Evolution of Three-Dimensional Computed Tomography Imaging in Thoracic Surgery
Abstract
Simple Summary
Abstract
1. Introduction
2. Current Status of 3D CT Images in Thoracic Surgery
2.1. History of 3D CT Imaging
2.2. 3D Imaging with a Wide Variety of Use
2.3. Currently Available 3D CT Software
2.4. Three-Dimensional CT Imaging Recognized by Three Dimensions
2.5. Current Issues to Be Resolved for Future Improvement
3. Evolution of 3D CT Imaging
3.1. Three-Dimensional CT Images Reconstructed from Nonenhanced CT Data
3.2. Development of Variable Virtual 3D Images
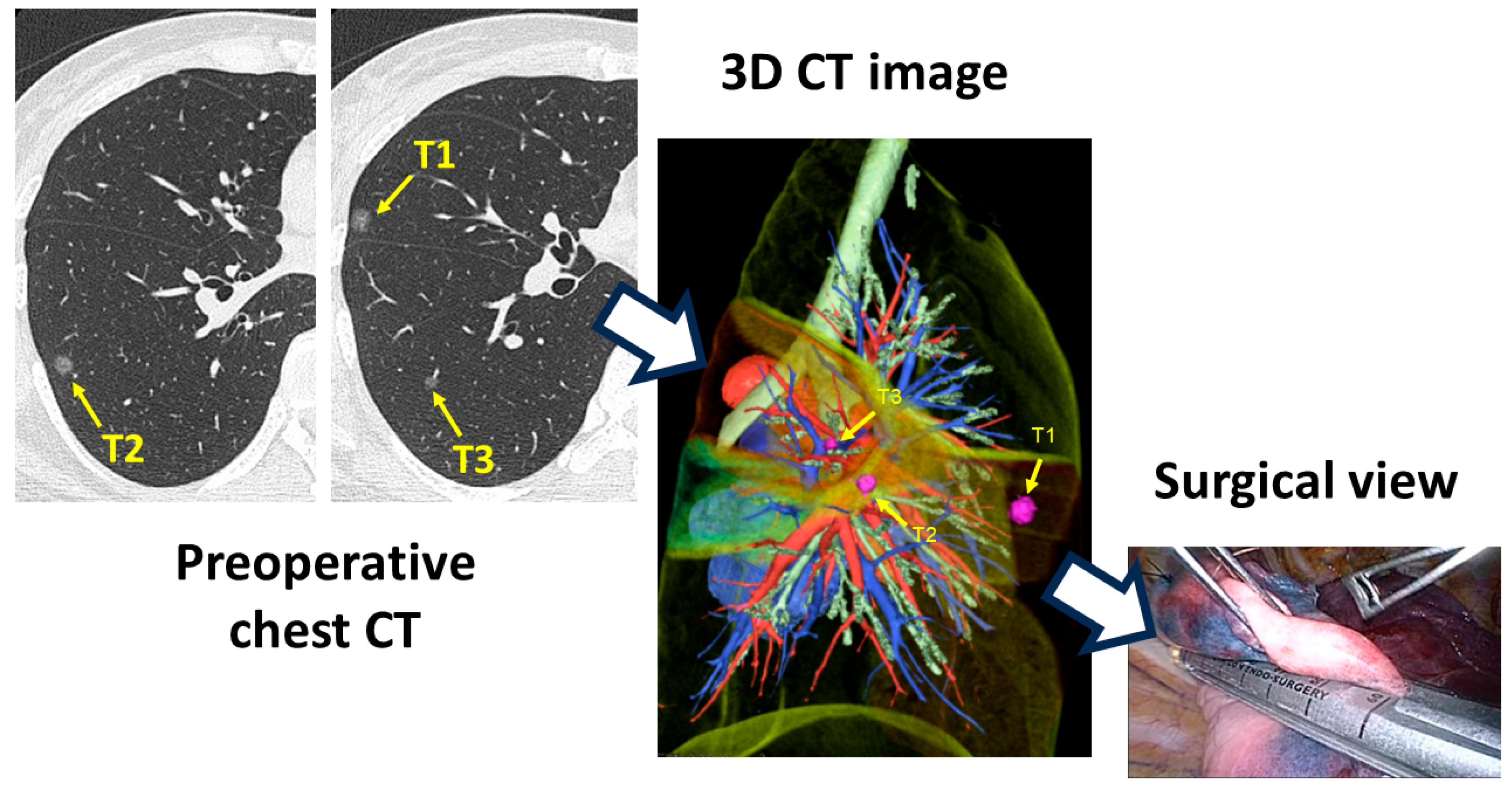
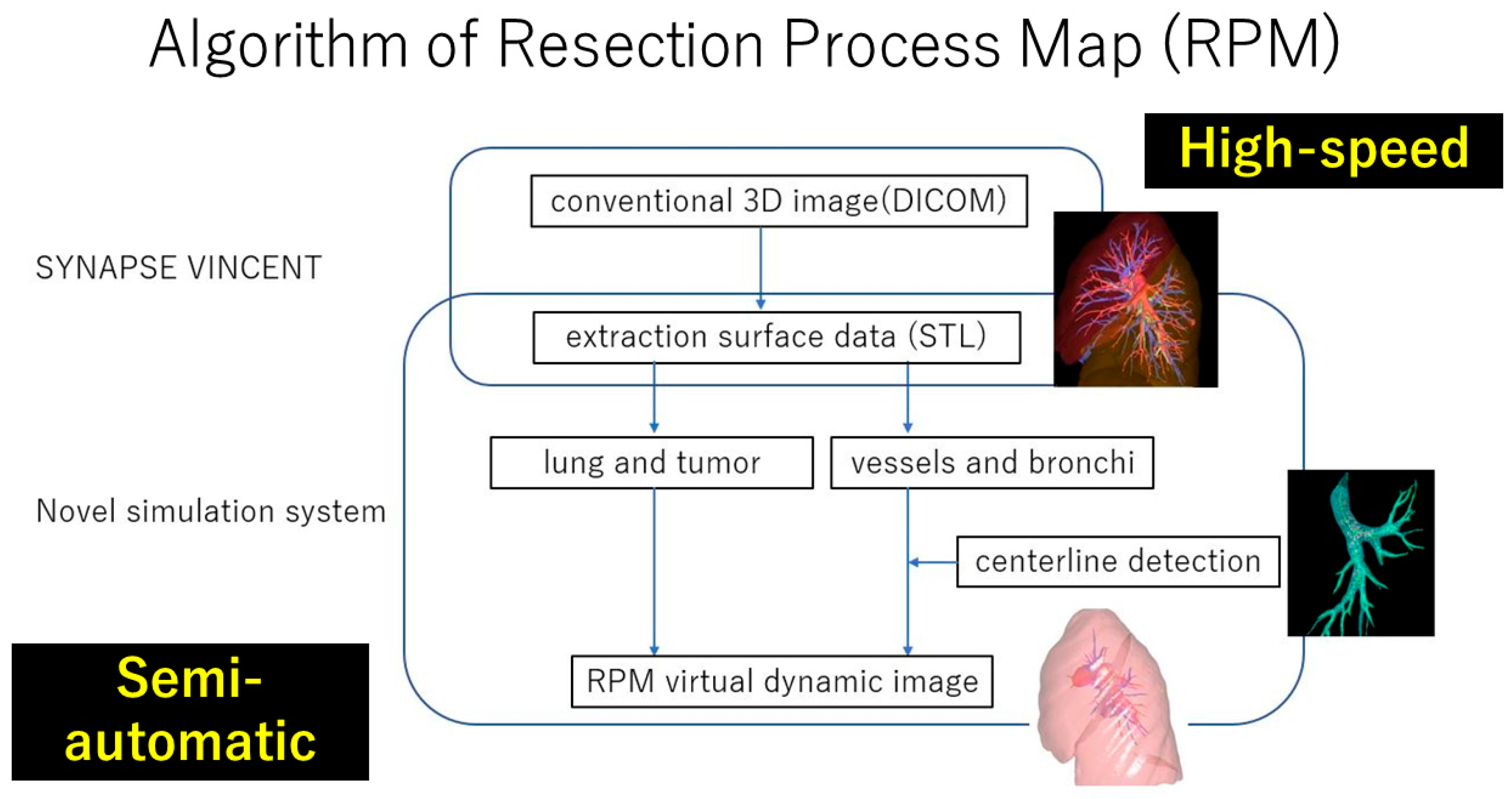
3.3. RPM Aiming at Real-Time Surgical Guide
3.4. RPM with Deformation by Deflation
4. Future Perspectives
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen-Yoshikawa, T.F.; Fukui, T.; Nakamura, S.; Ito, T.; Kadomatsu, Y.; Tsubouchi, H.; Ueno, H.; Sugiyama, T.; Goto, M.; Mori, S.; et al. Current Trends in Thoracic Surgery. Nagoya J. Med. Sci. 2020, 82, 161–174. [Google Scholar]
- Berzenji, L.; Wen, W.; Verleden, S.; Claes, E.; Yogeswaran, S.K.; Lauwers, P.; Van Schil, P.; Hendriks, J.M.H. Minimally invasive surgery in non-small cell lung cancer: Where do we stand? Cancers 2023, 15, 4281. [Google Scholar] [CrossRef] [PubMed]
- Chen-Yoshikawa, T.F.; Date, H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J. Thorac. Dis. 2016, 8 (Suppl. S3), S295–S301. [Google Scholar] [PubMed]
- Hamanaka, K.; Miura, K.; Eguchi, T.; Shimizu, K. Harnessing 3D-CT simulation and planning for enhanced precision surgery: A review of applications and advancements in lung cancer treatment. Cancers 2023, 15, 5400. [Google Scholar] [CrossRef]
- Nagano, M.; Sato, M. Ten-year outcome and development of virtual-assisted lung mapping in thoracic surgery. Cancers 2023, 15, 1971. [Google Scholar] [CrossRef]
- Sato, M.; Kuwata, T.; Yamanashi, K.; Kitamura, A.; Misawa, K.; Imashimizu, K.; Kobayashi, M.; Ikeda, M.; Koike, T.; Kosaka, S.; et al. Safety and reproducibility of virtual-assisted lung mapping: A multicentre study in Japan. Eur. J. Cardiothorac. Surg. 2017, 51, 861–868. [Google Scholar] [CrossRef]
- Tokuno, J.; Chen-Yoshikawa, T.F.; Nakajima, D.; Aoyama, A.; Motoyama, H.; Tanaka, S.; Yamada, Y.; Yutaka, Y.; Ohsumi, A.; Hamaji, M.; et al. Improvement of Visualization of Virtual-Assisted Lung Mapping by Indocyanine Green in the Near-Infrared Fluorescence Technique. JTCVS Tech. 2021, 10, 542–549. [Google Scholar] [CrossRef]
- Rad, A.A.; Vardanyan, R.; Thavarajasingam, S.G.; Zubarevich, A.; Eynde, J.V.D.; Sa, M.P.B.O.; Zhigalov, K.; Nia, P.S.; Ruhparwar, A.; Weymann, A. Extended, virtual and augmented reality in thoracic surgery: A systemic review. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 201–211. [Google Scholar]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectony versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomized, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jpnes, D.R.; Conti, M.; Ashrafi, H.; et al. Lobar or sublobar resection peripheral stage IA non-small cell lung cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Nakao, M.; Omura, K.; Hashimoto, K.; Ichinose, J.; Matsuura, Y.; Okumura, S.; Mun, M. Novel three-dimensional image simulation for lung segmentectomy developed with surgeons’ perspective. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; Shimada, Y.; Kato, Y.; Nawa, K.; Makino, Y.; Furumoto, H.; Akata, S.; Kakihana, M.; Kajiwara, N.; Ohira, T.; et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: Results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery. Eur. J. Cardiothorac. Surg. 2014, 46, e120–e126. [Google Scholar] [CrossRef]
- Grossi, S.; Cattoni, M.; Rotolo, N.; Imperatori, A. Video-assisted thoracoscopic surgery simulation and training: A comprehensive literature review. BMC Med. Educ. 2023, 23, 535. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.I.; Arai, K.; Watanabe, T.; Koda, W.; Urayama, H. Use of three-dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann. Thorac. Surg. 2003, 75, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Salvolini, L.; Secchi, E.B.; Costarelli, L.; Nicola, M.D. Clinical applications of 2D and 3D CT imaging of the airways—A review. Eur. J. Radiol. 2000, 34, 9–25. [Google Scholar] [CrossRef]
- Akiba, T.; Marushima, H.; Okada, M.; Harada, J.; Kobayashi, S.; Morikawa, T. Pulmonary vein analysis uding three-dimensional computed tomography angiography for thoracic surgery. Gen. Thorac. Cardiovasc. Surg. 2010, 58, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Oizumi, H.; Kanauchi, N.; Kato, H.; Endoh, M.; Suzuki, J.; Fukaya, K.; Sadahiro, M. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: A report of 52 consecutive cases. J. Thorac. Cardiovasc. Surg. 2011, 141, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Shimizu, K.; Ohtaki, Y.; Obayashi, K.; Kakegawa, S.; Nakazawa, S.; Kamiyoshihara, M.; Igai, H.; Takeyoshi, I. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Nagashima, T.; Ohtaki, Y.; Obayashi, K.; Nakazawa, S.; Kamiyoshihara, M.; Igai, H.; Takeyoshi, I.; Mogi, A.; Kuwano, H. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 604–611. [Google Scholar] [CrossRef]
- Kanzaki, M.; Maeda, H.; Wachi, N.; Kikkawa, T.; Komine, H.; Oyama, K.; Murasugi, M.; Onuki, T. Complete video-assisted thoracoscopic multi-subsegmentectomy based on patients’ specific virtual 3-D pulmonary models. Asian J. Endosc. Surg. 2013, 6, 110–115. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Zhang, X.; Yin, H.; Fu, Y.; Cao, M.; Zhao, X. Application of three-dimensional (3D) reconstruction in the treatment of video-assisted thoracoscopic complex segmentectomy of the lower lobe.: A retrospective study. Front. Surg. 2022, 9, 968199. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Shi, Q.T.; Zhang, Y.; Wang, Y.L. Thoracoscopic segmentectomy and lobectomy assisted by three-dimensional computed-tomography bronchography and angiography for the treatment of primary lung cancer. Word J. Clin. Cases 2021, 9, 10494–10506. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Du, J.; Chen, C.; Zheng, W.; Chen, H.; Xiao, J.; Wu, W. Intersegmental plane simulation based on the bronchus-vein-artery triad in pulmonary segmentectomy. Transl. Cancer Res. 2021, 10, 4702–4713. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, S.; Nagashima, T.; Kawatani, N.; Gedeon, P.C.; DeSimone, A.K.; Igai, H.; Kosaka, T.; Shirabe, K. Anatomy of the lung revisited by 3D-CT imaging. Video Assist. Thorac. Surg. 2023, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, W.J.; Wu, Q.C.; Ge, M.J. Boyden’s triad in the left lung: An interesting phenomenon. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac082. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, H.; Bian, C.; Chen, Z.; Huang, J.; Xia, Y.; Wu, W.; Zhu, Q.; Yuan, M.; Chen, L. A modified system for classifying the bilateral superior pulmonary veins using three-dimensional computed tomography bronchography and angiography images. J. Thorac. Dis. 2021, 13, 5933–5941. [Google Scholar] [CrossRef] [PubMed]
- Sugai, K.; Sekine, Y.; Kawamura, T.; Yanagihara, T.; Saeki, Y.; Kitazawa, S.; Kobayashi, N.; Kikuchi, S.; Goto, Y.; Ichimura, H.; et al. Sphericity of lymph nodes using 3D-CT predicts metastasis in lung cancer patients. Cancer Imaging 2023, 34, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Chu, X.P.; Zhang, J.T.; Fu, R.; Kang, J.; Chen, J.H.; Jiang, B.Y.; Wu, Y.L.; Zhong, W.Z.; Nie, Q. the regularity of anatomical variations of dominant pulmonary segments in the right upper lobe. Thorac. Cancer 2023, 14, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Mimae, T.; Miyata, Y.; Kumada, T.; Tsutani, Y.; Okada, M. The intersegmental pulmonary vein is not always located on the intersegmental plane of the lung: Evaluation with 3-dimensional volume-rendering image reconstruction. JTCVS Tech. 2022, 16, 132–138. [Google Scholar] [CrossRef]
- Shibazaki, T.; Mori, S.; Noda, Y.; Tsukamoto, Y.; Kato, D.; Nakada, T.; Yabe, M.; Matsudaira, H.; Hirano, J.; Ohtsuka, T. Effect of resected lung lobe on the prediction of postoperative pulmonary function. Eur. J. Cardiothorac. Surg. 2022, 62, ezac480. [Google Scholar] [CrossRef]
- Matsuoka, S.; Eguchi, T.; Takeda, T.; Miura, K.; Hamanaka, K.; Shimizu, K. Three-dimensional computed tomography and indocyanine green-guided technique for pulmonary sequestration surgery. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Mun, M.; Okumura, S.; Nakao, M.; Matsuura, Y.; Nakagawa, K. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J. Vis. Surg. 2017, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Okamoto, T.; Tokunaga, Y.; Nakano, T. Intraoperative computed tomography navigation during thoracoscopic segmentectomy for small-sized lung tumors. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Yokomise, H.; Yokota, N.; Yoshida, C.; Katoh, A.; Misaki, N.; Go, T. Dual image navigation to secure surgical margins in thoracoscopic segmentectomy. Ann. Surg. Oncol. 2023, 30, 843–849. [Google Scholar] [CrossRef]
- Nakamura, S.; Hayashi, Y.; Kawaguchi, K.; Fukui, T.; Hakiri, S.; Ozeki, N.; Mori, S.; Goto, M.; Mori, K.; Yokoi, K. Clinical application of a surgical navigation system based on virtual thoracoscopy for lung cancer patients: Real time visualization of area of lung cancer before induction therapy and optimal resection line for obtaining a safe surgical margin during surgery. J. Thorac. Dis. 2020, 12, 672–679. [Google Scholar] [PubMed]
- Young, J.S.; McAllister, M.; Marshall, M.B. Three-dimensional technologies in chest wall resection and reconstruction. J. Surg. Oncol. 2023, 127, 336–342. [Google Scholar] [CrossRef]
- Pontiki, A.A.; Natarajan, S.; Parker, F.N.H.; Mukhammadaminov, A.; Dibblin, C.; Housden, R.; Benedetti, G.; Rhode, K.; Bille, A. Chest wall reconstruction using 3-Dimensiona; printing: Functional and mechanical results. Ann. Thorac. Surg. 2022, 114, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.; Bizekis, C.; Dellis, S.L.; Donington, J.S.; Oliker, A.; Balsam, L.B.; Zervos, M.; Galloway, A.C.; Pass, H.; Grossi, E.A. Simulating video-assisted thoracoscopic lobectomy: A virtual reality cognitive task simulation. J. Thorac. Cardiovasc. Surg. 2011, 141, 249–255. [Google Scholar] [CrossRef]
- Jensen, K.; Bjerrum, F.; Hansen, H.J.; Petersen, R.H.; Pedersen, J.K.; Konge, L. Using virtual reality simulation to assess competence in video-assisted thoracoscopic surgery (VATS) lobectomy. Surg. Endosc. 2017, 31, 2520–2528. [Google Scholar] [CrossRef]
- Haidari, T.A.; Bjerrum, F.; Hansen, H.J.; Konge, L.; Petersen, R.H. Simulation-based VATS resection of the five lung lobes: A technical skills test. Surg. Endosc. 2022, 36, 1234–1242. [Google Scholar] [CrossRef]
- Bedetti, B.; Bertolaccini, L.; Patrini, D.; Schmidt, J.; Scarci, M. Virtual simulation and learning new skills in video-assisted thoracic surgery. Video-Assist. Thorac. Surg. 2018, 3, 35. [Google Scholar] [CrossRef]
- Han, K.N.; Kim, H.K.; Choi, Y.H. Application of a three-dimensional video system in the training for uniportal thoracoscopic surgery. J. Thorac. Dis. 2018, 10, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Cannone, G.; Verzeletti, V.; Busetto, A.; Lione, L.; Bonis, A.; Nicotra, S.; Rebusso, A.; Mammana, M.; Schiavon, M.; Dell’Amore, A.; et al. Three-dimensional imaging-guided lung anatomic segmentectomy: A single-center preliminary experiment. Medicina 2023, 59, 2079. [Google Scholar] [CrossRef] [PubMed]
- Moal, J.L.; Peillon, C.; Dacher, J.N.; Baste, J.M. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: A pilot study. J. Thorac. Dis. 2018, 10, 196–200. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, F.; Wang, P.Y.; Chen, P.; Li, W.W.L.; Perroni, G.; Liu, S.Y. Anatomical analysis of variations in the bronchus pattern of the left upper lobe using three-dimensional computed tomography angiography and bronchography. Ann. Transl. Med. 2022, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xu, W.Z.; Li, Z.H.; Chen, L. Analysis of bronchial and vascular patterns in left upper lobes to explore the genesis of mediastinal lingular artery and its influence on pulmonary anatomical variation. J. Cardiothorac. Surg. 2021, 16, 306. [Google Scholar] [CrossRef] [PubMed]
- Nia, P.S.; Olsthoorn, J.R.; Heuts, S.; Maessen, J.G. Interactive 3D reconstruction of pulmonary anatomy for preoperative planning, virtual simulation, and intraoperative guiding in video-assisted thoracoscopic lung surgery. Innovations 2019, 14, 17–26. [Google Scholar]
- Liu, X.; Zhao, Y.; Xuan, Y.; Lan, X.; Zhao, J.; Lan, X.; Han, B.; Jiao, W. Three-dimensional printing in the preoperative planning of thoracoscopic pulmonary segmentectomy. Transl. Lung Cancer Res. 2019, 8, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Ji, Y.; He, H.; Zhao, J.; Xue, Q.; Gao, S. Three-dimensional reconstruction/personalized three-dimensional printed model for thoracoscopic anatomical partial-lobectomy in stage I lung cancer: A retrospective study. Transl. Lung Cancer Res. 2020, 9, 1235–1246. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Chen, Q.; Li, T.; Chen, K.; Yu, Q.; Lin, X. Three-dimensional printing technology for localized thoracoscopic segmental resection for lung cancer: A quasi-randomised clinical trial. World J. Surg. Oncol. 2020, 18, 223. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, K.; Han, X.; Zhao, J.; Wang, G.; Yuan, S.; He, B. Three-dimensional computed tomography angiography and bronchography combined with three-dimensional printing for thoracoscopic pulmonary segmentectomy in stage IA non-small cell lung cancer. J. Thorac. Dis. 2021, 13, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Tongxin, L.; Jing, X.; Runyuan, W.; Wei, W.; Yu, Z.; Dong, W.; Wang, H.; Yi, W.; Ping, H.; Yong, F. Application research of three-dimensional printing technology and three-dimensional computed tomography in segmentectomy. Front. Surg. 2022, 9, 881076. [Google Scholar] [CrossRef]
- Chen, F.; Miyamoto, E.; Takemoto, M.; Minakata, K.; Yamada, T.; Sato, M.; Aoyama, A.; Date, H. Right and Left Inverted Lobar Lung Transplantation. Am. J. Transplant. 2015, 15, 1716–1721. [Google Scholar] [CrossRef]
- Hong, D.; Kim, H.; Kim, T.; Kim, Y.H.; Kim, N. Development of patient specific, realistic, and reusable video assisted thoracoscopic surgery using 3D printing and pediatric computed tomography images. Sci. Rep. 2021, 11, 6191. [Google Scholar] [CrossRef]
- Kanzaki, M.; Isaka, T.; Kikkawa, T.; Sakamoto, K.; Yoshiya, T.; Mitsuboshi, S.; Oyama, K.; Murasugi, M.; Onuki, T. Binocular stereo-navigation for three-dimensional thoracoscopic lung resection. BMC Surg. 2015, 15, 56. [Google Scholar] [CrossRef]
- Ujiie, H.; Yamaguchi, A.; Gregor, A.; Chan, H.; Kato, T.; Hida, Y.; Kaga, K.; Wakasa, S.; Eitel, C.; Clapp, T.R.; et al. Developing virtual reality simulation system for preoperative planning of thoracoscopic thoracic surgery. J. Thorac. Dis. 2021, 13, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, S.; Hanawa, R.; Nagashima, T.; Shimizu, K.; Yajima, T.; Shirabe, K. Segmentectomy guided by 3-dimensional images reconstructed from nonenhanced computed tomographic data. Ann. Thorac. Surg. 2021, 111, e301–e304. [Google Scholar] [CrossRef]
- Nakao, M.; Omura, K.; Hashimoto, K.; Ichinose, J.; Matsuura, Y.; Okumura, S.; Mun, M. Three-dimensional image simulation for lung segmentectomy from unenhanced computed tomography data. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Qi, Q.; Zhang, K.; Sui, X.; Wang, X.; Weng, W.; Wang, S.; Zhao, H.; Sun, C.; et al. A fully automated noncontrast CT 3-D reconstruction algorithm enabled accurate anatomical demonstration for lung segmentectomy. Thorac. Cancer 2022, 13, 795–803. [Google Scholar] [CrossRef]
- Jensen, K.; Bjerrum, F.; Hansen, H.J.; Petersen, R.H.; Pedersen, J.H.; Konge, L. A new possibility in thoracoscopic virtual reality simulation training: Development and testing of a novel virtual reality simulator for video-assisted thoracoscopic surgery lobectomy. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 420–426. [Google Scholar] [CrossRef]
- Tokuno, J.; Chen-Yoshikawa, T.F.; Nakao, M.; Matsuda, T.; Date, H. Resection Process Map: A novel dynamic simulation system for pulmonary resection. J. Thorac. Cardiovasc. Surg. 2020, 159, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Oda, Y.; Taura, K.; Minato, K. Direct volume manipulation for visualizing intraoperative liver resection process. Comput. Methods Programs Biomed. 2014, 113, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, J.; Chen-Yoshikawa, T.F.; Nakao, M.; Iwakura, M.; Motoki, T.; Matsuda, T.; Date, H. Creation of a video library for education and virtual simulation of anatomical lung resection. Interact. Thorac. Cardiovasc. Surg. 2022, 34, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Kadomatsu, Y.; Nakao, M.; Ueno, H.; Nakamura, S.; Fukumoto, K.; Chen-Yoshikawa, T.F. Clinical application of resection process map as a novel surgical guide in thoracic surgery. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad059. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Tokuno, J.; Chen-Yoshikawa, T.; Date, H.; Matsuda, T. Surface deformation analysis of collapsed lungs using model-based shape matching. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, H.; Nakao, M.; Mineura, K.; Chen-Yoshikawa, T.F.; Matsuda, T. Model-based registration for pneumothorax deformation analysis using intraoperative cone-beam CT images. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 5818–5821. [Google Scholar]
- Okado, S.; Kadomatsu, Y.; Nakao, M.; Ueno, H.; Fukumoto, K.; Nakamura, S.; Chen-Yoshikawa, T.F. New method for delineation of the intersegmental line in a deflated lung. J. Thorac. Dis. 2023, 15, 4736–4744. [Google Scholar] [CrossRef]
- Hofman, J.; Backer, P.D.; Manghi, I.; Simoens, J.; Groote, R.D.; Bossche, H.V.D.; D’Hondt, M.; Oosterlinck, T.; Lippens, J.; Praet, C.V. First-in-human real-time AI-assisted instrument deocclusion during augmented reality robotic surgery. Healthc. Technol. Lett. 2023, 11, 33–39. [Google Scholar] [CrossRef]
- Sadeghi, A.H.; Mank, Q.; Tuzcu, A.S.; Hofman, J.; Siregar, S.; Maat, A.; Mottrie, A.; Kluin, J.; Backer, P.D. Artificial intelligence-assisted augmented reality robotic lung surgery; navigating the future of thoracic surgery. JTCVS Tech. 2024; in press. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen-Yoshikawa, T.F. Evolution of Three-Dimensional Computed Tomography Imaging in Thoracic Surgery. Cancers 2024, 16, 2161. https://doi.org/10.3390/cancers16112161
Chen-Yoshikawa TF. Evolution of Three-Dimensional Computed Tomography Imaging in Thoracic Surgery. Cancers. 2024; 16(11):2161. https://doi.org/10.3390/cancers16112161
Chicago/Turabian StyleChen-Yoshikawa, Toyofumi Fengshi. 2024. "Evolution of Three-Dimensional Computed Tomography Imaging in Thoracic Surgery" Cancers 16, no. 11: 2161. https://doi.org/10.3390/cancers16112161
APA StyleChen-Yoshikawa, T. F. (2024). Evolution of Three-Dimensional Computed Tomography Imaging in Thoracic Surgery. Cancers, 16(11), 2161. https://doi.org/10.3390/cancers16112161






