Differences in Breast Cancer Subtypes among Racial/Ethnic Groups
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Troester, M.A.; Sun, X.; Allott, E.H.; Geradts, J.; Cohen, S.M.; Tse, C.K.; Kirk, E.L.; Thorne, L.B.; Mathews, M.; Perou, C.M.; et al. Racial Differences in PAM50 Subtypes in the Carolina Breast Cancer Study. J. Natl. Cancer Inst. 2018, 110, 176–182. [Google Scholar] [CrossRef]
- Loo, L.W.M.; Williams, M.; Hernandez, B.Y.; Rhee, C.S.; Castillo, E.; Maskarinec, G.; Shvetsov, Y.B.; Le Marchand, L.; Wilkens, L.; Kolonel, L.N. The high and heterogeneous burden of breast cancer in Hawaii: A unique multiethnic U.S. Population. Cancer Epidemiol. 2019, 58, 71–76. [Google Scholar] [CrossRef]
- Shoemaker, M.L.; White, M.C.; Wu, M.; Weir, H.K.; Romieu, I.; Chen, V.W.; Anderson, R.N.; Singh, S.D.; Pulte, D.; Yabroff, K.R. Differences in breast cancer incidence among young women aged 20–49 years by stage and tumor characteristics, age, race, and ethnicity, 2004–2013. Breast Cancer Res. Treat. 2018, 169, 595–606. [Google Scholar] [CrossRef]
- Sparano, J.A.; Brawley, O.W. Deconstructing Racial and Ethnic Disparities in Breast Cancer. JAMA Oncol. 2021, 7, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Heller, S.L. Health Disparity and Breast Cancer Outcomes in Asian Women. Radiographics 2022, 42, 1912–1924. [Google Scholar] [CrossRef] [PubMed]
- Fane, L.; Biswas, T.; Jindal, C.; Choi, Y.M.; Efird, J.T. Breast Cancer Disparities in Asian Women: The Need for Disaggregated Research. Int. J. Environ. Res. Public Health 2022, 19, 9790. [Google Scholar] [CrossRef]
- Conroy, S.M.; Maskarinec, G.; Wilkens, L.R.; White, K.K.; Henderson, B.E.; Kolonel, L.N. Obesity and breast cancer survival in ethnically diverse postmenopausal women: The Multiethnic Cohort Study. Breast Cancer Res. Treat. 2011, 129, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Shvetsov, Y.B.; Conroy, S.M.; Haiman, C.A.; Setiawan, V.W.; Le Marchand, L.; Park, S.Y.; Monroe, K.R.; Wilkens, L.R.; Henderson, B.E. Type 2 diabetes as a predictor of survival among breast cancer patients: The Multiethnic Cohort. Breast Cancer Res. Treat. 2019, 173, 637–645. [Google Scholar] [CrossRef]
- Stringer-Reasor, E.M.; Reid, S.D.; Hall, C.M.; Fraser, K.D.; Chisolm, D.J.; Sullivan, M.; Legendre, M.; McNulty, D.; Padmore, R.F.; Humphrey, J.L.; et al. Disparities in Breast Cancer Associated with African American Identity. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e29–e46. [Google Scholar] [CrossRef]
- Hawaii Tumor Registry, University of Hawaii Cancer Center. Hawaii Cancer at a Glance: 2012–2016; Hawaii Tumor Registry, University of Hawaii Cancer Center: Honolulu, HI, USA, 2020. [Google Scholar]
- Yu, A.Y.L.; Thomas, S.M.; DiLalla, G.D.; Greenup, R.A.; Hwang, E.S.; Hyslop, T.; Menendez, C.S.; Plichta, J.K.; Tolnitch, L.A.; Fayanju, O.M. Disease characteristics and mortality among Asian women with breast cancer. Cancer 2022, 128, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Ihenacho, U.; McKinley, M.A.; Vu, A.; Ding, J.; Park, Y.H.; Rhee, C.S.; Fujita, S.; Goyal, R.K.; Kwan, M.L.; Killeen, J.K.; et al. Characterizing breast cancer incidence and trends among Asian American, Native Hawaiian, and non-Hispanic White women in Hawai’i, 1990–2014. Cancer Causes Control 2023, 34, 241–249. [Google Scholar] [CrossRef]
- Kong, X.; Liu, Z.; Cheng, R.; Wang, M.; Zhao, H.; Lin, Y.; Huang, J.; Ma, J.; Li, C.; Fan, P.; et al. Variation in Breast Cancer Subtype Incidence and Distribution by Race/Ethnicity in the United States From 2010 to 2015. JAMA Netw. Open 2020, 3, e2020303. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Fidler-Benaoudia, M.M.; Jemal, A.; Siegel, R.L.; Hyuna, S.; Taylor, K.; et al. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Fong, M.; Henson, D.E.; Devesa, S.S.; Anderson, W.F. Inter- and intra-ethnic differences for female breast carcinoma incidence in the continental United States and in the state of Hawaii. Breast Cancer Res. Treat. 2006, 97, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Taparra, K.; Fukui, J.; Killeen, J.; Sumida, K.; Loo, L.W.M.; Hernandez, B.Y.; Williams, M.J.; Aflague, T.; Manog, S.; Chee, S.; et al. Racial and Ethnic Disparities in Rates of Invasive Second Breast Cancer among Women with Ductal Carcinoma In Situ in Hawaii. JAMA Netw. Open 2021, 4, e2128977. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Telli, M.L.; Chang, E.T.; Kurian, A.W.; Keegan, T.H.; McClure, L.A.; Shema, S.J.; Clarke, C.A.; Gomez, S.L.; Glaser, S.L.; Kwan, M.L.; et al. Asian ethnicity and breast cancer subtypes: A study from the California Cancer Registry. Breast Cancer Res. Treat. 2011, 127, 471–478. [Google Scholar] [CrossRef]
- Parise, C.; Caggiano, V. Disparities in the risk of the ER/PR/HER2 breast cancer subtypes among Asian Americans in California. Cancer Epidemiol. 2014, 38, 556–562. [Google Scholar] [CrossRef]
- Gomez, S.L.; Clarke, C.A.; Shema, S.J.; Chang, E.T.; Keegan, T.H.M.; Glaser, S.L.; Babcock, B.; Kwan, M.L.; Monroe, K.R.; Lee, M.M.; et al. Disparities in Breast Cancer Survival among Asian Women by Ethnicity and Immigrant Status: A Population-Based Study. Am. J. Public Health 2010, 100, 861–869. [Google Scholar] [CrossRef]
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A.; Warner, E.; Foulkes, W.D.; Kong, X.; Cheng, R.; Shao, Y.; et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015, 313, 2287. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Diamant, A.L.; Kagawa-Singer, M.; Pourat, N.; Wold, C. Disaggregating data on Asian and Pacific Islander women to assess cancer screening. Am. J. Prev. Med. 2004, 27, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Elewonibi, B.R.; Thierry, A.D.; Miranda, P.Y. Examining mammography use by breast cancer risk, race, nativity, and socioeconomic status. J. Immigr. Minor. Health 2019, 21, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.; Breen, N.; Liu, B.; Lee, R.; Kagawa-Singer, M. Asian American women in California: A pooled analysis of predictors for breast and cervical cancer screening. Am. J. Public Health 2015, 105, e98–e109. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Pagano, I.; Lurie, G.; Bantum, E.; Gotay, C.C.; Issell, B.F.; Stram, D.O.; Kolonel, L.N.; Monroe, K.R.; Le Marchand, L. Factors affecting survival among women with breast cancer in Hawaii. J. Womens Health 2011, 20, 231–237. [Google Scholar] [CrossRef]
- Gopalani, S.V.; Qin, J.; Baksa, J.; Ding, J.; Fujita, S.; Park, Y.H.; Kwan, M.L.; Killeen, J.K.; Choi, Y.M.; Killeen, M.L.; et al. Breast cancer incidence and stage at diagnosis in the six US-Affiliated Pacific Islands. Cancer Epidemiol. 2024, 92, 102611. [Google Scholar] [CrossRef]
- Goodman, M.J. Breast cancer in multi-ethnic populations: The Hawaii perspective. Breast Cancer Res. Treat. 1991, 18 (Suppl. S1), S5–S9. [Google Scholar] [CrossRef]
- Hirko, K.A.; Rocque, G.; Reasor, E.; Taye, A.; Daly, A.; Cutress, R.I.; Copson, E.R.; Lee, D.W.; Lee, K.H.; Im, S.A.; et al. The impact of race and ethnicity in breast cancer—Disparities and implications for precision oncology. BMC Med. 2022, 20, 72. [Google Scholar] [CrossRef]
- Sentell, T.; Dela Cruz, M.R.; Heo, H.H.; Braun, K.L. Health literacy, health communication challenges, and cancer screening among rural Native Hawaiian and Filipino women. J. Cancer Educ. 2013, 28, 325–334. [Google Scholar] [CrossRef]
- Aflague, T.F.; Hammond, K.; Delos Reyes, B.; Pangelinan, M.M.; Castro, T.; Aflague, J.; Mosier, S.L.; McConnell, D.; Epinoza, K.J.; Goenaga-Infante, F.; et al. Barriers, facilitators, and strategies for developing a culturally informed lifestyle intervention for Native Hawaiian, CHamoru, and Filipino breast cancer survivors: Mixed-methods findings from focus group participants. Int. J. Environ. Res. Public Health 2023, 20, 6075. [Google Scholar] [CrossRef]
- Sohn, Y.J.; Johnson, C.B.; Leung, C.; Williams, M.J.; Hernandez, B.Y.; Ghassemi, E.; Kim, Y.H.; Picon-Ruiz, M.; Setiawan, V.W.; Woo, Y.H.; et al. The role of AI in predicting breast cancer treatment. J. Am. Coll. Radiol. 2021, 18, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Taparra, K.; Fukui, J.; Aflague, T.; Sumida, K.; Williams, M.J.; Loo, L.W.M.; Hernandez, B.Y.; Chee, S.; Sakoda, L.; Davis, D.; et al. Disaggregation of Asian American and Pacific Islander women with stage 0-II breast cancer unmasks disparities in survival and surgery-to-radiation intervals: A national cancer database analysis from 2004 to 2017. JCO Oncol. Pract. 2022, 18, e1255–e1264. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Kyalwazi, B.; Yau, C.; Campbell, M.J.; Almeda, A.; Park, J.W.; Gomez, S.L.; Habel, L.A.; Kumar, S.; Wara, W.; Esserman, L.J.; et al. Race, gene expression signatures, and clinical outcomes of patients with high-risk early breast cancer. JAMA Netw. Open 2023, 6, e2349646. [Google Scholar] [CrossRef]
- Shadbad, M.A.; Safaei, S.; Brunetti, O.; Minervini, G.; Vitale, G.; Mastronuzzi, A.; Sacco, A.; Tafuto, S.; Prantera, T.; Di Martino, R.; et al. A systematic review on the therapeutic potentiality of PD-L1-inhibiting microRNAs for triple-negative breast cancer: Toward single-cell sequencing-guided biomimetic delivery. Genes 2021, 12, 1206. [Google Scholar] [CrossRef]

| Total | Premenopausal (Age < 50) | Postmenopausal (Age ≥ 50) | ||||||
|---|---|---|---|---|---|---|---|---|
| N = 4591 | N = 902 | N = 3689 | ||||||
| Variable | n | Col% | n | Row% | n | Row% | p | |
| Age at Diagnosis | 18–39 | 221 | 4.8 | 221 | 100.0 | 0 | 0.0 | <0.0001 |
| 40–49 | 681 | 14.8 | 681 | 100.0 | 0 | 0.0 | ||
| 50–59 | 993 | 21.6 | 0 | 0.0 | 993 | 100.0 | ||
| 60–69 | 1398 | 30.5 | 0 | 0.0 | 1398 | 100.0 | ||
| 70+ | 1298 | 28.3 | 0 | 0.0 | 1298 | 100.0 | ||
| Race | White | 979 | 21.3 | 190 | 19.4 | 789 | 80.6 | 0.5 |
| Asian | 1799 | 39.2 | 319 | 17.7 | 1480 | 82.3 | ||
| Filipino | 815 | 17.8 | 179 | 22.0 | 636 | 78.0 | ||
| NHPI | 909 | 19.8 | 193 | 21.2 | 716 | 78.8 | ||
| other | 89 | 1.9 | 21 | 23.6 | 68 | 76.4 | ||
| Subtype | Triple Positive | 242 | 5.3 | 74 | 30.6 | 168 | 69.4 | <0.0001 |
| HR+ HER2− | 3746 | 81.6 | 682 | 18.2 | 3064 | 81.8 | ||
| HR− HER2+ | 188 | 4.1 | 54 | 28.7 | 134 | 71.3 | ||
| Triple Negative | 415 | 9.0 | 92 | 22.2 | 323 | 77.8 | ||
| Histology | Ductal | 3873 | 84.4 | 790 | 20.4 | 3083 | 79.6 | 0.15 |
| Lobular | 434 | 9.5 | 66 | 15.2 | 368 | 84.8 | ||
| Mucinous | 153 | 3.3 | 26 | 17.0 | 127 | 83.0 | ||
| Tubular | 12 | 0.3 | 2 | 16.7 | 10 | 83.3 | ||
| Metaplastic | 15 | 0.3 | 3 | 20.0 | 12 | 80.0 | ||
| Mixed | 23 | 0.5 | 4 | 17.4 | 19 | 82.6 | ||
| other | 81 | 1.8 | 11 | 13.6 | 70 | 86.4 | ||
| County | Hawai’i | 96 | 2.1 | 31 | 32.3 | 65 | 67.7 | 0.01 |
| Honolulu | 2213 | 48.2 | 447 | 20.2 | 1766 | 79.8 | ||
| Kauai | 352 | 7.7 | 58 | 16.5 | 294 | 83.5 | ||
| Maui | 136 | 3.0 | 24 | 17.6 | 112 | 82.4 | ||
| unknown | 1794 | 39.1 | 342 | 19.1 | 1452 | 80.9 | ||
| Year | 2015 | 685 | 14.9 | 134 | 19.6 | 551 | 80.4 | 0.53 |
| 2016 | 672 | 14.6 | 117 | 17.4 | 555 | 82.6 | ||
| 2017 | 756 | 16.5 | 140 | 18.5 | 616 | 81.5 | ||
| 2018 | 714 | 15.6 | 139 | 19.5 | 575 | 80.5 | ||
| 2019 | 628 | 13.7 | 128 | 20.4 | 500 | 79.6 | ||
| 2020 | 430 | 9.4 | 96 | 22.3 | 334 | 77.7 | ||
| 2021 | 400 | 8.7 | 87 | 21.8 | 313 | 78.3 | ||
| 2022 | 306 | 6.7 | 61 | 19.9 | 245 | 80.1 |
| Total | Triple Positive | HR+ HER2− | HR− HER2+ | Triple Negative | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Col% | N | Row% | OR | LCL | UCL | p | N | Row% | N | Row% | OR | LCL | UCL | p | N | Row% | OR | LCL | UCL | p | ||
| Total | 902 | 100 | 74 | 8.2 | 682 | 75.6 | 54 | 6.0 | 92 | 10.2 | |||||||||||||
| Age | 18–39 | 221 | 24.5 | 31 | 14.0 | 1.00 | 137 | 62.0 | 22 | 10.0 | 1.00 | 31 | 14.0 | 1.00 | |||||||||
| Age | 40–49 | 681 | 75.5 | 43 | 6.8 | 0.38 | 0.23 | 0.63 | 0.0002 | 545 | 79.7 | 32 | 4.7 | 0.36 | 0.20 | 0.65 | 0.0007 | 61 | 8.9 | 0.49 | 0.30 | 0.81 | 0.005 |
| Race | White | 190 | 21.1 | 17 | 8.9 | 1.00 | 135 | 71.1 | 9 | 4.7 | 1.00 | 29 | 15.3 | 1.00 | |||||||||
| Race | Asian | 319 | 35.4 | 16 | 4.7 | 0.49 | 0.23 | 1.02 | 0.06 | 251 | 77.2 | 16 | 5.8 | 1.13 | 0.48 | 2.66 | 0.79 | 36 | 12.3 | 0.74 | 0.43 | 1.28 | 0.28 |
| Race | Filipino | 179 | 19.8 | 20 | 11.2 | 1.25 | 0.61 | 2.54 | 0.55 | 130 | 71.3 | 14 | 8.8 | 1.85 | 0.76 | 4.48 | 0.18 | 15 | 8.8 | 0.57 | 0.29 | 1.14 | 0.11 |
| Race | NHPI | 193 | 21.4 | 18 | 8.8 | 0.88 | 0.43 | 1.81 | 0.73 | 153 | 79.7 | 13 | 7.0 | 1.31 | 0.54 | 3.21 | 0.55 | 9 | 4.5 | 0.26 | 0.12 | 0.58 | 0.001 |
| Race | other | 21 | 2.3 | 3 | 12.4 | 1.58 | 0.39 | 6.41 | 0.52 | 13 | 62.1 | 2 | 10.8 | 2.60 | 0.50 | 13.65 | 0.26 | 3 | 14.8 | 1.11 | 0.29 | 4.27 | 0.88 |
| Year | 2015 | 134 | 14.9 | 7 | 5.2 | 1.00 | 108 | 80.6 | 6 | 4.5 | 1.00 | 13 | 9.7 | 1.00 | |||||||||
| Year | 2016 | 117 | 13.0 | 7 | 6.0 | 1.14 | 0.38 | 3.42 | 0.81 | 95 | 81.3 | 8 | 7.0 | 1.54 | 0.51 | 4.67 | 0.44 | 7 | 5.7 | 0.58 | 0.22 | 1.54 | 0.28 |
| Year | 2017 | 140 | 15.5 | 5 | 3.4 | 0.64 | 0.20 | 2.11 | 0.47 | 115 | 82.5 | 11 | 7.9 | 1.73 | 0.61 | 4.89 | 0.30 | 9 | 6.1 | 0.62 | 0.25 | 1.52 | 0.29 |
| Year | 2018 | 139 | 15.4 | 11 | 8.0 | 1.50 | 0.55 | 4.08 | 0.42 | 113 | 82.0 | 6 | 4.1 | 0.90 | 0.28 | 2.93 | 0.87 | 9 | 5.9 | 0.59 | 0.24 | 1.46 | 0.26 |
| Year | 2019 | 128 | 14.2 | 15 | 10.9 | 2.34 | 0.89 | 6.12 | 0.08 | 88 | 71.9 | 7 | 4.9 | 1.23 | 0.39 | 3.88 | 0.72 | 18 | 12.3 | 1.42 | 0.65 | 3.13 | 0.38 |
| Year | 2020 | 96 | 10.6 | 7 | 6.6 | 1.40 | 0.46 | 4.26 | 0.55 | 67 | 72.8 | 8 | 8.0 | 1.98 | 0.64 | 6.09 | 0.23 | 14 | 12.5 | 1.43 | 0.62 | 3.30 | 0.40 |
| Year | 2021 | 87 | 9.6 | 16 | 18.5 | 4.32 | 1.65 | 11.31 | 0.003 | 57 | 66.2 | 5 | 5.6 | 1.52 | 0.44 | 5.27 | 0.51 | 9 | 9.7 | 1.22 | 0.49 | 3.07 | 0.67 |
| Year | 2022 | 61 | 6.8 | 6 | 9.4 | 2.29 | 0.71 | 7.37 | 0.16 | 39 | 63.3 | 3 | 4.9 | 1.40 | 0.33 | 5.96 | 0.65 | 13 | 22.4 | 2.94 | 1.23 | 7.02 | 0.02 |
| Total | Triple Positive | HR+ HER2− | HR− HER2+ | Triple Negative | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Col% | N | Row% | OR | LCL | UCL | p | N | Row% | N | Row% | OR | LCL | UCL | p | N | Row% | OR | LCL | UCL | p | ||
| Total | 3689 | 100 | 168 | 4.6 | 3064 | 83.1 | 134 | 3.6 | 323 | 8.8 | |||||||||||||
| Age | 50–59 | 993 | 26.9 | 72 | 7.3 | 1.00 | 803 | 80.9 | 44 | 4.4 | 1.00 | 74 | 7.5 | 1.00 | |||||||||
| Age | 60–69 | 1398 | 37.9 | 58 | 4.4 | 0.60 | 0.41 | 0.87 | 0.006 | 1170 | 83.1 | 49 | 3.7 | 0.81 | 0.53 | 1.24 | 0.34 | 121 | 8.7 | 1.14 | 0.84 | 1.55 | 0.41 |
| Age | 70+ | 1298 | 35.2 | 38 | 3.1 | 0.41 | 0.27 | 0.62 | <0.0001 | 1091 | 84.0 | 41 | 3.2 | 0.70 | 0.45 | 1.10 | 0.12 | 128 | 9.7 | 1.25 | 0.92 | 1.71 | 0.15 |
| Race | White | 789 | 21.4 | 43 | 5.4 | 1.00 | 641 | 81.2 | 23 | 2.9 | 1.00 | 82 | 10.4 | 1.00 | |||||||||
| Race | Asian | 1480 | 40.1 | 51 | 4.1 | 0.72 | 0.46 | 1.14 | 0.16 | 1247 | 84.0 | 54 | 3.2 | 1.07 | 0.63 | 1.79 | 0.81 | 128 | 8.7 | 0.81 | 0.59 | 1.11 | 0.19 |
| Race | Filipino | 636 | 17.2 | 38 | 5.6 | 1.04 | 0.64 | 1.68 | 0.89 | 504 | 80.5 | 36 | 4.8 | 1.66 | 0.95 | 2.89 | 0.07 | 58 | 9.1 | 0.89 | 0.61 | 1.28 | 0.52 |
| Race | NHPI | 716 | 19.4 | 33 | 4.6 | 0.80 | 0.48 | 1.32 | 0.37 | 621 | 86.9 | 20 | 2.5 | 0.79 | 0.42 | 1.48 | 0.46 | 42 | 6.0 | 0.54 | 0.36 | 0.80 | 0.002 |
| Race | other | 68 | 1.8 | 3 | 5.9 | 1.19 | 0.33 | 4.24 | 0.79 | 51 | 73.4 | 1 | 1.5 | 0.56 | 0.07 | 4.25 | 0.57 | 13 | 19.3 | 2.06 | 1.05 | 4.03 | 0.03 |
| Histology | Ductal | 3083 | 83.6 | 155 | 5.0 | 1.00 | 2519 | 81.7 | 127 | 4.1 | 1.00 | 282 | 9.1 | 1.00 | |||||||||
| Histology | Lobular | 368 | 10.0 | 4 | 1.0 | 0.17 | 0.06 | 0.46 | 0.0005 | 347 | 94.7 | 3 | 0.8 | 0.17 | 0.05 | 0.54 | 0.003 | 14 | 3.5 | 0.33 | 0.19 | 0.57 | 0.0001 |
| Histology | Mucinous | 127 | 3.4 | 3 | 2.2 | 0.37 | 0.11 | 1.20 | 0.10 | 123 | 97.0 | 0 | 0.0 | 0.00 | 0.96 | 1 | 0.8 | 0.07 | 0.01 | 0.53 | 0.009 | ||
| Histology | other | 111 | 3.0 | 6 | 4.3 | 1.02 | 0.43 | 2.44 | 0.96 | 75 | 68.6 | 4 | 3.5 | 1.00 | 0.35 | 2.81 | 0.99 | 26 | 23.7 | 3.08 | 1.92 | 4.95 | <0.0001 |
| County | Hawai’i | 65 | 1.8 | 8 | 10.5 | 1.47 | 0.66 | 3.28 | 0.35 | 49 | 77.0 | 3 | 5.0 | 1.12 | 0.33 | 3.77 | 0.86 | 5 | 7.5 | 0.82 | 0.31 | 2.12 | 0.68 |
| County | Honolulu | 1766 | 47.9 | 129 | 7.3 | 1.00 | 1391 | 78.8 | 81 | 4.6 | 1.00 | 165 | 9.3 | 1.00 | |||||||||
| County | Kauai | 294 | 8.0 | 15 | 4.4 | 0.59 | 0.33 | 1.04 | 0.07 | 236 | 81.6 | 10 | 3.5 | 0.74 | 0.37 | 1.49 | 0.40 | 33 | 10.4 | 1.08 | 0.71 | 1.64 | 0.72 |
| County | Maui | 112 | 3.0 | 14 | 10.4 | 1.40 | 0.75 | 2.63 | 0.30 | 88 | 80.2 | 1 | 0.9 | 0.19 | 0.03 | 1.43 | 0.11 | 9 | 8.5 | 0.89 | 0.43 | 1.83 | 0.75 |
| County | unknown | 1452 | 39.4 | 2 | 0.1 | 0.02 | 0.00 | 0.07 | <0.0001 | 1300 | 89.9 | 39 | 2.4 | 0.46 | 0.31 | 0.70 | 0.0003 | 111 | 7.6 | 0.71 | 0.54 | 0.94 | 0.02 |
| Year | 2015 | 551 | 14.9 | 21 | 3.8 | 1.00 | 475 | 86.2 | 11 | 2.0 | 1.00 | 44 | 8.0 | 1.00 | |||||||||
| Year | 2016 | 555 | 15.0 | 11 | 2.0 | 0.54 | 0.25 | 1.14 | 0.11 | 474 | 84.6 | 18 | 3.3 | 1.69 | 0.78 | 3.63 | 0.18 | 52 | 10.1 | 1.29 | 0.84 | 1.98 | 0.24 |
| Year | 2017 | 616 | 16.7 | 30 | 5.6 | 1.52 | 0.84 | 2.77 | 0.17 | 515 | 82.4 | 25 | 4.3 | 2.24 | 1.08 | 4.63 | 0.03 | 46 | 7.8 | 1.02 | 0.66 | 1.58 | 0.93 |
| Year | 2018 | 575 | 15.6 | 13 | 2.1 | 0.57 | 0.28 | 1.18 | 0.13 | 486 | 84.5 | 33 | 5.7 | 2.91 | 1.45 | 5.85 | 0.003 | 43 | 7.7 | 0.98 | 0.63 | 1.53 | 0.93 |
| Year | 2019 | 500 | 13.6 | 23 | 3.6 | 1.00 | 0.53 | 1.87 | 0.99 | 405 | 81.9 | 16 | 3.1 | 1.61 | 0.74 | 3.54 | 0.23 | 56 | 11.4 | 1.50 | 0.98 | 2.30 | 0.06 |
| Year | 2020 | 334 | 9.1 | 28 | 5.0 | 1.39 | 0.75 | 2.55 | 0.29 | 256 | 81.6 | 14 | 3.7 | 1.95 | 0.86 | 4.43 | 0.11 | 36 | 9.7 | 1.28 | 0.79 | 2.07 | 0.32 |
| Year | 2021 | 313 | 8.5 | 19 | 2.9 | 0.75 | 0.39 | 1.45 | 0.39 | 260 | 87.8 | 9 | 2.3 | 1.11 | 0.45 | 2.77 | 0.82 | 25 | 7.0 | 0.86 | 0.51 | 1.48 | 0.59 |
| Year | 2022 | 245 | 6.6 | 23 | 4.9 | 1.31 | 0.69 | 2.46 | 0.41 | 193 | 84.7 | 8 | 2.8 | 1.40 | 0.55 | 3.61 | 0.48 | 21 | 7.6 | 0.97 | 0.55 | 1.71 | 0.92 |
| Premenopausal (Age < 50) | Postmenopausal (Age ≥ 50) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RACE | Year | Cases | LCL | UCL | p | Cases | LCL | UCL | p |
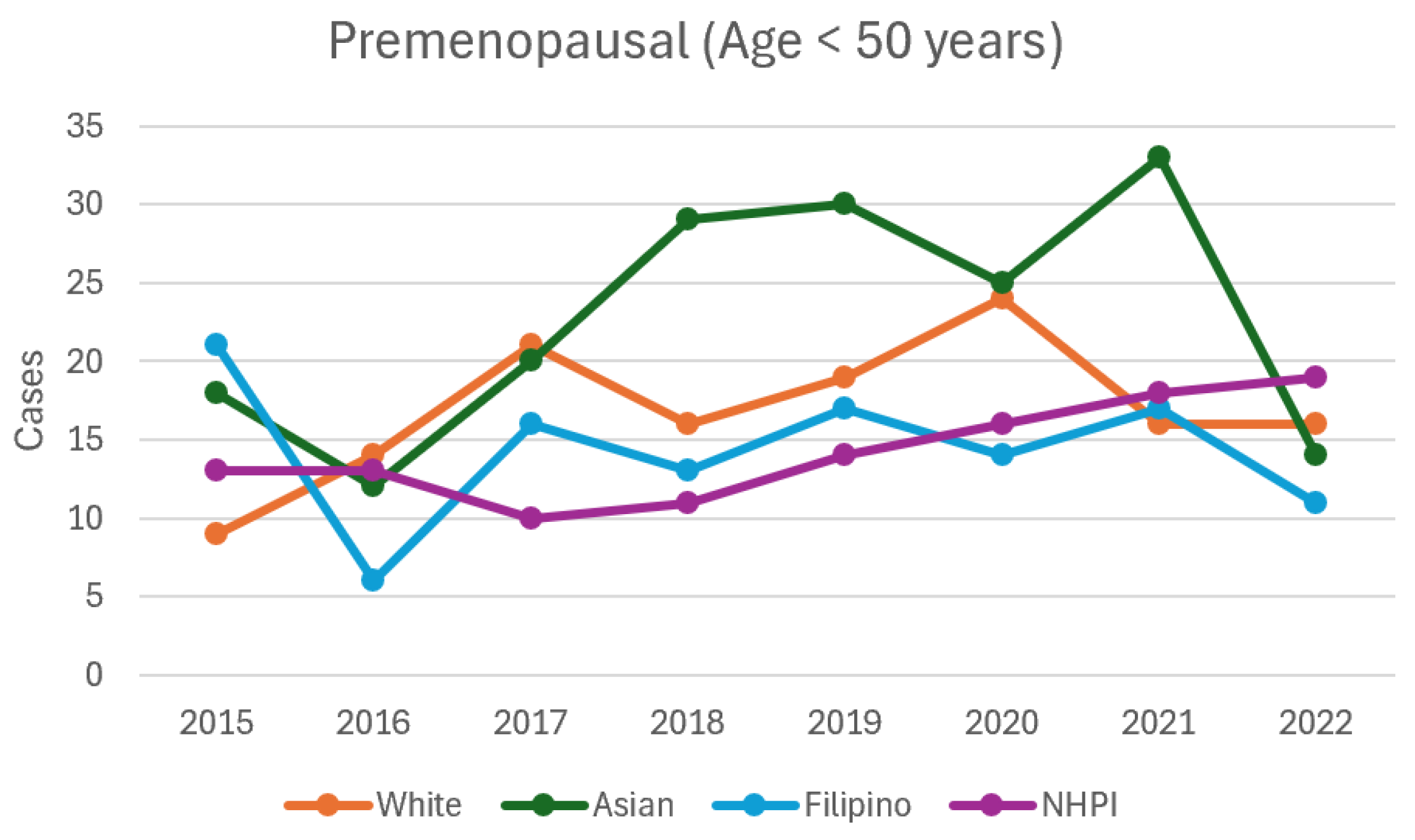
| All | 2015 | 62 | 48.3 | 79.5 | 271 | 240.6 | 305.3 | ||
| All | 2016 | 46 | 34.5 | 61.4 | 0.13 | 253 | 223.7 | 286.2 | 0.43 |
| All | 2017 | 69 | 54.5 | 87.4 | 0.54 | 281 | 250.0 | 315.9 | 0.67 |
| All | 2018 | 72 | 57.2 | 90.7 | 0.39 | 296 | 264.1 | 331.7 | 0.29 |
| All | 2019 | 83 | 66.9 | 102.9 | 0.08 | 313 | 280.2 | 349.7 | 0.08 |
| All | 2020 | 82 | 66.0 | 101.8 | 0.10 | 279 | 248.1 | 313.7 | 0.73 |
| All | 2021 | 87 | 70.5 | 107.3 | 0.04 | 313 | 280.2 | 349.7 | 0.08 |
| All | 2022 | 61 | 47.5 | 78.4 | 0.93 | 245 | 216.2 | 277.7 | 0.25 |
| White | 2015 | 9 | 4.7 | 17.3 | 84 | 67.8 | 104.0 | ||
| White | 2016 | 14 | 8.3 | 23.6 | 0.30 | 62 | 48.3 | 79.5 | 0.07 |
| White | 2017 | 21 | 13.7 | 32.2 | 0.03 | 61 | 47.5 | 78.4 | 0.06 |
| White | 2018 | 16 | 9.8 | 26.1 | 0.17 | 70 | 55.4 | 88.5 | 0.26 |
| White | 2019 | 19 | 12.1 | 29.8 | 0.06 | 84 | 67.8 | 104.0 | 0.99 |
| White | 2020 | 24 | 16.1 | 35.8 | 0.01 | 71 | 56.3 | 89.6 | 0.30 |
| White | 2021 | 16 | 9.8 | 26.1 | 0.17 | 69 | 54.5 | 87.4 | 0.23 |
| White | 2022 | 16 | 9.8 | 26.1 | 0.17 | 57 | 44.0 | 73.9 | 0.02 |
| Asian | 2015 | 18 | 11.3 | 28.6 | 88 | 71.4 | 108.4 | ||
| Asian | 2016 | 12 | 6.8 | 21.1 | 0.28 | 83 | 66.9 | 102.9 | 0.70 |
| Asian | 2017 | 20 | 12.9 | 31.0 | 0.75 | 113 | 94.0 | 135.9 | 0.08 |
| Asian | 2018 | 29 | 20.2 | 41.7 | 0.11 | 107 | 88.5 | 129.3 | 0.17 |
| Asian | 2019 | 30 | 21.0 | 42.9 | 0.09 | 118 | 98.5 | 141.3 | 0.04 |
| Asian | 2020 | 25 | 16.9 | 37.0 | 0.29 | 106 | 87.6 | 128.2 | 0.20 |
| Asian | 2021 | 33 | 23.5 | 46.4 | 0.04 | 117 | 97.6 | 140.2 | 0.04 |
| Asian | 2022 | 14 | 8.3 | 23.6 | 0.48 | 82 | 66.0 | 101.8 | 0.65 |
| Filipino | 2015 | 21 | 13.7 | 32.2 | 53 | 40.5 | 69.4 | ||
| Filipino | 2016 | 6 | 2.7 | 13.4 | 0.007 | 60 | 46.6 | 77.3 | 0.51 |
| Filipino | 2017 | 16 | 9.8 | 26.1 | 0.41 | 45 | 33.6 | 60.3 | 0.42 |
| Filipino | 2018 | 13 | 7.5 | 22.4 | 0.17 | 59 | 45.7 | 76.1 | 0.57 |
| Filipino | 2019 | 17 | 10.6 | 27.3 | 0.52 | 57 | 44.0 | 73.9 | 0.70 |
| Filipino | 2020 | 14 | 8.3 | 23.6 | 0.24 | 50 | 37.9 | 66.0 | 0.77 |
| Filipino | 2021 | 17 | 10.6 | 27.3 | 0.52 | 59 | 45.7 | 76.1 | 0.57 |
| Filipino | 2022 | 11 | 6.1 | 19.9 | 0.08 | 49 | 37.0 | 64.8 | 0.69 |
| NHPI | 2015 | 13 | 7.5 | 22.4 | 41 | 30.2 | 55.7 | ||
| NHPI | 2016 | 13 | 7.5 | 22.4 | 0.99 | 44 | 32.7 | 59.1 | 0.74 |
| NHPI | 2017 | 10 | 5.4 | 18.6 | 0.53 | 59 | 45.7 | 76.1 | 0.07 |
| NHPI | 2018 | 11 | 6.1 | 19.9 | 0.68 | 55 | 42.2 | 71.6 | 0.15 |
| NHPI | 2019 | 14 | 8.3 | 23.6 | 0.85 | 48 | 36.2 | 63.7 | 0.46 |
| NHPI | 2020 | 16 | 9.8 | 26.1 | 0.58 | 48 | 36.2 | 63.7 | 0.46 |
| NHPI | 2021 | 18 | 11.3 | 28.6 | 0.37 | 67 | 52.7 | 85.1 | 0.01 |
| NHPI | 2022 | 19 | 12.1 | 29.8 | 0.29 | 48 | 36.2 | 63.7 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, T.; Liyanage, A.; Bansil, S.; Silva, A.; Pagano, I.; Hidalgo, E.Y.; Jones, C.; Ueno, N.T.; Takahashi, Y.; Fukui, J. Differences in Breast Cancer Subtypes among Racial/Ethnic Groups. Cancers 2024, 16, 3462. https://doi.org/10.3390/cancers16203462
Sasaki T, Liyanage A, Bansil S, Silva A, Pagano I, Hidalgo EY, Jones C, Ueno NT, Takahashi Y, Fukui J. Differences in Breast Cancer Subtypes among Racial/Ethnic Groups. Cancers. 2024; 16(20):3462. https://doi.org/10.3390/cancers16203462
Chicago/Turabian StyleSasaki, Tamlyn, Akash Liyanage, Surbhi Bansil, Anthony Silva, Ian Pagano, Elena Y. Hidalgo, Corinne Jones, Naoto T. Ueno, Yoko Takahashi, and Jami Fukui. 2024. "Differences in Breast Cancer Subtypes among Racial/Ethnic Groups" Cancers 16, no. 20: 3462. https://doi.org/10.3390/cancers16203462
APA StyleSasaki, T., Liyanage, A., Bansil, S., Silva, A., Pagano, I., Hidalgo, E. Y., Jones, C., Ueno, N. T., Takahashi, Y., & Fukui, J. (2024). Differences in Breast Cancer Subtypes among Racial/Ethnic Groups. Cancers, 16(20), 3462. https://doi.org/10.3390/cancers16203462







