. Radiol. 2021, 32, e1–e10. [Google Scholar] [CrossRef]
- Venturini, M.; Cariati, M.; Marra, P.; Masala, S.; Pereira, P.L.; Carrafiello, G. CIRSE standards of practice on thermal ablation of primary and secondary lung tumours. Cardiovasc. Intervent Radiol. 2020, 43, 667–683. [Google Scholar] [CrossRef]
- Liu, H.; Song, Y. MDT is still important in the treatment of early-stage lung cancer. J. Thorac. Dis. 2018, 10, S3984–S3985. [Google Scholar] [CrossRef]
- Abbas, G.; Pennathur, A.; Landreneau, R.J.; Luketich, J.D. Radiofrequency and microwave ablation of lung tumors. J. Surg. Oncol. 2009, 100, 645–650. [Google Scholar] [CrossRef]
- Fan, W.; Li, X.; Zhang, L.; Jiang, H.; Zhang, J. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am. J. Roentgenol. 2012, 198, W46–W50. [Google Scholar] [CrossRef]
- Andreano, A.; Huang, Y.; Meloni, M.F.; Lee, F.T., Jr.; Brace, C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med. Phys. 2010, 37, 2967–2973. [Google Scholar] [CrossRef]
- Crocetti, L.; Bozzi, E.; Faviana, P.; Cioni, D.; Della Pina, C.; Sbrana, A.; Fontanini, G.; Lencioni, R. Thermal ablation of lung tissue: In vivo experimental comparison of microwave and radiofrequency. Cardiovasc. Interv. Radiol. 2010, 33, 818–827. [Google Scholar] [CrossRef]
- Li, H.W.; Long, Y.J.; Yan, G.W.; Bhetuwal, A.; Zhuo, L.H.; Yao, H.C.; Zhang, J.; Zou, X.X.; Hu, P.X.; Yang, H.F.; et al. Microwave ablation vs. cryoablation for treatment of primary and metastatic pulmonary malignant tumors. Mol. Clin. Oncol. 2022, 16, 62. [Google Scholar] [CrossRef]
- Chaudhry, A.; Grechushkin, V.; Hoshmand, M.; Kim, C.W.; Pena, A.; Huston, B.; Chaya, Y.; Bilfinger, T.; Moore, W. Characteristic CT findings after percutaneous cryoablation treatment of malignant lung nodules. Medicine 2015, 94, e1672. [Google Scholar] [CrossRef]
- Liu, S.; Liang, B.; Li, Y.; Xu, J.; Qian, W.; Lin, M.; Xu, M.; Niu, L. CT-guided percutaneous cryoablation in patients with lung nodules mainly composed of ground-glass opacities. J. Vasc. Interv. Radiol. 2022, 33, 942–948. [Google Scholar] [CrossRef]
- Chang, L.K.; Yang, S.M.; Chien, N.; Chang, C.C.; Fang, H.Y.; Liu, M.C.; Wang, K.L.; Lin, W.C.; Lin, F.C.F.; Chuang, C.Y.; et al. 2024 multidisciplinary consensus on image-guided lung tumor ablation from the Taiwan Academy of Tumor Ablation. Thorac. Cancer 2024, 15, 1607–1613. [Google Scholar] [CrossRef]
- Murphy, M.C.; Wrobel, M.M.; Fisher, D.A.; Cahalane, A.M.; Fintelmann, F.J. Update on image-guided thermal lung ablation: Society guidelines, therapeutic alternatives, and Postablation imaging findings. Am. J. Roentgenol. 2022, 219, 471–485. [Google Scholar] [CrossRef]
- Ng, C.S.H.; Krimsky, W.S.; Yasufuku, K. The hybrid operating room in modern thoracic surgery. Front. Surg. 2021, 8, 725897. [Google Scholar] [CrossRef]
- Yang, S.M.; Chung, W.Y.; Ko, H.J.; Chen, L.C.; Chang, L.K.; Chang, H.C.; Kuo, S.W.; Ho, M.C. Single-stage augmented fluoroscopic bronchoscopy localization and thoracoscopic resection of small pulmonary nodules in a hybrid operating room. Eur. J. Cardiothorac. Surg. 2022, 63, ezac541. [Google Scholar] [CrossRef]
- Spenkelink, I.M.; Heidkamp, J.; Futterer, J.J.; Rovers, M.M. Image guided procedures in the hybrid operating room: A systematic scoping review. PLoS ONE 2022, 17, e0266341. [Google Scholar] [CrossRef]
- Harrison, O.J.; Sarvananthan, S.; Tamburrini, A.; Peebles, C.; Alzetani, A. Image-guided combined ablation and resection in thoracic surgery for the treatment of multiple pulmonary metastases: A preliminary case series. JTCVS Tech. 2021, 9, 156–162. [Google Scholar] [CrossRef]
- Chang, L.K.; Yang, S.M.; Chung, W.Y.; Chen, L.C.; Chang, H.C.; Ho, M.C.; Chang, Y.C.; Yu, C.J. Cone-beam computed tomography image-guided percutaneous microwave ablation for lung nodules in a hybrid operating room: An initial experience. Eur. Radiol. 2024, 34, 3309–3319. [Google Scholar] [CrossRef]
- Chan, P.S.; Chang, L.K.; Malwade, S.; Chung, W.Y.; Yang, S.M. Cone beam CT derived laser-guided percutaneous lung ablation: Minimizing needle-related complications under general anesthesia with lung separation. Acad. Radiol. 2024; in press. [Google Scholar] [CrossRef]
- Yang, S.M.; Malwade, S.; Chung, W.Y.; Wu, W.T.; Chen, L.C.; Chang, L.K.; Change, H.C.; Chan, P.S.; Kuo, S.W. Augmented fluoroscopy-guided dye localization for small pulmonary nodules in hybrid operating room: Intrathoracic stamping versus transbronchial marking. Int. J. Comput. Assist. Radiol. Surg. 2024, 1–11. [Google Scholar] [CrossRef]
- Yang, S.M.; Malwade, S.; Chung, W.Y.; Chen, L.C.; Chang, L.K.; Chang, H.C.; Chan, P.S.; Kuo, S.W. Nontraumatic intraoperative pulmonary nodule localization with laser guide stamping in a hybrid operating room. Updates Surg. 2024, 1–10. [Google Scholar] [CrossRef]
- Loverdos, K.; Fotiadis, A.; Kontogianni, C.; Iliopoulou, M.; Gaga, M. Lung nodules: A comprehensive review on current approach and management. Ann. Thorac. Med. 2019, 14, 226–238. [Google Scholar] [CrossRef]
- Gill, R.R.; Barlow, J.; Jaklitsch, M.T.; Schmidlin, E.J.; Hartigan, P.M.; Bueno, R. Image-guided video-assisted thoracoscopic resection (iVATS): Translation to clinical practice—Real-world experience. J. Surg. Oncol. 2020, 121, 1225–1232. [Google Scholar] [CrossRef]
- Owen, D.; Olivier, K.R.; Mayo, C.S.; Miller, R.C.; Nelson, K.; Bauer, H.; Brown, P.D.; Park, S.S.; Ma, D.J.; Garces, Y.I. Outcomes of stereotactic body radiotherapy (SBRT) treatment of multiple synchronous and recurrent lung nodules. Radiat. Oncol. 2015, 10, 43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
