Simple Summary
Breast cancer continues to represent a leading cause of death among women globally. Recent studies have shown a growing link between breast cancer and the living microorganisms from the tumoral breast tissue, known as microbiota. This review looks at how the microbiota is found in healthy breast tissue and the changes observed during cancer development and progression. The microbiota has been shown to affect cancer growth, spread, and resistance to treatments by interacting with the tumor’s environment. Moreover, this review explores how different breast cancer types have distinct microbial profiles. Future studies could improve breast cancer care by endowing the microbiota as a diagnostic, prediction and treatment marker.
Abstract
Breast cancer remains one of the leading causes of death among women worldwide, and recent research highlights its growing connection to alterations in the microbiota. This review delves into the intricate relationship between microbiotas and breast cancer, exploring its presence in healthy breast tissue, its changes during cancer progression, and its considerable impact on both the tumor microenvironment (TME) and the tumor immune microenvironment (TIME). We extensively analyze how the microbiota influences cancer growth, invasion, metastasis, resistance to drugs, and the evasion of the immune system, with a special focus on its effects on the TIME. Furthermore, we investigate distinct microbial profiles associated with the four primary molecular subtypes of breast cancer, examining how the microbiota in tumor tissues compares with that in adjacent normal tissues. Emerging studies suggest that microbiotas could serve as valuable diagnostic and prognostic biomarkers, as well as targets for therapy. This review emphasizes the urgent need for further research to improve strategies for breast cancer prevention, diagnosis, and treatment. By offering a detailed examination of the microbiota’s critical role in breast cancer, this review aims to foster the development of novel microbiota-based approaches for managing the disease.
1. Introduction
Breast cancer is of great interest to many physicians, surgeons, and pathologists because it has the highest prevalence among the site-specific and gender-specific cancers in women. Recent data has shown that breast cancer malignancy exceeds that of lung cancer regarding incidence in 2020; therefore, it is the main cause of cancer worldwide [1].
The majority of malignant breast tumors are adenocarcinomas, and their origin is in the terminal duct lobular unit (TDLU). Carcinoma of the breast is classified as ductal or lobular based on the anatomical and histological structure from which the malignancy arises [2]. When referring to tumoral behavior, both ductal and lobular carcinomas can be defined as in situ carcinoma or invasive carcinoma. Invasive carcinomas have metastatic potential, and they encompass invasive breast carcinoma of no special type (IDC in the past), invasive lobular carcinoma (ILC), tubular carcinoma, mucinous carcinoma, invasive micropapillary carcinoma, and many more [3]. Invasive breast carcinoma of no special type (IDC-NST) is the most frequent form of invasive breast cancer (70–80% of all invasive breast cancers), followed by ILC (which accounts for 10% of all invasive malignancies of the breast) [2,4,5].
The molecular classification of breast cancer and more specifically the immunohistochemical-based substitute molecular classification has been supported because it offers guidance to the physician in selecting the best treatment. It is a major prognostic and predictive role in the tumoral response to hormonal therapy [6]. ER, PR, HER2, and Ki-67 proteins are IHC markers that are used for the identification of the following molecular subtypes of breast cancer: luminal A (ER+, PR ≥ 20%, HER2−, Ki-67 low), luminal B (ER+, PR < 20% and/ or HER2+ and/or Ki-67 high), HER2-overexpression (ER−, PR−, HER2+), and triple-negative breast cancer (ER−, PR−, HER2−). The most usual molecular subtype of breast cancer is represented by the luminal A subtype (which accounts for 40–50% of all invasive breast cancers) and has the best outcome among all molecular subtypes [5,7,8].
A variety of risk factors are associated with the development of breast cancer, such as the early onset of puberty, early menarche, late age at first pregnancy, lack of breastfeeding, late onset of menopause, familial history of breast malignancy (linked to pathogenic variants especially in high-penetrance genes, most frequently BRCA1, BRCA2, and PALB2), alcohol consumption, obesity, dietary fat intake, hormone replacement therapy, the intake of contraceptives containing estrogen and progesterone, and environmental factors [9,10,11,12,13]. All the risk factors mentioned above are well-known and thoroughly studied; though, not all cases of breast malignancies can be explained through these factors.
Recent studies have highlighted a connection between the local microbiota of the breast and the occurrence of malignant breast tumors, suggesting that the population of bacteria colonizing the mammary gland might represent a new risk factor for breast cancer. Previously, it was thought that breast tissue had no microbial colonization, but now it is understood that the mammary gland has a unique microbiome. The rich adipose tissue, the abundant blood supply, and lymphatic drainage of the breast makes it a perfect environment for the proliferation of microorganisms [14,15,16].
In the same line, an association has been observed between the specific microbial pattern of the four breast cancer subtypes and the outcome of the malignant disease [17]. Furthermore, the intratumoral microbiota has a crucial role in promoting the spreading and the endurance of tumor cells, encouraging metastatic colonization of different organs in breast cancer [18,19]. The changes and the imbalance occurring in the local microbiota when breast cancer develops were also analyzed. It was found that a certain type of bacteria (Sphingomonas yanoikuyae) is predominant in normal breast tissue (surrounding the tumoral tissue). Another type of bacteria (Methylobacterium radiotolerans) is most prevalent in the breast tissue with malignant transformation. The dysbiosis in the microbiota is explained by the fact that the amount of S. yanoikuyae decreases considerably in breast cancer while the amount of M. radiotolerans remains the same [20]. These findings emphasize the importance of the microbiome and microbiota as potential diagnostic markers for malignant breast disease.
All the aspects mentioned above confirm that the microbiota has a significant role in breast cancer regarding risk, diagnosis, therapy, and outcome. It is of vital importance to research and comprehend new associations between microbiotas and breast cancer in order to improve diagnostic and therapeutic algorithms. The aim of this review is to emphasize the involvement of microbiotas in the aforementioned aspects of breast cancer.
Key findings:
- The microbiota has a significant role in breast cancer, regarding risk, diagnosis, therapy, and outcome;
- The microbiota modulates the TME and TIME, which have a major influence on malignant cell viability, cancer proliferation, and invasion;
- Breast tissue has a unique microbiota (in terms of healthy or tumoral tissue), and shifts in bacterial taxa and metabolic reprogramming of the microbiota are preliminary events in malignancy development;
- The composition of the breast tissue resident microbiota changes dramatically as the breast tissue begins to suffer malignant transformation, holding specific microbial patterns;
- Breast-cancer-tissue-associated microbiotas may provide the role of a biomarker or therapeutic target in the future.
2. Materials and Methods
Literature research was conducted using PubMed and Google Scholar databases from 2014 onwards. The keywords used for database interrogations were as follows: microbiota; breast cancer; tumor microenvironment (TME); bacterial taxa; metabolic reprogramming of microbiota; host–microbiota interactions; microbial signatures; microbial dysbiosis; cancer proliferation; cancer metastasis; drug resistance; immune escape; diagnostic biomarkers; prognostic biomarkers; therapeutic targets; immunotherapy. A total of 6439 publications were found. The search results were reviewed, with the inclusion criteria of studies placing emphasis on breast cancer tissue resident microbiotas, microbial dysbiosis in breast cancer, specific microbial signatures in breast cancer, a correlation between breast microbiotas and cancer development, and the potential of microbiotas as diagnostic and prognostic biomarkers in breast cancer. Three of the authors independently selected potential articles for inclusion based on the title, with further refinement of the selection process based on abstract review. Following the PRISMA guidelines, after the exclusion of duplicates, the abstract review for suitability, and finally the full-text analysis of the remaining papers, a total number of 35 articles were selected for inclusion in the present review, as can be observed in the flow diagram (Figure 1). This study was not registered.
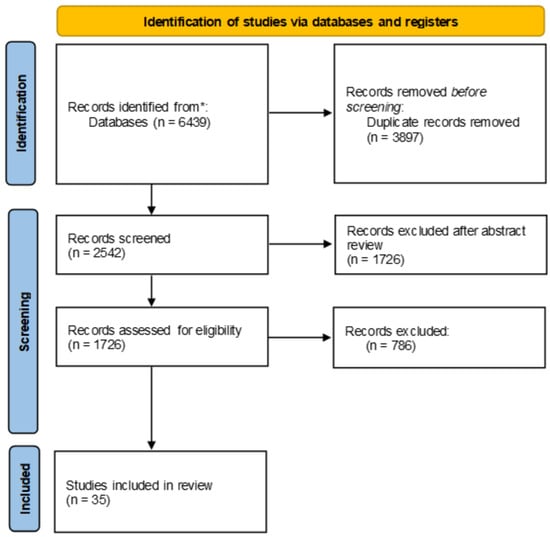
Figure 1.
PRISMA flow diagram of article selection process.
3. The Connection between Microbiota and Cancer
3.1. Relationship between Microbiota and Human Health
The interactions between microbiotas and human organisms are utterly important for the maturation of the immune system. It is acknowledged that any imbalance in the microbiota can lead to conditions mediated by an abnormal host immune response: allergic and autoimmune disorders, and metabolic and chronic inflammatory diseases, including various cancer type [21]. The influence of dysbiosis in the microbiota towards immune-mediated disorders, including cancer, surpasses the organs commonly colonized by extensive microbial communities (such as gastrointestinal tract, skin, mucosal tissue, and the respiratory tract) and includes conditions localized in non-barrier tissues or with less microbial colonization [21,22,23].
Gut microbiotas have significant roles in the human body, influencing the maturation of the immune system and cellular homeostasis, modulating neurologic signaling and host–cell multiplication, maintaining a barrier against pathogens, regulating the endocrine functions of the gut, participating in the synthesis or degradation of many substances (for instance, the synthesis of vitamin K2, the metabolism of bile salts, dietary constituents, medications, etc.) [24,25,26,27,28]. Disturbances in the human host–microbiota relationship in the gastrointestinal tract can lead to exaggerated immune responses against the gut microbiota, promoting the pathogenesis of IBD (inflammatory bowel disease), and the imbalance in the gut microbiota between commensal microorganisms and opportunistic pathogens is associated with colorectal cancer (CRC) [21,29]. These findings highlight a strong relationship between the microbiota and human health.
3.2. Impact of the Microbiota on the Tumor Microenvironment (TME) and Tumor Immune Microenvironment (TIME)
The tumor microenvironment (TME) has a major influence on malignant cell viability, cancer proliferation, and invasion [30]. The TME incorporates cellular and non-cellular components. The cellular components of TME are cancer cells, cells of the innate immune system (e.g., neutrophils, macrophages, NK cells, dendritic, and myeloid-derived suppressor cells), lymphocytes B and T, stromal cells (e.g., fibroblasts and adipocytes), and endothelial cells [30,31]. The extracellular matrix (ECM) represents a non-cellular constituent of the TME, which integrates molecules like collagen, laminin, hyaluronan, fibronectin, and elastin. The TME also includes soluble factors (e.g., cytokines, hormones, chemokines, growth factors, matrix remodeling enzymes, and mediators of inflammation), which enable cellular interactions. Other ways of intercellular communication in the TME include exosomes, apoptotic bodies, circulating tumor cells, and cell-free DNA [30,31,32,33,34]. The TME is shaped by the microbiota-derived metabolites, and the intercellular communication within TME is influenced by the microbiota, as it modulates the main functions in carcinogenesis: inflammation, proliferation, angiogenesis, the initiation of the epithelial–mesenchymal transition, invasion, metastasis, and immune escape [30,35]. In a study conducted by Xuan et al., the composition of the microbiota in malignant tissue and in paired normal tissue adjacent to the tumor within samples from 20 patients diagnosed with ER-positive breast cancer was analyzed and compared (both qualitatively and quantitatively). Next-generation sequencing techniques were used in order to complete a high-resolution scrutiny of the microbiota in tumor and paired normal tissue samples [20]. From a qualitative perspective, Sphingomonas yanoikuyae prevailed in paired normal tissue, as it was detected in 95% of samples. On the other hand, Methylobacterium radiotolerans prevailed (as it was detected in 100% of samples) in the malignant breast tissue [20,30]. From a quantitative perspective, the levels of S. yanoikuyae were considerably elevated in the paired normal tissue, as it was not found in the tumor tissue samples. M. radiotolerans was found in both the paired normal and malignant tissue samples, but between the absolute levels in these types of samples, a significant difference was not demonstrated. In the tumor tissue samples, the relative predominance of M. radiotolerans is explained by a reduction in other bacterial species. In the malignant tissue, compared to the paired normal tissue, the level of S. yanoikuyae decreases notably as the quantity of M. radiotolerans remains at a constant value. An obvious connection between the TME and microbiota is highlighted by the fact that the bacterium Sphingomonas yanoikuyae expresses certain glycosphingolipid ligands for invariant natural killer T cells. The glycosphingolipids from S. yanoikuyae induce the activation of invariant natural killer T cells, as these lymphocytes have important roles in tumor rejection and immunological surveillance [20,30,36].
The tumor immune microenvironment (TIME) encompasses a variety of cellular components and substances, such as malignant cells, immune cells, enzymes, and cytokines. These components are either anti-tumor factors or pro-tumor factors. Tumors have the ability to escape from immune surveillance by promoting an immunosuppressive tumor microenvironment and encouraging cancer progression. The anti-tumor immune response is driven mainly by T lymphocytes, such as cytotoxic T lymphocytes (CTL) and T helper cells [37]. By understanding the complex process of eluding the immune system by tumors, various antibody-based immunotherapies have been discovered in order to reset the TIME and undermine malignant cells. For instance, immune checkpoint inhibitors act by blocking CTLA-4 and PD-1/PD-L1, thereby diminishing the functional inhibition of T lymphocytes [37,38,39]. It was discovered that triple-negative breast cancer (TNBC) expresses an increased level of PD-L1, leading to the use of a PD-1 immune checkpoint blockade for treating TNBC [40,41]. Pembrolizumab was used in addition to neoadjuvant chemotherapy and also as an adjuvant treatment after performing breast surgery. Pembrolizumab-based therapy was applied to patients with incipient cancer, improving progression free survival and increasing the pathological complete response rate to more than 60% [40,42].
The TIME is strongly influenced by systemic patient-dependent factors such as age, gender, dietary habits, physical activity, adiposity, and the composition of microbiota. The inflammatory state of the patient can also shape the nature of the TIME in premalignant lesions; therefore, two scenarios are possible: supporting cancer proliferation and progression or promoting tumoral clearance by the immune system [38].
Certain mechanisms can describe the relationship between the microbiota and TIME [43]. One hypothesis is that microbial antigens can imitate tumor antigens. The microbial antigens can be presented to immune cells and tumor cells, and this process can activate a response from the immune system, as effector T cells can recognize and destroy the antigen-presenting cell. As a result of antigen similarity, T lymphocytes can identify malignant cells that present similar antigenic determinants and can eliminate them [43,44,45]. Another hypothesis is that microbial residents can interact with pattern recognition receptors (PRRs) and so modulate the TIME [43,46].
3.3. The Link between Microbiotas and Cancer Proliferation, Invasion, Metastasis, Drug Resistance, and Immune Escape
As exemplified before, breast microbiotas can have either a protective role against cancer proliferation or an inductive role in tumor cell multiplication [43]. In terms of the influence exerted by microbiotas on cancer metastasis, Fu et al. showed that malignant cells transported bacteria from the primary tumor to the site of the metastasis, as illustrated in Figure 2. Using a 16s rRNA sequencing technique, various tissues were analyzed: the primary breast tumor, normal breast tissue, normal lung tissue, metastasis-adjacent lung with early micro-metastasis, and lung macro-metastasis. It was observed that the breast tumor microbiota resembled the microbiota found in the metastasis-adjacent lung but was completely different from the one found in the normal breast tissue or in the normal lung tissue. The microbiota discovered in the lung macro-metastasis had characteristics from both breast tumor tissue and normal tissues. Further analysis revealed that aerobic types of bacteria were expanding in the lung metastasis, while the amount of facultative anaerobic microorganisms was diminishing [18].
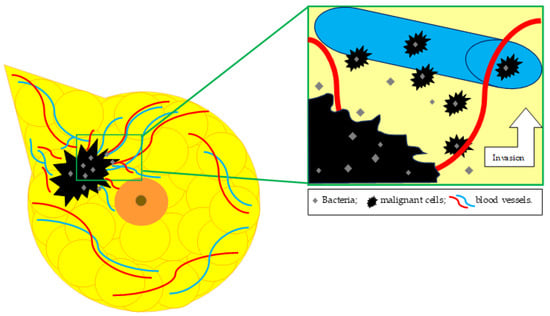
Figure 2.
Breast tumoral tissue microbiota in relation to metastatic spread.
The impact of microbiotas on drug resistance is of great interest, as this interaction between microbiotas and anticancer drugs modulates the immune system, influences side and adverse effects, and eventually can lead to drug resistance as the patient becomes unresponsive to conventional treatment. Rao Malla et al. reported that Doxorubicin is inactivated by streptomyces WAC04685 via a biochemical mechanism of deglycosylation [47,48]. The therapy with selective estrogen receptor modulators (SERMs), Tamoxifen and Raloxifene, used in hormone-receptor-positive breast cancer seemed to modulate the microbiota. It was demonstrated that SERMs exert a harmful effect on different bacteria such as Pseudomonas aeruginosa, Bacillus stearothermophilus, Klebsiella pneumoniae, Enterococcus faecium, and many more [47,49]. On the other hand, malignant cells can develop drug resistance to Tamoxifen, as this process is influenced by the microbiota. Gemcitabine, a chemotherapeutic agent used in many types of cancers (including breast cancer), loses its cytotoxic properties when it interacts with microorganisms from gammaproteobacteria class [47,50].
4. Microbiota in Healthy and Tumoral Breast Tissue
As stated before, breast tissue has a specific microbiota and it is no longer considered to be sterile, since the local tissular environment (abundant adiposity, rich vasculature, and lymphatic drainage) promotes bacterial growth [14]. In German et al., fresh frozen mammary tissue cores were obtained from 403 healthy (cancer-free) individuals and analyzed for microbial signatures [51]. In terms of family taxonomic rank, Acetobacterraceae (Proteobacteria phylum), Lactobacillaceae (Firmicutes phylum), and Xanthomonadaceae (Proteobacteria phylum) were the most abundant in normal breast tissue. The healthy mammary tissue is mostly colonized by the Acetobacter genus (Proteobacteria phylum) and Liquorilactobacillus genus (Firmicutes phylum) [51,52]. Vitorino et al. showed that healthy breast tissue is populated by the following bacterial genera: Actinobacteria, Lactococcus, Streptococcus, Prevotella, and Sphingomonas [43].
4.1. Changes in Bacterial Taxa
It was observed that the composition of microbiotas in patients with breast cancer can vary depending on the type of tissue invaded by the malignant cells. German et al. studied a cohort consisting of 76 breast cancer patients. The fresh frozen tissue cores from the 76 individuals with breast malignancies incorporated primary tumor tissue cores, normal tissue cores adjacent to the tumor mass, and distant-metastasis tissue cores [51].
In the normal breast tissue adjacent to the tumor, in terms of the bacterial family level, Cyanobacteria and Corynebacteriaceae were dominant compared to that in the healthy breast tissue. The Lactobacillaceae, Acetobacterraceae, and Xanthomonadaceae family showed a diminished presence in the normal breast tissue samples adjacent to the tumor compared to the healthy mammary tissue samples. The normal breast tissue adjacent to the tumor was intensely colonized by the Ralstonia genus (Proteobacteria phylum) in contrast with the normal breast tissue from cancer-free individuals. The Acetobacter genus and Liquorilactobacillus genus were significantly less abundant in the samples obtained from the normal tissue adjacent to the tumor in contrast to that in the samples obtained from healthy breast tissue [51,52].
In terms of the bacterial family taxonomic rank, the primary tumor tissue cores were enriched in Staphylococcaceae and Corynebacteriaceae in comparison with normal mammary tissue. Lactobacillaceae, Acetobacterraceae, and Xanthomonadaceae were in remarkably lower concentrations in the primary malignant tumor tissue. The Acetobacter genus and Liquorilactobacillus genus were almost absent in the majority of the primary tumor tissue samples. On the other hand, the Ralstonia genus was detected in strikingly high abundance in most of the tumor tissue cores when compared with the abundance in normal breast tissue cores [51].
Hoskinson et al. studied and compared the resident microbiotas of healthy breast tissue from cancer-free individuals with the resident microbiota of tissues collected before (prediagnostic) and after (adjacent normal and tumor tissue) breast cancer diagnosis. They highlighted the existence of a transitional taxonomic signature in the prediagnostic tissue compared to that of the healthy breast tissue. The microbiota found in the prediagnostic tissue was an intermediate compositional trademark, indicating the genesis of dysbiosis in the breast tissue prior to malignancy development. In the prediagnostic tissue were discovered several bacterial taxa with similarly higher abundances as in the adjacent normal or tumor tissue, indicating shifts in bacterial taxa (for instance, in Bacillaceae, Burkholderiaceae, Corynebacteriaceae, Enterobacteriaceae, Streptococcaceae, Staphylococcaceae, and Xanthobacteriaceae) [53].
The shifts in bacterial taxa and bacterial abundance and the metabolic reprogramming of the microbiota found in breast tissue are preliminary events in malignancy development [53,54]. In terms of metabolic reprogramming of the breast tissue resident microbiota, a downgrading in glutathione metabolism in the adjacent normal and tumor tissue compared to healthy breast tissue was noticed. Glutathione is an important antioxidant chemical compound found in the intracellular fluid, as it regulates cell differentiation, multiplication and apoptosis, and immune defense. Disruptions in glutathione metabolism are involved in tumor growth, development, progression, and therapeutic response [53,55].
4.2. Correlation between Host Transcriptome Profiling and Breast Microbiota in Precancerous Tissue
The host–microbiota interactions in breast cancer were highlighted by the correlations found between the host transcriptome and microbial taxa and genes. It was discovered that there was an inverse (negative) host–microbiota correlation pattern between a group of prediagnostic tissues and healthy breast tissues. Most of the correlations between microbial taxa and host transcriptomes and microbial KOs (KEGG orthologs) and host transcriptomes were positive in the prediagnostic tissue, but in the healthy breast tissue, most of these correlations were negative [53,56]. For instance, the CYP24A1 gene, a protein-encoding gene for the 24-hydroxylase enzyme, was one of the host genes positively correlated with microbial genes in prediagnostic tissue and negatively (inversely) linked with microbial genes in healthy mammary tissue [53]. The 24-hydroxylase enzyme is part of the cytochrome P450 family of enzymes [53,57]. These enzymes take part in the metabolic pathway of steroid hormones and xenobiotic substances (drugs, carcinogens, pollutants, and additives) [53,57]. It was observed that cytochrome p40 genes manifest an increased expression in breast malignancies, as we can notice a correlated bacterial response to a transforming tissue microenvironment in the prediagnostic tissue in the incipient stages of breast cancer development [53,57]. All in all, an inverse association between host gene expression and functions of the microbiota can be highlighted [53].
5. Microbial Patterns in Breast Cancer
As stated before, the composition of the breast tissue resident microbiota changes dramatically as the breast tissue begins to suffer malignant transformation. Healthy breast tissue hosts mainly three bacterial families: Acetobacterraceae, Lactobacillaceae, and Xanthomonadaceae. The precancerous and cancerous breast tissues (adjacent normal and tumor tissue) are colonized by Cyanobacteria, Corynebacteriaceae, and Staphylococcaceae, in terms of bacterial family taxonomic rank. Breast tissue collected from cancer-free individuals is populated by the Acetobacter genus and Liquorilactobacillus genus. On the other hand, the normal breast tissue adjacent to the tumor and the tumor tissue are inhabited by the Ralstonia genus. The microbiota of the breast tissue collected in a prediagnostic stage acquires a transitional taxonomic signature as shifts in bacterial taxa are made (e.g., Bacillaceae, Burkholderiaceae, Corynebacteriaceae, Enterobacteriaceae, Streptococcaceae, Staphylococcaceae, and Xanthobacteriaceae) [51,52,53] (Table 1).

Table 1.
Comparison between the composition of resident microbiota in normal breast tissue, normal breast tissue adjacent to the tumor, and tumor tissue.
In a study performed by Kartti et al., 94 fresh samples of tumor and adjacent normal tissue were collected from 47 patients [58]. The researchers investigated the breast cancer tissue samples by the four main molecular subtypes of breast cancer: luminal A, luminal B, HER2-overexpression, and triple-negative breast cancer [5,58]. The composition of the breast microbiota was investigated in tumor tissue and adjacent normal tissue. The main phyla in the two tissue groups are Proteobacteria (class Gammaproteobacteria), Firmicutes (class Bacilli), and Actinobacteria (class Actinobacteria) [15,20,58]. The normal breast tissue adjacent to the tumor was colonized in a higher proportion by microorganisms from the Gammaproteobacteria class (37.5%). In the tumor tissue, bacteria from the Bacilli class (18.8%) and Actinobacteria class (17.2%) were most abundant. It was found that in terms of family taxonomic rank, Moraxellaceae prevailed over other bacterial families (Micrococcaceae, Enterobacteriaceae, and Staphylococcaceae) in tumor tissue (19.67%) and adjacent normal tissue (22.32%). Psychrobacter, Streptococcus, Acinetobacter, and Corynebacterium were the main bacterial genera discovered in the tumor tissue of more than 80% of the individuals selected for this study. The Streptococcus, Rothia, and Staphylococcus genera were detected in a much higher proportion in tumor tissue compared to that in adjacent normal tissue (which was enriched with the Escherichia-shigella genus) [58].
The four main molecular subtypes of breast cancer were examined in terms of resident microbiota diversity. The Genus Alloiococcus was dominant in tumor tissue of luminal B subtype. In luminal A subtype, the genus Corynebacterium was plentiful in tumor tissue, while the Lawsonella genus colonized the adjacent normal tissue in a higher proportion. The Sporosarcina genus is more abundant in the adjacent normal tissue in the triple-negative breast cancer subtype (TNBC). Another disclosure related to TNBC is that the Sphingomonadaceae family was the main component of the microbiota in this type of breast malignancy [58]. Breast cancer tissue with HER2 overexpression is mostly colonized by the Thermus genus, which includes thermophilic bacteria [58,59].
6. Microbiota as a Potential Diagnostic and Prognostic Biomarker
The potential of breast microbiota as a biomarker can be exploited during the various stages of breast cancer development and management: before starting the treatment, in order to establish the molecular profile of the breast malignant tumor; in the course of the treatment, allowing changes in therapy when it becomes ineffective; and during disease progression, in order to determine the gained drug resistance of the tumor [60,61].
As a pretreatment biomarker, the breast microbiota would provide valuable information about tumor behavior and tumor response to different antineoplastic therapies. In order to include a biomarker into clinical practice, five stages should be reached: preclinical studies, clinical trials, retrospective, prospective, and control studies [60,62]. At the present time, studies concerning breast microbiotas as biomarkers are still in the preclinical stage; therefore, further research is required in this field [60].
Banerjee et al. analyzed the correlation between microbial signatures of each breast cancer subtype and clinical outcome. This study used 95–105 formalin-fixed paraffin-embedded samples for each breast cancer subtype, 20 paired control specimens, and 68 non-matched control samples obtained from breast reduction interventions [17].
The pan-pathogen microarray (PathoChip) was used to analyze tissue samples (tumor and control samples), highlighting a correlation between distinctive microbial colonization in different breast cancer subtypes and disease prognosis [17,63]. In triple-negative breast cancer tissue samples, higher average hybridization signals of various microorganisms, Bacillus, Mucor, Toxocara, Trichophyton, and Nodaviridae, could be seen that correlated with prolonged survival time. In the samples collected from “ER + breast cancer” (ER+ and/or PR+ and HER2 -) patients, the analysis performed showed higher average hybridization signals for Klebsiella, Stenotrophomonas, and Neodiplostomum. This finding was also correlated with extended disease-free time after treatment. For “triple positive” (ER+, PR+ and HER2 +) breast cancer tumor tissue samples, the examination revealed higher average hybridization signals for Orientia, Klebsiella, Fusobacterium, Azorhizobium, Yersinia, Arthroderma (which are all bacterial genera), Anelloviridae (a family of viruses), Angiostrongylus, and Toxocara (parasitic genera). The presence of these microorganisms in “triple positive” breast cancer tissue was strongly correlated with reduced disease-free time after treatment and reduced survival interval. In terms of HER2-overexpression breast cancer, an important correlation between a higher detection of certain microorganisms and a favorable disease prognosis was not found. Nevertheless, some data show that lower average hybridization signals for Pseudoterranova, Trichuris, Ancylostoma, and Issatchenkia were correlated with prolonged disease-free period after anticancer therapy [17,64].
Tzeng et al. performed a cross-sectional study which incorporated fresh-frozen breast tissue samples from 221 patients diagnosed with breast cancer and 87 patients without breast malignancy [65]. They described correlations between specific microbial signatures and prognostic features in breast cancer, such as stage, histologic grade, receptor status, and lymphovascular invasion [64,65,66,67,68]. It was found that Porphyromonas, Lacibacter, Fusobacterium, and Ezakiella genera were detected in a greater proportion in advanced stage tumors compared to lower stage tumors. They also identified correlations between specific bacterial taxa and markers of tumor metastatic potential. For instance, lymphovascular invasion and node-positive status in breast cancer were associated with a decreased presence of the Oblitimonas genus. Furthermore, lymphovascular invasion was positively correlated with the presence of the Lactobacillus genus and negatively correlated with the existence of the Alkanindiges genus. A node-positive status in breast cancer was positively correlated with the residence of bacteria from Acinetobacter and Bacteroides genera and negatively correlated with the presence of microorganisms from the Achromobacter genus [65] (Table 2).

Table 2.
Correlations between tumoral tissue microbiota and BC prognosis.
Microbiotas from breast tumoral tissue have the potential to complement existing diagnostic and therapeutic methods; however, current data, although promising, do not offer sufficient evidence with regard to the reliability of this method. As such, the application of the routine clinical use of microbiotas as biomarkers for breast cancer requires further research.
7. Emerging Role of Microbiota as Therapeutic Target
There is emerging evidence that breast cancer microbiotas can be modulated by antitumor therapy. In certain situations, patients diagnosed with breast cancer receive neoadjuvant chemotherapy (usually a combination of anthracycline, alkylating agents, and taxanes) in order to reduce the size of the tumor before surgery, so that breast-conserving interventions and limited axillary lymph node dissections are feasible [69,70,71]. Chiba et al. investigated if neoadjuvant chemotherapy modulates the tumor-resident microbiota in breast cancer. They collected snap-frozen tumor tissue from patients who received neoadjuvant chemotherapy and from patients who did not undergo prior treatment at the time of surgery. The researchers found that the administration of neoadjuvant chemotherapy diminishes the diversity of microbiota within tumor tissue. The analysis in terms of genus-level differences showed an increased abundance of Pseudomonas and a reduced abundance of Prevotella in tumor tissue collected from patients who underwent neoadjuvant chemotherapy [69].
Local microbiotas also influence the pathogenesis of hormone-receptor-positive breast cancer in certain ways, by modulating the TME and interfering with the malignant-cell-inner functions [72,73]. These events have an impact as well on the therapeutic efficacy of aromatase inhibitors and estrogen receptor antagonists used in the treatment of hormone-receptor-positive breast cancer [72,74]. As stated before, estrogen receptor antagonists exert toxic effects on various microorganisms related to breast cancer (Porphyromonas gingivaliis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, Streptococcus mutants, Bacillus stearothemphilus, and Enterococcus faecium) [47,49].
Immunotherapy has become an important constituent in the treatment protocol for many specific cancers, such as melanoma, renal cell carcinoma, non-small-cell lung cancer, gastric cancer, head and neck cancers, and even breast cancer [75].
The use of immune checkpoint inhibitors (ICIs) in the treatment of breast malignancies showed better results compared to conventional chemotherapy, in several clinical trials [76,77,78,79,80,81,82]. Immune checkpoint inhibitors have effectiveness mainly in the triple-negative breast cancer subtype. A phase II clinical trial (KEYNOTE-086) in which Pembrolizumab was used as a frontline treatment for metastatic TNBC revealed an overall response rate of 23%, a good safety profile, and antitumor effectiveness [83,84]. Currently, Pembrolizumab is used in HR (hormone receptor)-negative and HER2-negative breast cancers as a peri-operative therapy in association with neoadjuvant chemotherapy and as an adjuvant systemic therapy, in particular cases, with notable results [85,86,87,88].
It has been discovered that the gut microbiota can influence the potency and toxicity of anticancer treatments, including ICIs. In studies using murine models, a correlation was observed between the existence of certain bacteria within the gut microbiota and a positive response to immunotherapy. A more favorable response to PD-(L)1 inhibitors was seen in murine models colonized by certain bacterial species: Akkermansia muciniphila, Collinsella aerofaciens, Bifidobacterium longem, and Faecalibacterium prausnitzii [84,89,90,91,92].
Local breast tissue microbiotas and gut microbiotas exert great influence on breast cancer therapy (chemotherapy, hormonotherapy, immunotherapy, and radiotherapy) outcome. Also, a bidirectional interaction between malignant cells and anticancer therapies was observed on one hand and the microbiota on the other [93,94,95,96,97,98]. The estrobolome, which represents the bacterial genes whose products are involved in estrogen metabolism, may increase the risk of breast cancer and may fulfil the role of a biomarker or therapeutic target in the future [99,100,101,102].
8. Conclusions
Exploring microbiomes and microbiotas in the context of breast cancer is still a challenging task, but of tremendous importance. It was demonstrated that microbiotas have multiple roles, such as defining healthy and malignant transformed breast tissue, modulating the TME and TIME, or influencing the efficacy of anticancer therapies. There is a strong urge to elucidate the capacity of microbiotas as a large-scale diagnostic and prognostic biomarker in breast cancer. So far, important steps have been taken in this field, analyzing the multifaceted traits of the microbiota in breast malignancies. Still, studies implying the functions of microbiomes and microbiotas deserve more investigation, reflection, and awareness.
All in all, microbiotas have a great impact on every aspect regarding cancer, exerting influence on the TME, TIME, cancer growth, metastasis, and impacting the outcome of antitumor therapy.
Author Contributions
Methodology, O.C.N. and A.P.T.; validation, M.I. and A.P.T.; investigation, C.-X.-R.N. and M.I.; resources, C.-X.-R.N.; data curation, M.I.; writing—original draft preparation, C.-X.-R.N. and M.I.; writing—review and editing, C.-X.-R.N. and O.C.N.; supervision, O.C.N.; project administration, A.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge VICTOR BABES UNIVERSITY OF MEDICINE AND PHARMACY TIMIȘOARA for their support in covering the costs of publication for this research paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 7, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Makki, J. Diversity of breast carcinoma: Histological subtypes and clinical relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica 2020, 112, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.J. Overview of breast cancer. J. Am. Acad. Physician Assist. 2019, 32, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. Am. Soc. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- Voduc, K.D.; Cheang, M.C.U.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef]
- Bernard, P.S.; Parker, J.S.; Mullins, M.; Cheung, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P.; et al. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Majeed, W.; Aslam, B.; Javed, I.; Khaliq, T.; Muhammad, F.; Ali, A.; Raza, A. Breast cancer: Major risk factors and recent developments in treatment. Asian Pac. J. Cancer Prev. 2014, 15, 3353–3358. [Google Scholar] [CrossRef]
- Poorolajal, J.; Heidarimoghis, F.; Karami, M.; Cheraghi, Z.; Gohari-Ensaf, F.; Shahbazi, F.; Zareie, B.; Ameri, P.; Sahraei, F. Factors for the primary prevention of breast cancer: A meta-analysis of prospective cohort studies. J. Res. Health Sci. 2021, 21, e00520. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.; Vatten, L.J. Reproductive and hormonal risk factors of breast cancer: A historical perspective. Int. J. Women’s Health 2017, 9, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Eslami-S, Z.; Majidzadeh-A, K.; Halvaei, S.; Babapirali, F.; Esmaeili, R. Microbiome and Breast Cancer: New Role for an Ancient Population. Front. Oncol. 2020, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of human breast tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef]
- O’Connor, H.; MacSharry, J.; Bueso, Y.F.; Lindsay, S.; Kavanagh, E.L.; Tangney, M.; Clyne, M.; Saldova, R.; McCann, A. Resident bacteria in breast cancer tissue: Pathogenic agents or harmless commensals? Discov. Med. 2018, 26, 93–102. [Google Scholar] [PubMed]
- Banerjee, S.; Wei, Z.; Tian, T.; Bose, D.; Shih, N.N.C.; Feldman, M.D.; Khoury, T.; De Michele, A.; Robertson, E.S. Prognostic correlations with the microbiome of breast cancer subtypes. Cell Death Dis. 2021, 12, 831. [Google Scholar] [CrossRef]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y.; et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022, 185, 1356–1372. [Google Scholar] [CrossRef]
- Fu, A.; Yao, B.; Dong, T.; Cai, S. Emerging roles of intratumor microbiota in cancer metastasis. Trends Cell Biol. 2023, 33, 583–593. [Google Scholar] [CrossRef]
- Xuan, C.; Shamonki, J.M.; Chung, A.; DiNome, M.L.; Chung, M.; Sieling, P.A.; Lee, D.J. Microbial dysbiosis is associated with human breast cancer. PLoS ONE 2014, 9, e83744. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Dehner, C.; Fine, R.; Kriegel, M.A. The microbiome in systemic autoimmune disease: Mechanistic insights from recent studies. Curr. Opin. Rheumatol. 2019, 31, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ruff, W.E.; Kriegel, M.A. Autoimmune host–microbiota interactions at barrier sites and beyond. Trends Mol. Med. 2015, 21, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; van Mil, S.W.C.; Müller, M.; Kleerebezem, M.; van der Meer, R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y.; et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Devlin, A.S.; Fischbach, M.A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor microbiome—An integral part of the tumor microenvironment. Front. Oncol. 2022, 12, 1063100. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Pascut, D.; Pratama, M.Y.; Vo, N.V.T.; Masadah, R.; Tiribelli, C. The Crosstalk between Tumor Cells and the Microenvironment in Hepatocellular Carcinoma: The Role of Exosomal microRNAs and Their Clinical Implications. Cancers 2020, 12, 823. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Gao, W.; Chen, L.; Xu, W.; Zhu, X. Exosome-Mediated Crosstalk Between Tumor and Tumor-Associated Macrophages. Front. Mol. Biosci. 2021, 8, 764222. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Vergara, D.; Fanini, F.; Maffia, M.; Bravaccini, S.; Pirini, F. Microbiota-derived metabolites in tumor progression and metastasis. Int. J. Mol. Sci. 2020, 21, 5786. [Google Scholar] [CrossRef] [PubMed]
- Pilones, K.A.; Aryankalayil, J.; Babb, J.S.; Demaria, S. Invariant natural killer T cells regulate anti-tumor immunity by controlling the population of dendritic cells in tumor and draining lymph nodes. J. Immunother. Cancer 2014, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- LV, B.; Wang, Y.; Ma, D.; Cheng, W.; Liu, J.; Yong, T.; Chen, H.; Wang, C. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 844142. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- So, J.Y.; Ohm, J.; Lipkowitz, S.; Yang, L. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol. Ther. 2022, 237, 108253. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, M.; Alpuim Costa, D.; Vicente, R.; Caleça, T.; Santos, C. Local Breast Microbiota: A “New” Player on the Block. Cancers 2022, 14, 3811. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef]
- Liwinski, T.; Zheng, D.; Elinav, E. The microbiome and cytosolic innate immune receptors. Immunol. Rev. 2020, 297, 207–224. [Google Scholar] [CrossRef]
- Rao Malla, R.; Marni, R.; Kumari, S.; Chakraborty, A.; Lalitha, P. Microbiome Assisted Tumor Microenvironment: Emerging Target of Breast Cancer. Clin. Breast Cancer 2022, 22, 200–211. [Google Scholar] [CrossRef]
- Westman, E.L.; Canova, M.J.; Radhi, I.J.; Koteva, K.; Kireeva, I.; Waglechner, N.; Wright, G.D. Bacterial inactivation of the anticancer drug doxorubicin. Chem. Biol. 2012, 19, 1255–1264. [Google Scholar] [CrossRef]
- Bao, L.; Peng, R.; Ren, X.; Ma, R.; Li, J.; Wang, Y. Analysis of some common pathogens and their drug resistance to antibiotics. Pak. J. Med. Sci. 2013, 29, 135–139. [Google Scholar] [CrossRef]
- Sawasdee, N.; Thepmalee, C.; Sujjitjoon, J.; Yongpitakwattana, P.; Junking, M.; Poungvarin, N.; Yenchitsomanus, P.T.; Panya, A. Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int. Immunopharmacol. 2020, 78, 106006. [Google Scholar] [CrossRef]
- German, R.; Marino, N.; Hemmerich, C.; Podicheti, R.; Rusch, D.B.; Stiemsma, L.T.; Gao, H.; Xuei, X.; Rockey, P.; Storniolo, A.M.; et al. Exploring breast tissue microbial composition and the association with breast cancer risk factors. Breast Cancer Res. 2023, 25, 82. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Magno, S.; Albanese, D.; Donati, C.; Molinari, R.; Filippone, A.; Masetti, R.; Merendino, N. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci. Rep. 2018, 8, 16893. [Google Scholar] [CrossRef] [PubMed]
- Hoskinson, C.; Zheng, K.; Gabel, J.; Kump, A.; German, R.; Podicheti, R.; Marino, N.; Stiemsma, L.T. Composition and Functional Potential of the Human Mammary Microbiota Prior to and Following Breast Tumor Diagnosis. mSystems 2022, 7, e0148921. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.; Das, G.M. Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells 2019, 8, 89. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-culf, M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Vaclavikova, R.; Hubackova, M.; Stribrna-Sarmanova, J.; Kodet, R.; Mrhalova, M.; Novotny, J.; Gut, I.; Soucek, P. RNA expression of cytochrome P450 in breast cancer patients. Anticancer Res. 2007, 27, 4443–4450. [Google Scholar] [PubMed]
- Kartti, S.; Bendani, H.; Boumajdi, N.; Bouricha, E.M.; Zarrik, O.; ELAgouri, H.; Fokar, M.; Aghlallou, Y.; EL Jaoudi, R.; Belyamani, L.; et al. Metagenomics Analysis of Breast Microbiome Highlights the Abundance of Rothia Genus in Tumor Tissues. J. Pers. Med. 2023, 13, 450. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Dieleman, S.; Aarnoutse, R.; Ziemons, J.; Kooreman, L.; Boleij, A.; Smidt, M. Exploring the Potential of Breast Microbiota as Biomarker for Breast Cancer and Therapeutic Response. Am. J. Pathol. 2021, 191, 968–982. [Google Scholar] [CrossRef]
- Olson, E.M.; Lin, N.U.; Krop, I.E.; Winer, E.P. The ethical use of mandatory research biopsies. Nat. Rev. Clin. Oncol. 2011, 8, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.S.; Etzioni, R.; Feng, Z.; Potter, J.D.; Thompson, M.L.; Thornquist, M.; Winget, M.; Yasui, Y. Phases of biomarker development for early detection of cancer. J. Natl. Cancer Inst. 2001, 93, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.A.; Feldman, M.; Alwine, J.C.; Robertson, E.S. Metagenomic Assay for Identification of Microbial Pathogens in Tumor Tissues. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct Microbial Signatures Associated With Different Breast Cancer Types. Front. Microbiol. 2018, 9, 951. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C.; et al. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef]
- Wang, H.; Altemus, J.; Niazi, F.; Green, H.; Calhoun, B.C.; Sturgis, C.; Grobmyer, S.R.; Eng, C. Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget 2017, 8, 88122–88138. [Google Scholar] [CrossRef]
- Meng, S.; Chen, B.; Yang, J.; Wang, J.; Zhu, D.; Meng, Q.; Zhang, L. Study of Microbiomes in Aseptically Collected Samples of Human Breast Tissue Using Needle Biopsy and the Potential Role of in situ Tissue Microbiomes for Promoting Malignancy. Front. Oncol. 2018, 8, 318. [Google Scholar] [CrossRef]
- Smith, A.; Pierre, J.F.; Makowski, L.; Tolley, E.; Lyn-Cook, B.; Lu, L.; Vidal, G.; Starlard-Davenport, A. Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci. Rep. 2019, 9, 11940. [Google Scholar] [CrossRef]
- Chiba, A.; Bawaneh, A.; Velazquez, C.; Clear, K.Y.J.; Wilson, A.S.; Howard-McNatt, M.; Levine, E.A.; Levi-Polyachenko, N.; Yates-Alston, S.A.; Diggle, S.P.; et al. Neoadjuvant chemotherapy shifts breast tumor microbiota populations to regulate drug responsiveness and the development of metastasis. Mol. Cancer Res. 2020, 18, 130–139. [Google Scholar] [CrossRef]
- Rubovszky, G.; Horváth, Z. Recent Advances in the Neoadjuvant Treatment of Breast Cancer. J. Breast Cancer 2017, 20, 119. [Google Scholar] [CrossRef]
- Jones, S.E.; Savin, M.A.; Holmes, F.A.; O’Shaughnessy, J.A.; Blum, J.L.; Vukelja, S.; McIntyre, K.J.; Pippen, J.E.; Bordelon, J.H.; Kirby, R.; et al. Phase III Trial Comparing Doxorubicin Plus Cyclophosphamide With Docetaxel Plus Cyclophosphamide As Adjuvant Therapy for Operable Breast Cancer. J. Clin. Oncol. 2006, 24, 5381–5387. [Google Scholar] [CrossRef] [PubMed]
- Terrisse, S.; Zitvogel, L.; Kroemer, G. Impact of microbiota on breast cancer hormone therapy. Cell Stress 2023, 7, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; McQuade, J.L.; Merad, M.; André, F.; Zitvogel, L. Bodywide ecological interventions on cancer. Nat. Med. 2023, 29, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Chan, L.C.; Song, M.S.; Hung, M.C. New Approaches on Cancer Immunotherapy. Cold Spring Harb. Perspect. Med. 2020, 10, a036863. [Google Scholar] [CrossRef]
- Loibl, S.; Schneeweiss, A.; Huober, J.; Braun, M.; Rey, J.; Blohmer, J.U.; Furlanetto, J.; Zahm, D.M.; Hanusch, C.; Thomalla, J.; et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann. Oncol. 2022, 33, 1149–1158. [Google Scholar] [CrossRef]
- Pusztai, L.; Yau, C.; Wolf, D.M.; Han, H.S.; Du, L.; Wallace, A.M.; String-Reasor, E.; Boughey, J.C.; Chien, A.J.; Elias, A.D.; et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell 2021, 39, 989–998. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Saji, S.; Ohsumi, S.; Ito, M.; Hayashi, N.; Kobayashi, K.; Masuda, N.; Niikura, N.; Yamashita, T.; Kiyama, K.; Hasegawa, A.; et al. Subgroup analysis of Japanese patients in a phase III randomized, controlled study of neoadjuvant atezolizumab or placebo, combined with nab -paclitaxel and anthracycline-based chemotherapy in early triple-negative breast cancer (IMpassion031). Jpn. J. Clin. Oncol. 2022, 52, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, M.; Baptista de Almeida, S.; Alpuim Costa, D.; Faria, A.; Calhau, C.; Azambuja Braga, S. Human Microbiota and Immunotherapy in Breast Cancer—A Review of Recent Developments. Front. Oncol. 2021, 11, 815772. [Google Scholar] [CrossRef] [PubMed]
- Rashmi Kumar, N.; Schonfeld, R.; Gradishar, W.J.; Lurie, R.H.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; et al. NCCN Guidelines Version 2.2024 Breast Cancer [Internet]. 2024. Available online: https://www.nccn.org (accessed on 30 September 2024).
- O’Rourke, H.; Hart, C.; De Boer, R.H. Current usage of pembrolizumab in triple negative breast cancer (TNBC). Expert Rev. Anticancer Ther. 2024, 24, 253–261. [Google Scholar] [CrossRef]
- Takahashi, M.; Cortés, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Denkert, C.; Park, Y.H.; Im, S.A.; Ahn, J.H.; et al. Pembrolizumab Plus Chemotherapy Followed by Pembrolizumab in Patients With Early Triple-Negative Breast Cancer. JAMA Netw. Open 2023, 6, e2342107. [Google Scholar] [CrossRef]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.; McArthur, H.; Kümmel, S.; Bergh, J.; Park, Y.H.; Hui, R.; et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: Exploratory analysis from KEYNOTE-522. Ann. Oncol. 2024, 35, 429–436. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Alpuim Costa, D.; Nobre, J.G.; Batista, M.V.; Ribeiro, C.; Calle, C.; Cortes, A.; Marhold, M.; Negreiros, I.; Borralho, P.; Brito, M.; et al. Human Microbiota and Breast Cancer—Is There Any Relevant Link?—A Literature Review and New Horizons Toward Personalised Medicine. Front. Microbiol. 2021, 12, 584332. [Google Scholar] [CrossRef]
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, E.M.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. Microbiota–drug interactions: Impact on metabolism and efficacy of therapeutics. Maturitas 2018, 112, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Gately, S. Human Microbiota and Personalized Cancer Treatments: Role of Commensal Microbes in Treatment Outcomes for cancer patients. In Precision Medicine in Cancer Therapy; Springer: Cham, Switzerland, 2019; Volume 178, pp. 253–264. [Google Scholar] [CrossRef]
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.B.; Bose, S.; et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 2021, 39, 1202–1213.e6. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mao, Q.; Xia, W.; Dong, G.; Yu, C.; Jiang, F. Gut Microbiota Shapes the Efficiency of Cancer Therapy. Front. Microbiol. 2019, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Nayak, N.; Rathi, P.; Verma, D.; Sharma, R.; Chaudhary, A.; Agarwal, A.; Tripathi, Y.B.; Garg, N. Microbiome and host crosstalk: A new paradigm to cancer therapy. Semin. Cancer Biol. 2021, 70, 71–84. [Google Scholar] [CrossRef]
- Fernández-Murga, M.L.; Gil-Ortiz, F.; Serrano-García, L.; Llombart-Cussac, A. A New Paradigm in the Relationship between Gut Microbiota and Breast Cancer: β-glucuronidase Enzyme Identified as Potential Therapeutic Target. Pathogens 2023, 12, 1086. [Google Scholar] [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor–Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar]
- Filippone, A.; Rossi, C.; Rossi, M.M.; Di Micco, A.; Maggiore, C.; Forcina, L.; Natale, M.; Costantini, L.; Merendino, N.; Di Leone, A.; et al. Endocrine Disruptors in Food, Estrobolome and Breast Cancer. J. Clin. Med. 2023, 12, 3158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).