Head and Neck Cancer and Sarcopenia: An Integrative Clinical and Functional Review
Abstract
Simple Summary
Abstract
1. Introduction
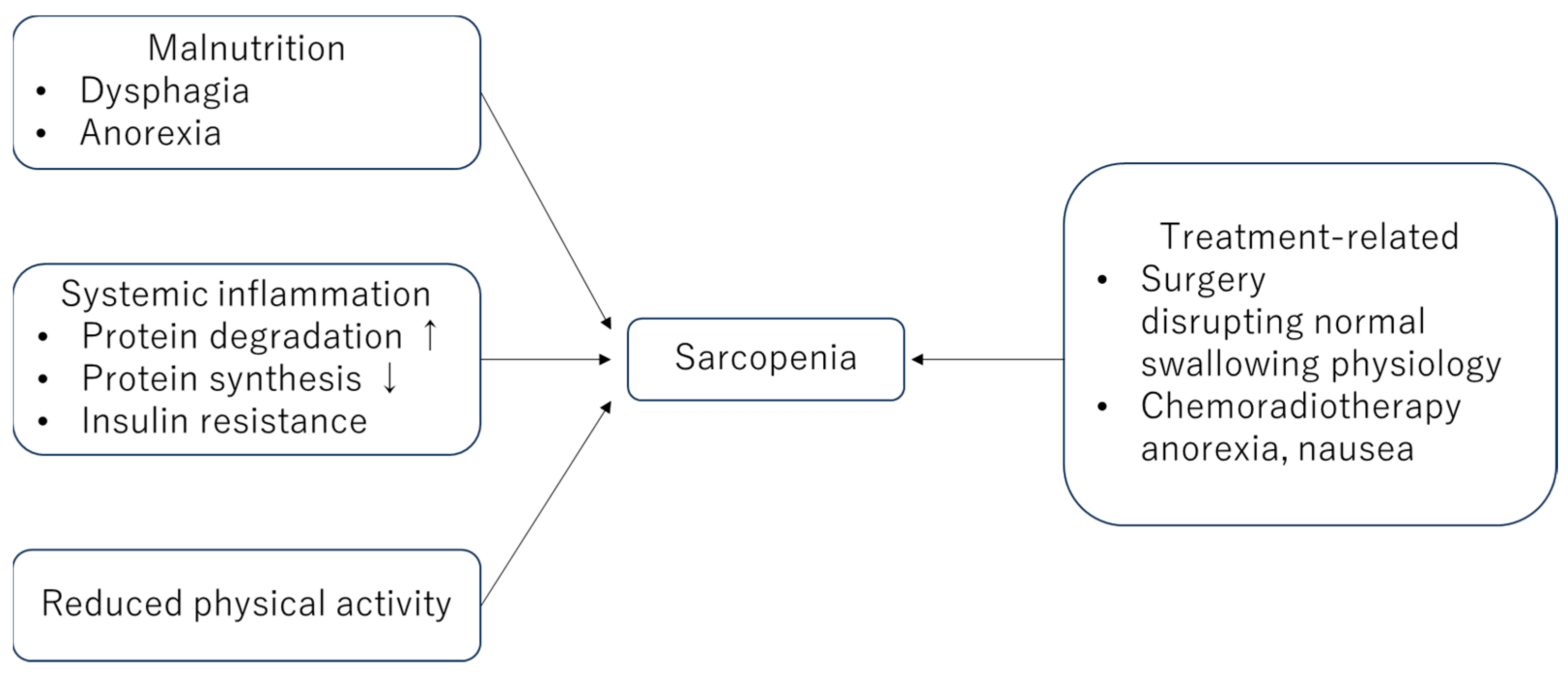
2. Mechanisms Underlying This Relationship
3. Clinical Implications
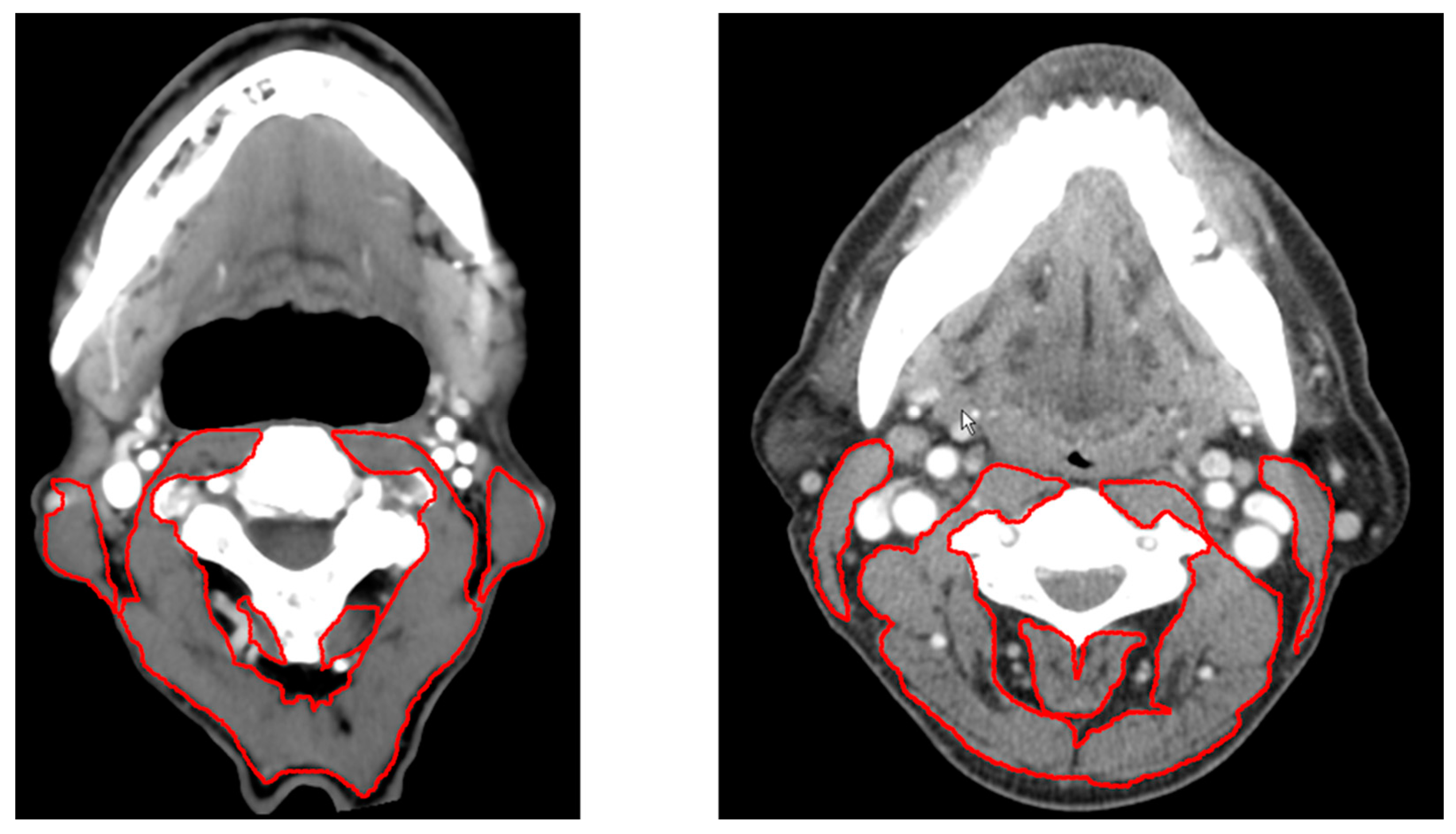
4. Assessment Methods
5. Interventions and Management
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, B.; Johnson, N.W.; Kumar, N. Global epidemiology of head and neck cancers: A continuing challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Rier, H.N.; Jager, A.; Sleijfer, S.; Maier, A.B.; Levin, M.D. The prevalence and prognostic value of low muscle mass in cancer patients: A review of the literature. Oncologist 2016, 21, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Pressoir, M.; Desne, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in french comprehensive cancer centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Prado, C.M.; Cushen, S.J.; Orsso, C.E.; Ryan, A.M. Sarcopenia and cachexia in the era of obesity: Clinical and nutritional impact. Proc. Nutr. Soc. 2016, 75, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Kaida, K.; Tanaka, T.; Itani, Y.; Ikegame, K.; Okada, M.; Ishii, S.; Kodama, N.; Ogawa, H.; Domen, K. Prevalence of sarcopenia and relevance of body composition, physiological function, fatigue, and health-related quality of life in patients before allogeneic hematopoietic stem cell transplantation. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2012, 20, 3161–3168. [Google Scholar] [CrossRef]
- Kilgour, R.D.; Vigano, A.; Trutschnigg, B.; Lucar, E.; Borod, M.; Morais, J.A. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2013, 21, 3261–3270. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhuang, C.L.; Huang, D.D.; Pang, W.Y.; Lou, N.; Chen, F.F.; Zhou, C.J.; Shen, X.; Yu, Z. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: A prospective study. Ann. Surg. Oncol. 2016, 23, 556–564. [Google Scholar] [CrossRef]
- Zwart, A.T.; van der Hoorn, A.; van Ooijen, P.M.A.; Steenbakkers, R.; de Bock, G.H.; Halmos, G.B. Ct-measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 1060–1069. [Google Scholar] [CrossRef]
- Köller, M. Sarcopenia-a geriatric pandemic: A narrative review. Wien. Med. Wochenschr. (1946) 2023, 173, 97–103. [Google Scholar] [CrossRef]
- Karsten, R.T.; Al-Mamgani, A.; Bril, S.I.; Tjon, A.J.S.; van der Molen, L.; de Boer, J.P.; Hilgers, F.J.M.; Smeele, L.E.; van den Brekel, M.W.M.; Stuiver, M.M. Sarcopenia, a strong determinant for prolonged feeding tube dependency after chemoradiotherapy for head and neck cancer. Head Neck 2019, 41, 4000–4008. [Google Scholar] [CrossRef] [PubMed]
- Karsten, R.T.; Stuiver, M.M.; van der Molen, L.; Navran, A.; de Boer, J.P.; Hilgers, F.J.M.; Klop, W.M.C.; Smeele, L.E. From reactive to proactive tube feeding during chemoradiotherapy for head and neck cancer: A clinical prediction model-based approach. Oral Oncol. 2019, 88, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Capuano, G.; Gentile, P.C.; Bianciardi, F.; Tosti, M.; Palladino, A.; Di Palma, M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2010, 18, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Bril, S.I.; Pezier, T.F.; Tijink, B.M.; Janssen, L.M.; Braunius, W.W.; de Bree, R. Preoperative low skeletal muscle mass as a risk factor for pharyngocutaneous fistula and decreased overall survival in patients undergoing total laryngectomy. Head Neck 2019, 41, 1745–1755. [Google Scholar] [CrossRef]
- Achim, V.; Bash, J.; Mowery, A.; Guimaraes, A.R.; Li, R.; Schindler, J.; Wax, M.; Andersen, P.; Clayburgh, D. Prognostic indication of sarcopenia for wound complication after total laryngectomy. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1159–1165. [Google Scholar] [CrossRef]
- Russi, E.G.; Corvò, R.; Merlotti, A.; Alterio, D.; Franco, P.; Pergolizzi, S.; De Sanctis, V.; Ruo Redda, M.G.; Ricardi, U.; Paiar, F.; et al. Swallowing dysfunction in head and neck cancer patients treated by radiotherapy: Review and recommendations of the supportive task group of the italian association of radiation oncology. Cancer Treat. Rev. 2012, 38, 1033–1049. [Google Scholar] [CrossRef]
- Brown, J.C.; Cespedes Feliciano, E.M.; Caan, B.J. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: Facts and numbers. J. Cachexia Sarcopenia Muscle 2018, 9, 1200–1208. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, L.; Huang, X.; Zeng, Y.; Xiong, Z.; Zuo, M. Contribution of skeletal muscle to cancer immunotherapy: A focus on muscle function, inflammation, and microbiota. Nutrition 2023, 105, 111829. [Google Scholar] [CrossRef]
- Friedlander, A.H.; Tajima, T.; Kawakami, K.T.; Wang, M.B.; Tomlinson, J. The relationship between measures of nutritional status and masticatory function in untreated patients with head and neck cancer. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2008, 66, 85–92. [Google Scholar] [CrossRef]
- Bosaeus, I. Nutritional support in multimodal therapy for cancer cachexia. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2008, 16, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; López-Soriano, F.J. The pivotal role of cytokines in muscle wasting during cancer. Int. J. Biochem. Cell Biol. 2005, 37, 2036–2046. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: A role for inflammation. Oxidative Med. Cell. Longev. 2017, 2017, 3292087. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E. Cancer-associated cachexia and underlying biological mechanisms. Annu. Rev. Nutr. 2006, 26, 435–461. [Google Scholar] [CrossRef]
- Zimmers, T.A. Tumours block protective muscle and nerve signals to cause cachexia. Nature 2021, 598, 37–38. [Google Scholar] [CrossRef]
- Bilgic, S.N.; Domaniku, A.; Toledo, B.; Agca, S.; Weber, B.Z.C.; Arabaci, D.H.; Ozornek, Z.; Lause, P.; Thissen, J.P.; Loumaye, A.; et al. Eda2r-nik signalling promotes muscle atrophy linked to cancer cachexia. Nature 2023, 617, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr. Opin. Pharmacol. 2015, 22, 100–106. [Google Scholar] [CrossRef]
- Papadogianni, G.; Ravens, I.; Dittrich-Breiholz, O.; Bernhardt, G.; Georgiev, H. Impact of aging on the phenotype of invariant natural killer t cells in mouse thymus. Front. Immunol. 2020, 11, 575764. [Google Scholar] [CrossRef]
- de Bree, R.; van Beers, M.A.; Schaeffers, A. Sarcopenia and its impact in head and neck cancer treatment. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 87–93. [Google Scholar] [CrossRef]
- Chen, Q.; Rong, P.; Zhu, S.; Yang, X.; Ouyang, Q.; Wang, H.Y.; Chen, S. Targeting ralgapα1 in skeletal muscle to simultaneously improve postprandial glucose and lipid control. Sci. Adv. 2019, 5, eaav4116. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.; Dakhil, S.R.; Nguyen, P.L.; Sloan, J.A.; Kugler, J.W.; Rowland, K.M., Jr.; Soori, G.S.; Wender, D.B.; Fitch, T.R.; Novotny, P.J.; et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: Results from n00c1 from the north central cancer treatment group. Cancer 2007, 110, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, T.J.; Smith, J.T.; Schuster, M.; Dragnev, K.H.; Rigas, J.R. A humanized anti-il-6 antibody (ald518) in non-small cell lung cancer. Expert. Opin. Biol. Ther. 2011, 11, 1663–1668. [Google Scholar] [CrossRef]
- Marceca, G.P.; Londhe, P.; Calore, F. Management of cancer cachexia: Attempting to develop new pharmacological agents for new effective therapeutic options. Front. Oncol. 2020, 10, 298. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Zhao, J.; Yang, L.; Liu, F.; Zheng, H.; Wang, Z.; Xu, Y. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J. Surg. Oncol. 2016, 14, 289. [Google Scholar] [CrossRef]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; O’Reilly, D.S.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. An inflammation-based prognostic score (mgps) predicts cancer survival independent of tumour site: A glasgow inflammation outcome study. Br. J. Cancer 2011, 104, 726–734. [Google Scholar] [CrossRef]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Tabuchi, K.; Hara, A. Clinical utility of the modified glasgow prognostic score in patients with advanced head and neck cancer. Head Neck 2015, 37, 1745–1749. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, Y.; Hou, L.S.; Xia, X.; Ge, M.L.; Liu, X.L.; Yue, J.R.; Dong, B.R. The association between systemic inflammatory markers and sarcopenia: Results from the west china health and aging trend study (wchat). Arch. Gerontol. Geriatr. 2021, 92, 104262. [Google Scholar] [CrossRef]
- Kakehi, S.; Wakabayashi, H.; Inuma, H.; Inose, T.; Shioya, M.; Aoyama, Y.; Hara, T.; Uchimura, K.; Tomita, K.; Okamoto, M.; et al. Rehabilitation nutrition and exercise therapy for sarcopenia. World J. Men’s Health 2022, 40, 1–10. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Perez-Schindler, J.; Philp, A.; Smith, K.; Atherton, P.J. Skeletal muscle homeostasis and plasticity in youth and ageing: Impact of nutrition and exercise. Acta Physiol. 2016, 216, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Cohen, A.A.; Xue, Q.L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Meerkerk, C.D.A.; Chargi, N.; de Jong, P.A.; van den Bos, F.; de Bree, R. Sarcopenia measured with handgrip strength and skeletal muscle mass to assess frailty in older patients with head and neck cancer. J. Geriatr. Oncol. 2021, 12, 434–440. [Google Scholar] [CrossRef]
- Joglekar, S.; Asghar, A.; Mott, S.L.; Johnson, B.E.; Button, A.M.; Clark, E.; Mezhir, J.J. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J. Surg. Oncol. 2015, 111, 771–775. [Google Scholar] [CrossRef]
- Lieffers, J.R.; Bathe, O.F.; Fassbender, K.; Winget, M.; Baracos, V.E. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br. J. Cancer 2012, 107, 931–936. [Google Scholar] [CrossRef]
- Surov, A.; Wienke, A. Low skeletal muscle mass predicts relevant clinical outcomes in head and neck squamous cell carcinoma. A meta analysis. Ther. Adv. Med. Oncol. 2021, 13, 17588359211008844. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Sohda, M.; Saito, H.; Ubukata, Y.; Nakazawa, N.; Kuriyama, K.; Hara, K.; Sano, A.; Ogata, K.; Yokobori, T.; et al. Impact of combined assessment of systemic inflammation and presarcopenia on survival for surgically resected esophageal cancer. Am. J. Surg. 2021, 221, 149–154. [Google Scholar] [CrossRef]
- Orzell, S.; Verhaaren, B.F.J.; Grewal, R.; Sklar, M.; Irish, J.C.; Gilbert, R.; Brown, D.; Gullane, P.; de Almeida, J.R.; Yu, E.; et al. Evaluation of sarcopenia in older patients undergoing head and neck cancer surgery. Laryngoscope 2022, 132, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Alwani, M.M.; Jones, A.J.; Novinger, L.J.; Pittelkow, E.; Bonetto, A.; Sim, M.W.; Moore, M.G.; Mantravadi, A.V. Impact of sarcopenia on outcomes of autologous head and neck free tissue reconstruction. J. Reconstr. Microsurg. 2020, 36, 369–378. [Google Scholar]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Barret, M.; Antoun, S.; Dalban, C.; Malka, D.; Mansourbakht, T.; Zaanan, A.; Latko, E.; Taieb, J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr. Cancer 2014, 66, 583–589. [Google Scholar] [CrossRef]
- Cushen, S.J.; Power, D.G.; Teo, M.Y.; MacEneaney, P.; Maher, M.M.; McDermott, R.; O’Sullivan, K.; Ryan, A.M. Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with sunitinib. Am. J. Clin. Oncol. 2017, 40, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Baracos, V.E.; Sawyer, M.B.; Bianchi, L.; Roberts, S.; Assenat, E.; Mollevi, C.; Senesse, P. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with folfox regimens. Cancer Med. 2016, 5, 607–616. [Google Scholar] [CrossRef]
- Ganju, R.G.; Morse, R.; Hoover, A.; TenNapel, M.; Lominska, C.E. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 137, 117–124. [Google Scholar] [CrossRef]
- Wendrich, A.W.; Swartz, J.E.; Bril, S.I.; Wegner, I.; de Graeff, A.; Smid, E.J.; de Bree, R.; Pothen, A.J. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017, 71, 26–33. [Google Scholar] [CrossRef]
- Huang, X.; Lv, L.N.; Zhao, Y.; Li, L.; Zhu, X.D. Is skeletal muscle loss associated with chemoradiotherapy toxicity in nasopharyngeal carcinoma patients? A prospective study. Clin. Nutr. 2021, 40, 295–302. [Google Scholar] [CrossRef]
- Bril, S.I.; Al-Mamgani, A.; Chargi, N.; Remeijer, P.; Devriese, L.A.; de Boer, J.P.; de Bree, R. The association of pretreatment low skeletal muscle mass with chemotherapy dose-limiting toxicity in patients with head and neck cancer undergoing primary chemoradiotherapy with high-dose cisplatin. Head Neck 2022, 44, 189–200. [Google Scholar] [CrossRef]
- Jung, A.R.; Roh, J.L.; Kim, J.S.; Kim, S.B.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Prognostic value of body composition on recurrence and survival of advanced-stage head and neck cancer. Eur. J. Cancer 2019, 116, 98–106. [Google Scholar] [CrossRef]
- Kumar, A.; Moynagh, M.R.; Multinu, F.; Cliby, W.A.; McGree, M.E.; Weaver, A.L.; Young, P.M.; Bakkum-Gamez, J.N.; Langstraat, C.L.; Dowdy, S.C.; et al. Muscle composition measured by ct scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol. Oncol. 2016, 142, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rier, H.N.; Jager, A.; Sleijfer, S.; van Rosmalen, J.; Kock, M.; Levin, M.D. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast 2017, 31, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tsukioka, T.; Nishiyama, N.; Izumi, N.; Mizuguchi, S.; Komatsu, H.; Okada, S.; Toda, M.; Hara, K.; Ito, R.; Shibata, T. Sarcopenia is a novel poor prognostic factor in male patients with pathological stage i non-small cell lung cancer. Jpn. J. Clin. Oncol. 2017, 47, 363–368. [Google Scholar] [CrossRef][Green Version]
- Järvinen, T.; Ilonen, I.; Kauppi, J.; Salo, J.; Räsänen, J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: A retrospective cohort study. World J. Surg. Oncol. 2018, 16, 27. [Google Scholar] [CrossRef]
- Hayashi, N.; Ando, Y.; Gyawali, B.; Shimokata, T.; Maeda, O.; Fukaya, M.; Goto, H.; Nagino, M.; Kodera, Y. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol. Rep. 2016, 35, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Van Rijssen, L.B.; van Huijgevoort, N.C.; Coelen, R.J.; Tol, J.A.; Haverkort, E.B.; Nio, C.Y.; Busch, O.R.; Besselink, M.G. Skeletal muscle quality is associated with worse survival after pancreatoduodenectomy for periampullary, nonpancreatic cancer. Ann. Surg. Oncol. 2017, 24, 272–280. [Google Scholar] [CrossRef]
- Antoun, S.; Lanoy, E.; Iacovelli, R.; Albiges-Sauvin, L.; Loriot, Y.; Merad-Taoufik, M.; Fizazi, K.; di Palma, M.; Baracos, V.E.; Escudier, B. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013, 119, 3377–3384. [Google Scholar] [CrossRef]
- Takenaka, Y.; Takemoto, N.; Oya, R.; Inohara, H. Prognostic impact of sarcopenia in patients with head and neck cancer treated with surgery or radiation: A meta-analysis. PLoS ONE 2021, 16, e0259288. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Pruthi, D.S.; Pandey, M.; Yadav, A.; Singh, H. Impact of sarcopenia in locally advanced head and neck cancer treated with chemoradiation: An indian tertiary care hospital experience. Oral Oncol. 2021, 121, 105483. [Google Scholar] [CrossRef]
- Findlay, M.; White, K.; Stapleton, N.; Bauer, J. Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin. Nutr. 2021, 40, 1711–1718. [Google Scholar] [CrossRef]
- Wong, A.; Zhu, D.; Kraus, D.; Tham, T. Radiologically defined sarcopenia affects survival in head and neck cancer: A meta-analysis. Laryngoscope 2021, 131, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Ueno, T.; Hirai, N.; Komori, T.; Nakanishi, Y.; Kondo, S.; Wakisaka, N.; Yoshizaki, T. Low skeletal muscle mass is a risk factor for aspiration pneumonia during chemoradiotherapy. Laryngoscope 2021, 131, E1524–E1529. [Google Scholar] [CrossRef]
- Lee, J.; Liu, S.H.; Chen, J.C.; Leu, Y.S.; Liu, C.J.; Chen, Y.J. Progressive muscle loss is an independent predictor for survival in locally advanced oral cavity cancer: A longitudinal study. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 158, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Harada, K.; Okawa, N.; Tamura, K.; Moriyama, H. Low body mass index negatively affects muscle mass and intramuscular fat of chronic stroke survivors. PLoS ONE 2019, 14, e0211145. [Google Scholar] [CrossRef]
- Wu, L.W.; Lin, Y.Y.; Kao, T.W.; Lin, C.M.; Liaw, F.Y.; Wang, C.C.; Peng, T.C.; Chen, W.L. Mid-arm muscle circumference as a significant predictor of all-cause mortality in male individuals. PLoS ONE 2017, 12, e0171707. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in asia: Consensus report of the asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Heymsfield, S.B. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: What are we really estimating? J. Cachexia Sarcopenia Muscle 2017, 8, 187–189. [Google Scholar] [CrossRef]
- Gonera-Furman, A.; Bolanowski, M.; Jędrzejuk, D. Osteosarcopenia-the role of dual-energy x-ray absorptiometry (dxa) in diagnostics. J. Clin. Med. 2022, 11, 2522. [Google Scholar] [CrossRef]
- Daly, L.E.; Prado, C.M.; Ryan, A.M. A window beneath the skin: How computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc. Nutr. Soc. 2018, 77, 135–151. [Google Scholar] [CrossRef]
- van Rijn-Dekker, M.I.; van den Bosch, L.; van den Hoek, J.G.M.; Bijl, H.P.; van Aken, E.S.M.; van der Hoorn, A.; Oosting, S.F.; Halmos, G.B.; Witjes, M.J.H.; van der Laan, H.P.; et al. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 147, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.E.; Pothen, A.J.; Wegner, I.; Smid, E.J.; Swart, K.M.; de Bree, R.; Leenen, L.P.; Grolman, W. Feasibility of using head and neck ct imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, N.; Chinnery, T.; Mattonen, S.A.; Palma, D.A.; Doyle, P.C.; Theurer, J.A. Sarcopenia in head and neck cancer: A scoping review. PLoS ONE 2022, 17, e0278135. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G. Epidemiology and consequences of sarcopenia. J. Nutr. Health Aging 2009, 13, 708–712. [Google Scholar] [CrossRef]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support. Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef]
- Roubenoff, R.; Parise, H.; Payette, H.A.; Abad, L.W.; D’Agostino, R.; Jacques, P.F.; Wilson, P.W.; Dinarello, C.A.; Harris, T.B. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The framingham heart study. Am. J. Med. 2003, 115, 429–435. [Google Scholar] [CrossRef]
- Dhillon, R.J.; Hasni, S. Pathogenesis and management of sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Chargi, N.; Bril, S.I.; Emmelot-Vonk, M.H.; de Bree, R. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur. Arch. Otorhinolaryngol. 2019, 276, 1475–1486. [Google Scholar] [CrossRef]
- Park, W.T.; Shon, O.J.; Kim, G.B. Multidisciplinary approach to sarcopenia: A narrative review. J. Yeungnam Med. Sci. 2023, 40, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1–553.e16. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate intensity resistive training reduces oxidative stress and improves muscle mass and function in older individuals. Antioxidants 2019, 8, 431. [Google Scholar] [CrossRef]
- Chen, H.T.; Wu, H.J.; Chen, Y.J.; Ho, S.Y.; Chung, Y.C. Effects of 8-week kettlebell training on body composition, muscle strength, pulmonary function, and chronic low-grade inflammation in elderly women with sarcopenia. Exp. Gerontol. 2018, 112, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Billis, E.; Tsepis, E.; Dimitriadis, Z.; Matzaroglou, C.; Tyllianakis, M.; Panagiotopoulos, E.; Gliatis, J. The effects of group and home-based exercise programs in elderly with sarcopenia: A randomized controlled trial. J. Clin. Med. 2018, 7, 480. [Google Scholar] [CrossRef]
- Bao, W.; Sun, Y.; Zhang, T.; Zou, L.; Wu, X.; Wang, D.; Chen, Z. Exercise programs for muscle mass, muscle strength and physical performance in older adults with sarcopenia: A systematic review and meta-analysis. Aging Dis. 2020, 11, 863–873. [Google Scholar] [CrossRef]
- Wang, R.; Liang, Y.; Jiang, J.; Chen, M.; Li, L.; Yang, H.; Tan, L.; Yang, M. Effectiveness of a short-term mixed exercise program for treating sarcopenia in hospitalized patients aged 80 years and older: A prospective clinical trial. J. Nutr. Health Aging 2020, 24, 1087–1093. [Google Scholar] [CrossRef]
- Makizako, H.; Nakai, Y.; Tomioka, K.; Taniguchi, Y.; Sato, N.; Wada, A.; Kiyama, R.; Tsutsumimoto, K.; Ohishi, M.; Kiuchi, Y.; et al. Effects of a multicomponent exercise program in physical function and muscle mass in sarcopenic/pre-sarcopenic adults. J. Clin. Med. 2020, 9, 1386. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Ottenbacher, M.E.; Ottenbacher, A.J.; Acha, A.A.; Ostir, G.V. Androgen treatment and muscle strength in elderly men: A meta-analysis. J. Am. Geriatr. Soc. 2006, 54, 1666–1673. [Google Scholar] [CrossRef]
- Corona, G.; Giagulli, V.A.; Maseroli, E.; Vignozzi, L.; Aversa, A.; Zitzmann, M.; Saad, F.; Mannucci, E.; Maggi, M. Testosterone supplementation and body composition: Results from a meta-analysis of observational studies. J. Endocrinol. Investig. 2016, 39, 967–981. [Google Scholar] [CrossRef]
- Snyder, P.J.; Bhasin, S.; Cunningham, G.R.; Matsumoto, A.M.; Stephens-Shields, A.J.; Cauley, J.A.; Gill, T.M.; Barrett-Connor, E.; Swerdloff, R.S.; Wang, C.; et al. Effects of testosterone treatment in older men. New Engl. J. Med. 2016, 374, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.T.; Barnette, K.G.; Bohl, C.E.; Hancock, M.L.; Rodriguez, D.; Dodson, S.T.; Morton, R.A.; Steiner, M.S. The selective androgen receptor modulator gtx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: Results of a double-blind, placebo-controlled phase ii trial. J. Cachexia Sarcopenia Muscle 2011, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Deng, K.L.; Xing, T.F.; Mei, Y.Q.; Xiao, S.M. Effect of hormone therapy on muscle strength in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Menopause 2020, 27, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.S.; Rizza, R.A.; O’Brien, P.; Dhatariya, K.; Short, K.R.; Nehra, A.; Vittone, J.L.; Klee, G.G.; Basu, A.; Basu, R.; et al. Dhea in elderly women and dhea or testosterone in elderly men. N. Engl. J. Med. 2006, 355, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Grunseich, C.; Miller, R.; Swan, T.; Glass, D.J.; El Mouelhi, M.; Fornaro, M.; Petricoul, O.; Vostiar, I.; Roubenoff, R.; Meriggioli, M.N.; et al. Safety, tolerability, and preliminary efficacy of an igf-1 mimetic in patients with spinal and bulbar muscular atrophy: A randomised, placebo-controlled trial. The Lancet. Neurology 2018, 17, 1043–1052. [Google Scholar] [CrossRef]
- Papadakis, M.A.; Grady, D.; Black, D.; Tierney, M.J.; Gooding, G.A.; Schambelan, M.; Grunfeld, C. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann. Intern. Med. 1996, 124, 708–716. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Arai, H.; Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in japan: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef]
- Rooks, D.; Swan, T.; Goswami, B.; Filosa, L.A.; Bunte, O.; Panchaud, N.; Coleman, L.A.; Miller, R.R.; Garcia Garayoa, E.; Praestgaard, J.; et al. Bimagrumab vs optimized standard of care for treatment of sarcopenia in community-dwelling older adults: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2020836. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Mozaffari, H.; Kord-Varkaneh, H.; Omidian, M.; Kruger, M.C. The effects of vitamin d supplementation on muscle strength and mobility in postmenopausal women: A systematic review and meta-analysis of randomised controlled trials. J. Hum. Nutr. Diet. 2020, 33, 207–221. [Google Scholar] [CrossRef]
- Sjúrðarson, T.; Bejder, J.; Breenfeldt Andersen, A.; Bonne, T.; Kyhl, K.; Róin, T.; Patursson, P.; Oddmarsdóttir Gregersen, N.; Skoradal, M.B.; Schliemann, M.; et al. Effect of angiotensin-converting enzyme inhibition on cardiovascular adaptation to exercise training. Physiol. Rep. 2022, 10, e15382. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Kwon, K.S. Pharmacological interventions for treatment of sarcopenia: Current status of drug development for sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 98–104. [Google Scholar] [CrossRef]
- Negm, A.M.; Lee, J.; Hamidian, R.; Jones, C.A.; Khadaroo, R.G. Management of sarcopenia: A network meta-analysis of randomized controlled trials. J. Am. Med. Dir. Assoc. 2022, 23, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Nutritional deterioration in cancer: The role of disease and diet. Clin. Oncol. 2003, 15, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Alshadwi, A.; Nadershah, M.; Carlson, E.R.; Young, L.S.; Burke, P.A.; Daley, B.J. Nutritional considerations for head and neck cancer patients: A review of the literature. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2013, 71, 1853–1860. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Soons, Z.; Aarnoutse, R.; van Dijk, D.P.J.; Penders, J.; van Helvoort, A.; Smidt, M.L.; Kruitwagen, R.; Baade-Corpelijn, L.; et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J. Cachexia Sarcopenia Muscle 2021, 12, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Avuthu, N.; Guda, C. Meta-analysis of altered gut microbiota reveals microbial and metabolic biomarkers for colorectal cancer. Microbiol. Spectr. 2022, 10, e0001322. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Van Hul, M.; Plovier, H.; Pachikian, B.; et al. Increased gut permeability in cancer cachexia: Mechanisms and clinical relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Claus, S.P.; Le Roy, C.I.; Grangette, C.; Pot, B.; Martinez, I.; Walter, J.; Cani, P.D.; Delzenne, N.M. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 2016, 10, 1456–1470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, K.; Ichinose, M.; Kobayashi, E.; Ueno, T.; Hirai, N.; Nakanishi, Y.; Kondo, S.; Yoshizaki, T. Head and Neck Cancer and Sarcopenia: An Integrative Clinical and Functional Review. Cancers 2024, 16, 3460. https://doi.org/10.3390/cancers16203460
Endo K, Ichinose M, Kobayashi E, Ueno T, Hirai N, Nakanishi Y, Kondo S, Yoshizaki T. Head and Neck Cancer and Sarcopenia: An Integrative Clinical and Functional Review. Cancers. 2024; 16(20):3460. https://doi.org/10.3390/cancers16203460
Chicago/Turabian StyleEndo, Kazuhira, Mariko Ichinose, Eiji Kobayashi, Takayoshi Ueno, Nobuyuki Hirai, Yosuke Nakanishi, Satoru Kondo, and Tomokazu Yoshizaki. 2024. "Head and Neck Cancer and Sarcopenia: An Integrative Clinical and Functional Review" Cancers 16, no. 20: 3460. https://doi.org/10.3390/cancers16203460
APA StyleEndo, K., Ichinose, M., Kobayashi, E., Ueno, T., Hirai, N., Nakanishi, Y., Kondo, S., & Yoshizaki, T. (2024). Head and Neck Cancer and Sarcopenia: An Integrative Clinical and Functional Review. Cancers, 16(20), 3460. https://doi.org/10.3390/cancers16203460





