Epidermolysis-Bullosa-Associated Squamous Cell Carcinomas Support an Immunosuppressive Tumor Microenvironment: Prospects for Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of SCCs
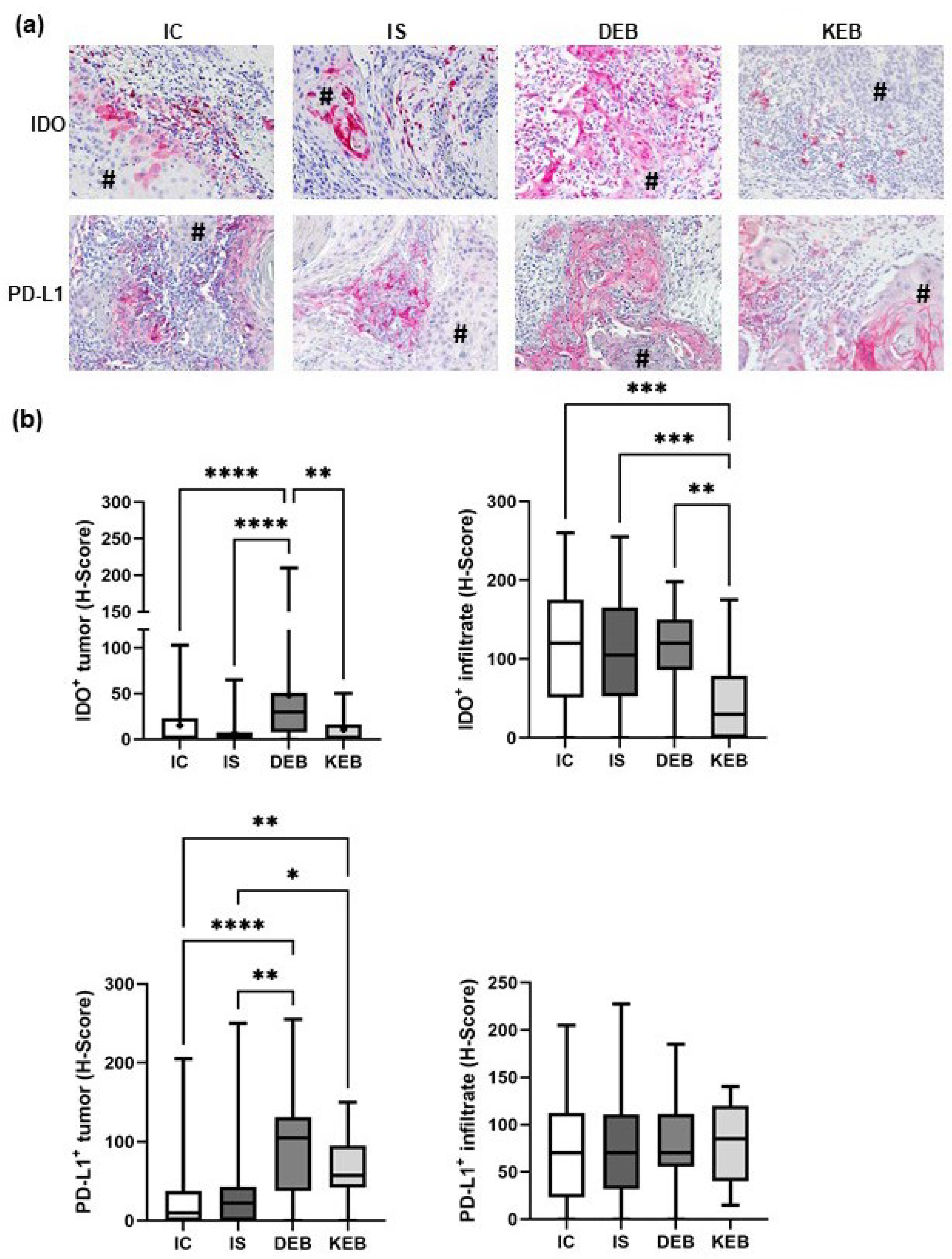
3.2. DEB-SCC Tumor Cells Express Significantly Higher Levels of IDO and PD-L1 Compared with IC- and IS-SCC Tumor Cells
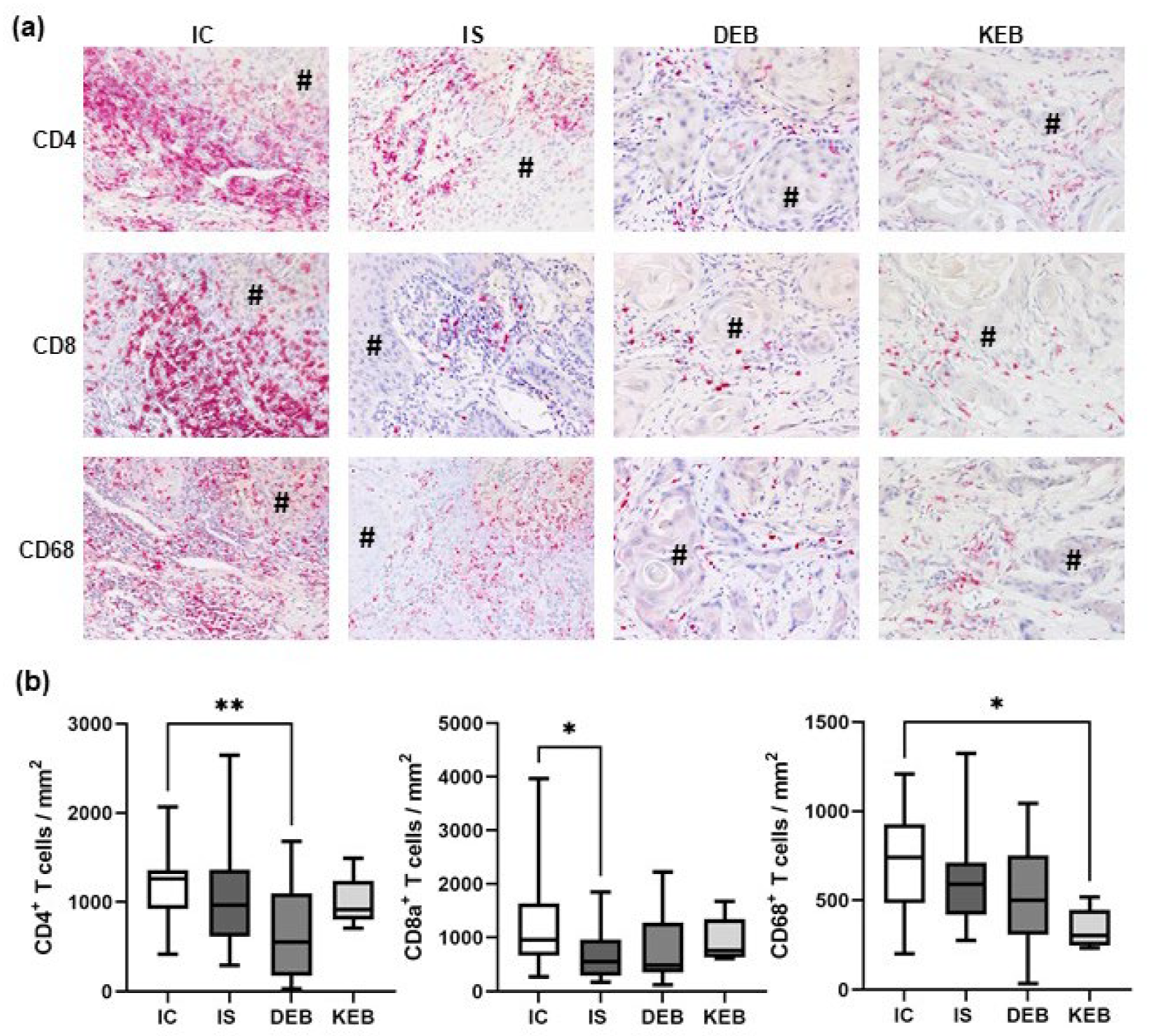
3.3. The TME Inflammatory Cell Infiltrate in EB-SCCs
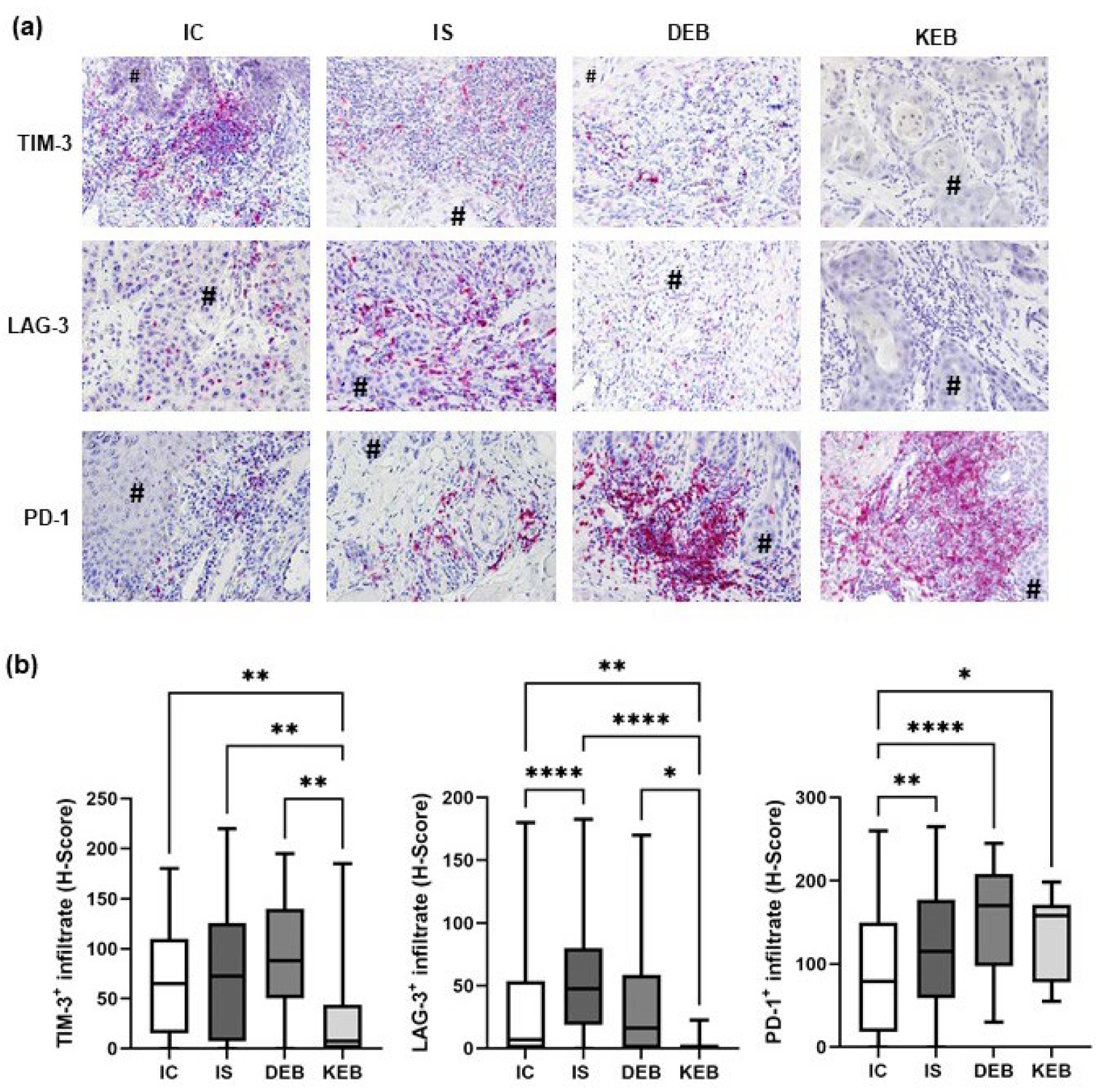
3.4. PD-1 Expression Was Significantly Upregulated in the TMEs of DEB-, KEB-, and IS-SCCs, While LAG-3 Was Increased in the TME of IS-SCCs
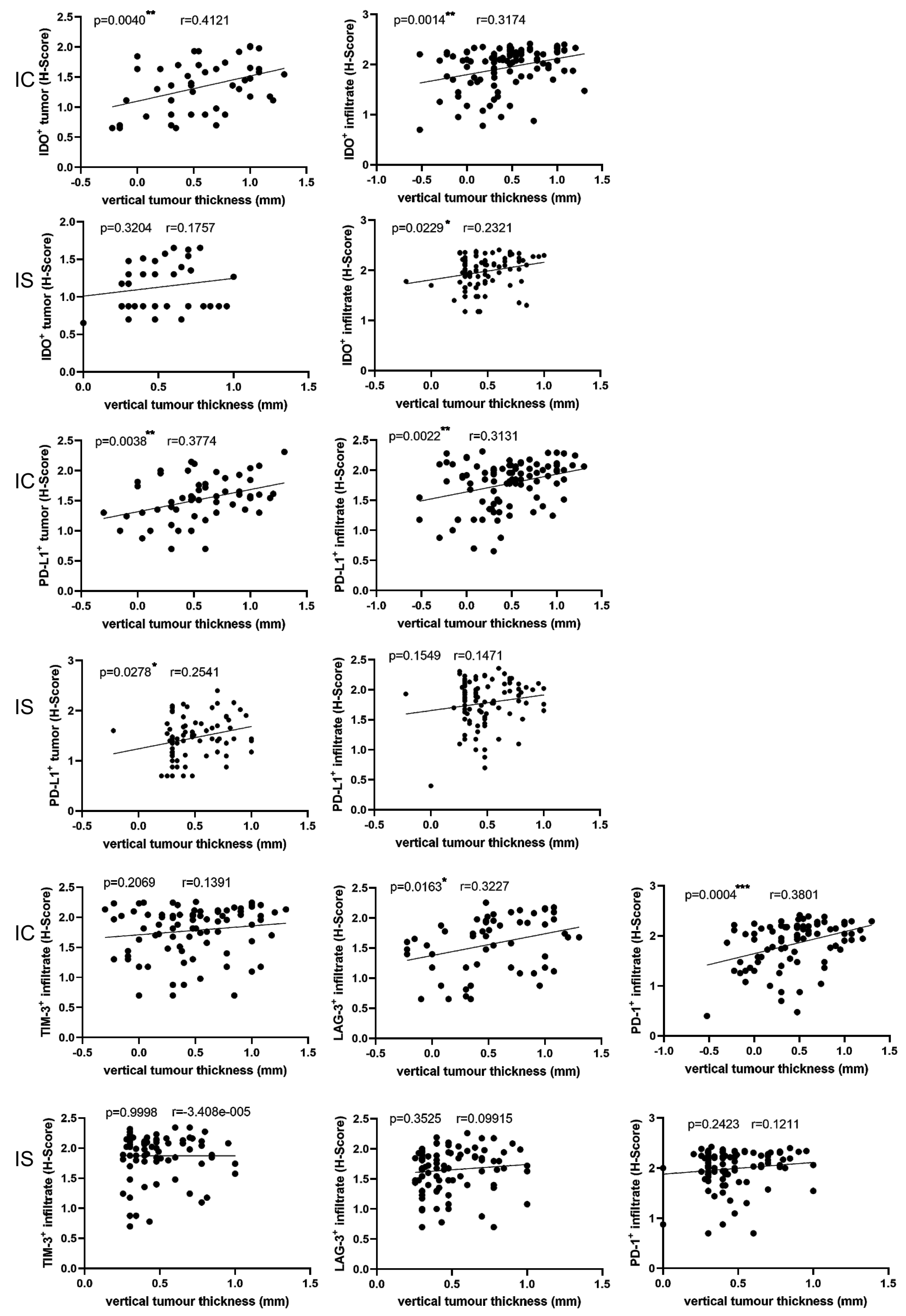
3.5. Correlations between Immune Markers and Clinicopathological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fine, J.-D.; Johnson, L.B.; Weiner, M.; Li, K.-P.; Suchindran, C. Epidermolysis Bullosa and the Risk of Life-Threatening Cancers: The National EB Registry Experience, 1986–2006. J. Am. Acad. Dermatol. 2009, 60, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Montaudié, H.; Chiaverini, C.; Sbidian, E.; Charlesworth, A.; Lacour, J.-P. Inherited Epidermolysis Bullosa and Squamous Cell Carcinoma: A Systematic Review of 117 Cases. Orphanet. J. Rare Dis. 2016, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Mallipeddi, R. Epidermolysis Bullosa and Cancer. Clin. Exp. Dermatol. 2002, 27, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Mellerio, J.E.; Robertson, S.J.; Bernardis, C.; Diem, A.; Fine, J.D.; George, R.; Goldberg, D.; Halmos, G.B.; Harries, M.; Jonkman, M.F.; et al. Management of Cutaneous Squamous Cell Carcinoma in Patients with Epidermolysis Bullosa: Best Clinical Practice Guidelines. Br. J. Dermatol. 2016, 174, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Yuen, W.Y.; Jonkman, M.F. Risk of Squamous Cell Carcinoma in Junctional Epidermolysis Bullosa, Non-Herlitz Type: Report of 7 Cases and a Review of the Literature. J. Am. Acad. Dermatol. 2011, 65, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Aspizua, S.; Conti, C.J.; Escamez, M.J.; Castiglia, D.; Zambruno, G.; Youssefian, L.; Vahidnezhad, H.; Requena, L.; Itin, P.; Tadini, G.; et al. Assessment of the Risk and Characterization of Non-Melanoma Skin Cancer in Kindler Syndrome: Study of a Series of 91 Patients. Orphanet. J. Rare Dis. 2019, 14, 183. [Google Scholar] [CrossRef]
- Harrs, C.; van den Akker, P.C.; Baardman, R.; Duipmans, J.C.; Horváth, B.; van Kester, M.S.; Lemmink, H.H.; Rácz, E.; Bolling, M.C.; Diercks, G.F.H. The Aggressive Behaviour of Squamous Cell Carcinoma in Epidermolysis Bullosa: Analysis of Clinical Outcomes and Tumour Characteristics in the Dutch EB Registry. Br. J. Dermatol. 2022, 187, 824–826. [Google Scholar] [CrossRef]
- Robertson, S.J.; Orrin, E.; Lakhan, M.K.; O’Sullivan, G.; Felton, J.; Robson, A.; Greenblatt, D.T.; Bernardis, C.; McGrath, J.A.; Martinez, A.E.; et al. Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: A 28-Year Retrospective Study. Acta Derm. Venereol. 2021, 101, adv00523. [Google Scholar] [CrossRef]
- Saleva, M.; Has, C.; He, Y.; Vassileva, S.; Balabanova, M.; Miteva, L. Natural History of Kindler Syndrome and Propensity for Skin Cancer—Case Report and Literature Review. J. Dtsch. Dermatol. Ges. 2018, 16, 338–341. [Google Scholar] [CrossRef]
- Shivaswamy, K.N.; Sumathy, T.K.; Shyamprasad, A.L.; Ranganathan, C. Squamous Cell Carcinoma Complicating Epidermolysis Bullosa in a 6-Year-Old Girl. Int. J. Dermatol. 2009, 48, 731–733. [Google Scholar] [CrossRef]
- Arnold, A.W.; Bruckner-Tuderman, L.; Zuger, C.; Itin, P.H. Cetuximab Therapy of Metastasizing Cutaneous Squamous Cell Carcinoma in a Patient with Severe Recessive Dystrophic Epidermolysis Bullosa. Dermatology 2009, 219, 80–83. [Google Scholar] [CrossRef]
- Medek, K.; Koelblinger, P.; Koller, J.; Diem, A.; Ude-Schoder, K.; Bauer, J.W.; Laimer, M. Wundheilungsstörungen Während Der Antitumorösen Therapie Mit Cetuximab Bei Schwerer Generalisierter Dystropher Epidermolysis Bullosa. J. Dtsch. Dermatol. Ges. 2019, 17, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, A.; Steinke, H.; Nyström, A.; Schwieger-Briel, A.; Meiss, F.; Pfannenberg, C.; Bruckner-Tuderman, L.; Ruf, J.; De Vito, R.; El Hachem, M.; et al. EGFR Inhibition for Metastasized Cutaneous Squamous Cell Carcinoma in Dystrophic Epidermolysis Bullosa. Orphanet. J. Rare Dis. 2019, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Li, M.; Intong, L.R.; Tran, K.; Melbourne, W.; Marucci, D.; Bucci, J.; de Souza, P.; Mallesara, G.; Murrell, D.F. Use of Cetuximab as an Adjuvant Agent to Radiotherapy and Surgery in Recessive Dystrophic Epidermolysis Bullosa with Squamous Cell Carcinoma. Br. J. Dermatol. 2013, 169, 208–210. [Google Scholar] [CrossRef]
- Reimer, A.; Lu, S.; He, Y.; Bruckner-Tuderman, L.; Technau-Hafsi, K.; Meiss, F.; Has, C.; von Bubnoff, D. Combined Anti-Inflammatory and Low Dose Antiproliferative Therapy for Squamous Cell Carcinomas in Recessive Dystrophic Epidermolysis Bullosa. J. Eur. Acad. Dermatol. Venereol. 2019, 34, E1–E3. [Google Scholar] [CrossRef]
- Piccerillo, A.; El Hachem, M.; De Vito, R.; De Luca, E.V.; Peris, K. Pembrolizumab for Treatment of a Patient with Multiple Cutaneous Squamous Cell Carcinomas and Recessive Dystrophic Epidermolysis Bullosa. JAMA Dermatol. 2020, 156, 708–710. [Google Scholar] [CrossRef]
- Duong, T.; Wong, D.; Barrett, A.; Price, H. Successful Use of Immunotherapy to Treat Advanced Cutaneous Squamous Cell Carcinoma in Recessive Dystrophic Epidermolysis Bullosa. BMJ Case Rep. 2021, 14, e238966. [Google Scholar] [CrossRef]
- Khaddour, K.; Gorell, E.S.; Dehdashti, F.; Tang, J.Y.; Ansstas, G. Induced Remission of Metastatic Squamous Cell Carcinoma with an Immune Checkpoint Inhibitor in a Patient with Recessive Dystrophic Epidermolysis Bullosa. Case Rep. Oncol. 2020, 13, 911–915. [Google Scholar] [CrossRef]
- Vasilev, P.; Kalev, D.; Karamanliev, M.; Dimitrov, D.; Troyanova, P.; Yordanova, I. Cemiplimab Treatment of Squamous Cell Carcinoma in a Patient with Severe Recessive Dystrophic Epidermolysis Bullosa. J. Dtsch. Dermatol. Ges. 2023, 21, 295–297. [Google Scholar] [CrossRef]
- Cho, R.J.; Alexandrov, L.B.; den Breems, N.Y.; Atanasova, V.S.; Farshchian, M.; Purdom, E.; Nguyen, T.N.; Coarfa, C.; Rajapakshe, K.; Prisco, M.; et al. APOBEC Mutation Drives Early-Onset Squamous Cell Carcinomas in Recessive Dystrophic Epidermolysis Bullosa. Sci. Transl. Med. 2018, 10, eaas9668. [Google Scholar] [CrossRef]
- Emmert, H.; Culley, J.; Brunton, V.G. Inhibition of Cyclin-Dependent Kinase Activity Exacerbates H2 O2 -Induced DNA Damage in Kindler Syndrome Keratinocytes. Exp. Dermatol. 2019, 28, 1074–1078. [Google Scholar] [CrossRef]
- Sun, Z.; Costell, M.; Fässler, R. Integrin Activation by Talin, Kindlin and Mechanical Forces. Nat. Cell Biol. 2019, 21, 25–31. [Google Scholar] [CrossRef]
- Plow, E.F.; Das, M.; Bialkowska, K.; Sossey-Alaoui, K. Of Kindlins and Cancer. Discoveries 2016, 4, e59. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, J.; Song, J.; Wang, Y.; Fang, W.; Liu, Z.; Zhang, H. Differential Expression of Kindlin-1 and Kindlin-2 Correlates with Esophageal Cancer Progression and Epidemiology. Sci. China Life Sci. 2017, 60, 1214–1222. [Google Scholar] [CrossRef]
- Kong, J.; Du, J.; Wang, Y.; Yang, M.; Gao, J.; Wei, X.; Fang, W.; Zhan, J.; Zhang, H. Focal Adhesion Molecule Kindlin-1 Mediates Activation of TGF-β Signaling by Interacting with TGF-ΒRI, SARA and Smad3 in Colorectal Cancer Cells. Oncotarget 2016, 7, 76224–76237. [Google Scholar] [CrossRef]
- Fritsch, A.; Loeckermann, S.; Kern, J.S.; Braun, A.; Bosl, M.R.; Bley, T.A.; Schumann, H.; von Elverfeldt, D.; Paul, D.; Erlacher, M.; et al. A Hypomorphic Mouse Model of Dystrophic Epidermolysis Bullosa Reveals Mechanisms of Disease and Response to Fibroblast Therapy. J. Clin. Investig. 2008, 118, 1669–1679. [Google Scholar] [CrossRef]
- Heinemann, A.; He, Y.; Zimina, E.; Boerries, M.; Busch, H.; Chmel, N.; Kurz, T.; Bruckner-Tuderman, L.; Has, C. Induction of Phenotype Modifying Cytokines by FERMT1 Mutations. Hum. Mutat. 2011, 32, 397–406. [Google Scholar] [CrossRef]
- Chacón-Solano, E.; Leon, C.; Díaz, F.; García-García, F.; García, M.; Escámez, M.J.; Aspizua, S.G.; Conti, C.J.; Mencía, Á.; Martínez-Santamaría, L.; et al. Fibroblasts Activation and Abnormal Extracellular Matrix Remodelling as Common Hallmarks in Three Cancer-Prone Genodermatoses. Br. J. Dermatol. 2019, 181, 512–522. [Google Scholar] [CrossRef]
- Mittapalli, V.R.; Madl, J.; Löffek, S.; Kiritsi, D.; Kern, J.S.; Römer, W.; Nyström, A.; Bruckner-Tuderman, L. Injury-Driven Stiffening of the Dermis Expedites Skin Carcinoma Progression. Cancer Res. 2016, 76, 940–951. [Google Scholar] [CrossRef]
- Ng, Y.-Z.; Pourreyron, C.; Salas-Alanis, J.C.; Dayal, J.H.S.; Cepeda-Valdes, R.; Yan, W.; Wright, S.; Chen, M.; Fine, J.-D.; Hogg, F.J.; et al. Fibroblast-Derived Dermal Matrix Drives Development of Aggressive Cutaneous Squamous Cell Carcinoma in Patients with Recessive Dystrophic Epidermolysis Bullosa. Cancer Res. 2012, 72, 3522–3534. [Google Scholar] [CrossRef]
- Martins, V.L.; Vyas, J.J.; Chen, M.; Purdie, K.; Mein, C.A.; South, A.P.; Storey, A.; McGrath, J.A.; O’Toole, E.A. Increased Invasive Behaviour in Cutaneous Squamous Cell Carcinoma with Loss of Basement-Membrane Type VII Collagen. J. Cell Sci. 2009, 122, 1788–1799. [Google Scholar] [CrossRef]
- Dayal, J.H.S.; Mason, S.M.; Salas-Alanis, J.C.; McGrath, J.A.; Taylor, R.G.; Mellerio, J.E.; Blyth, K.; South, A.P.; Inman, G.J. Heterogeneous Addiction to Transforming Growth Factor-Beta Signalling in Recessive Dystrophic Epidermolysis Bullosa-Associated Cutaneous Squamous Cell Carcinoma. Br. J. Dermatol. 2020, 184, 697–708. [Google Scholar] [CrossRef]
- Hoste, E.; Arwert, E.N.; Lal, R.; South, A.P.; Salas-Alanis, J.C.; Murrell, D.F.; Donati, G.; Watt, F.M. Innate Sensing of Microbial Products Promotes Wound-Induced Skin Cancer. Nat. Commun. 2015, 6, 5932. [Google Scholar] [CrossRef]
- Alexeev, V.; Huitema, L.; Phillips, T.; Cepeda, R.; de Los Cobos, D.; Perez, R.I.M.; Salas-Garza, M.; Fajardo-Ramirez, O.R.; Ringpfeil, F.; Uitto, J.; et al. T-Cell Activation and Bacterial Infection in Skin Wounds of Recessive Dystrophic Epidermolysis Bullosa Patients. Exp. Dermatol. 2022, 31, 1431–1442. [Google Scholar] [CrossRef]
- Fife, B.T.; Pauken, K.E. The Role of the PD-1 Pathway in Autoimmunity and Peripheral Tolerance. Ann. N. Y. Acad. Sci. 2011, 1217, 45–59. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Curran, T.-A.; Jalili, R.B.; Farrokhi, A.; Ghahary, A. IDO Expressing Fibroblasts Promote the Expansion of Antigen Specific Regulatory T Cells. Immunobiology 2014, 219, 17–24. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Li, Y.; Gasmi, B.; Munn, D.H.; Allison, J.P.; Merghoub, T.; Wolchok, J.D. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015, 13, 412–424. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and Its Role in Regulating Anti-Tumor Immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The Promising Immune Checkpoint LAG-3: From Tumor Microenvironment to Cancer Immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and Treatment of Invasive Squamous Cell Carcinoma of the Skin: European Consensus-Based Interdisciplinary Guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef]
- Föll, M.C.; Fahrner, M.; Gretzmeier, C.; Thoma, K.; Biniossek, M.L.; Kiritsi, D.; Meiss, F.; Schilling, O.; Nyström, A.; Kern, J.S. Identification of Tissue Damage, Extracellular Matrix Remodeling and Bacterial Challenge as Common Mechanisms Associated with High-Risk Cutaneous Squamous Cell Carcinomas. Matrix Biol. 2018, 66, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.; Bruckner-Tuderman, L.; Kiritsi, D. Dystrophic Epidermolysis Bullosa: Secondary Disease Mechanisms and Disease Modifiers. Front. Genet. 2021, 12, 737272. [Google Scholar] [CrossRef]
- Mittapalli, V.R.; Kühl, T.; Kuzet, S.E.; Gretzmeier, C.; Kiritsi, D.; Gaggioli, C.; Bruckner-Tuderman, L.; Nyström, A. STAT3 Targeting in Dystrophic Epidermolysis Bullosa. Br. J. Dermatol. 2020, 182, 1279–1281. [Google Scholar] [CrossRef]
- Haghighi Javid, A.; Li, D.; Technau-Hafsi, K.; Has, C. Interleukin-17A Immune Pattern across Genetic Acantholytic and Blistering Disorders. Clin. Exp. Dermatol. 2023, 48, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, G.; Morgese, M.G.; Esposito, S.; Lopalco, G.; Lattarulo, M.; Tampoia, M.; Bonamonte, D.; Brunetti, L.; Vitale, A.; Lapadula, G.; et al. Proinflammatory Cytokines and Antiskin Autoantibodies in Patients with Inherited Epidermolysis Bullosa. Medicine 2015, 94, e1528. [Google Scholar] [CrossRef] [PubMed]
- Zakharia, Y.; McWilliams, R.R.; Rixe, O.; Drabick, J.; Shaheen, M.F.; Grossmann, K.F.; Kolhe, R.; Pacholczyk, R.; Sadek, R.; Tennant, L.L.; et al. Phase II Trial of the IDO Pathway Inhibitor Indoximod plus Pembrolizumab for the Treatment of Patients with Advanced Melanoma. J. Immunother. Cancer 2021, 9, e002057. [Google Scholar] [CrossRef]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A Phase 1/2 Trial of an Immune-Modulatory Vaccine against IDO/PD-L1 in Combination with Nivolumab in Metastatic Melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef]
- Filoni, A.; Cicco, G.; Cazzato, G.; Bosco, A.; Lospalluti, L.; Tucci, M.; Cimmino, A.; Foti, C.; Marzullo, A.; Bonamonte, D. Immune Disregulation in Cutaneous Squamous Cell Carcinoma of Patients with Recessive Dystrophic Epidermolysis Bullosa: A Single Pilot Study. Life 2022, 12, 213. [Google Scholar] [CrossRef]
- Filoni, A.; Cicco, G.; Lospalluti, L.; Maglietta, A.; Foti, C.; Annichiarico, G.; Resta, L.; Bonamonte, D. Morphological and Morphometric Analysis of Cutaneous Squamous Cell Carcinoma in Patients with Recessive Dystrophic Epidermolysis Bullosa: A Retrospective Study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1707–1714. [Google Scholar] [CrossRef]
- Brummel, K.; Eerkens, A.L.; de Bruyn, M.; Nijman, H.W. Tumour-Infiltrating Lymphocytes: From Prognosis to Treatment Selection. Br. J. Cancer 2023, 128, 451–458. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Li, J.; Ferris, R.L. Differential Expression of PD-1 and Tim-3 Marks Activation versus Exhaustion Status of T Cells in the Tumor Microenvironment. J. ImmunoTher. Cancer 2014, 2, P220. [Google Scholar] [CrossRef][Green Version]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 Comes of Age as an Inhibitory Receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.-C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef]
- Wu, S.; Slater, N.A.; Sayed, C.J.; Googe, P.B. PD-L1 and LAG-3 Expression in Advanced Cutaneous Squamous Cell Carcinomas. J. Cutan. Pathol. 2020, 47, 882–887. [Google Scholar] [CrossRef]
- He, Y.; Rivard, C.J.; Rozeboom, L.; Yu, H.; Ellison, K.; Kowalewski, A.; Zhou, C.; Hirsch, F.R. Lymphocyte-Activation Gene-3, an Important Immune Checkpoint in Cancer. Cancer Sci. 2016, 107, 1193–1197. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tezlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant Relatlimab and Nivolumab in Resectable Melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef]
- Liu, J.; Luan, Y.; Deng, H.; Wang, F.; Wang, C.; Zhang, Z. A Bivalent Tim-3/PD-1 Bispecific Antibody for the Treatment of PD-1 Antibody Resistant or Refractory NSCLC. J. Clin. Oncol. 2022, 40, e14597. [Google Scholar] [CrossRef]
- Sung, E.; Ko, M.; Won, J.; Jo, Y.; Park, E.; Kim, H.; Choi, E.; Jung, U.; Jeon, J.; Kim, Y.; et al. LAG-3xPD-L1 Bispecific Antibody Potentiates Antitumor Responses of T Cells through Dendritic Cell Activation. Mol. Ther. 2022, 30, 2800–2816. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Zhou, Y.; Liu, Y.; Zhang, R.; Jiang, X.; Ren, C.; Gao, X.; Luo, L. PD-1/LAG-3 Bispecific Antibody Potentiates T Cell Activation and Increases Antitumor Efficacy. Front. Immunol. 2022, 13, 1047610. [Google Scholar] [CrossRef] [PubMed]
- Trefzer, L.; Hess, M.E.; Scholten, L.; Technau-Hafsi, K.; Meiss, F.; Boerries, M.; Has, C.; Rafei-Shamsabadi, D. Variable Outcome of Immunotherapy in Advanced Multiple Cutaneous Squamous Cell Carcinomas in Two Patients with Recessive Dystrophic Epidermolysis Bullosa. Acta Dermatovenerol. 2023, 103, 4870. [Google Scholar] [CrossRef]
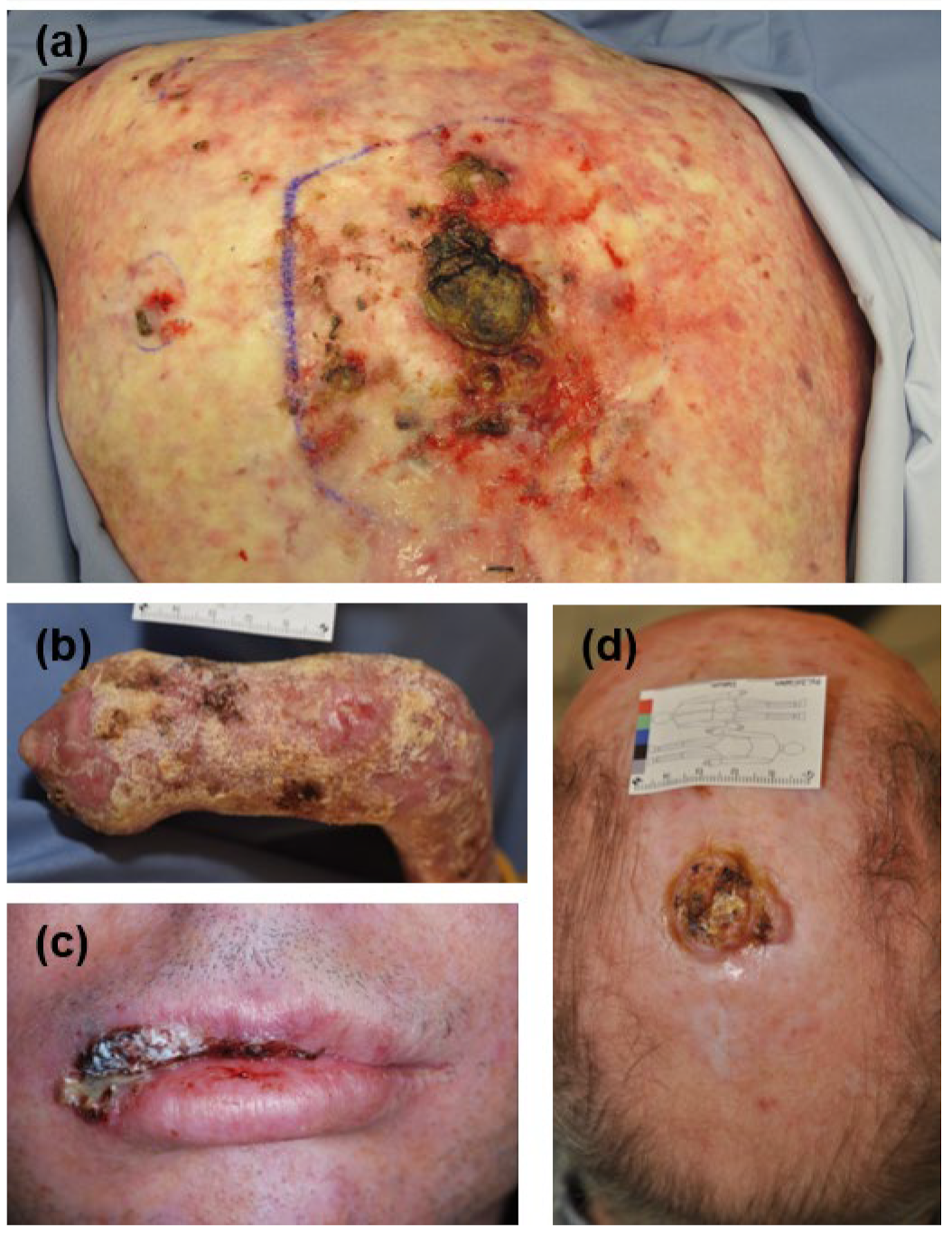
| DEB 1-SCC 2 | KEB 3-SCC | IC 4-SCC | IS 5-SCC | |
|---|---|---|---|---|
| Number of patients/SCC samples | 9/30 | 7/22 | 96/100 | 41/106 |
| Mean age at SCC diagnosis (range) in years | 32 (18–50) | 47 (30–65) | 80 (45–99) | 68 (41–87) |
| Gender (male:female) | 4:5 | 5:2 | 58:38 | 27:14 |
| Localization—number (%) | ||||
| Head and neck | 0 (0) | 7 (32) | 79 (79) | 54 (51) |
| Trunk | 0 (0) | 0 (0) | 0 (0) | 17 (16) |
| Upper extremities | 16 (53) | 5 (23) | 19 (19) | 24 (23) |
| Lower extremities | 14 (47) | 10 (45) | 2 (2) | 11 (10) |
| Histologic grading—number (%) | ||||
| G1 | 22 (73) | 8 (36) | 48 (48) | 38 (36) |
| G2 | 5 (17) | 3 (14) | 51 (51) | 60 (57) |
| G3 | 3 (10) | 11 (50) | 1 (1) | 8 (7) |
| Vertical tumor thickness in mm (%) | ||||
| ≤2 | 7 (23) | 6 (27) | 42 (42) | 35 (33) |
| 2.01–6 | 14 (47) | 16 (73) | 40 (40) | 58 (55) |
| >6 | 5 (17) | 0 (0) | 18 (18) | 9 (8) |
| NA 6 | 4 (13) | 0 (0) | 0 (0) | 4 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafei-Shamsabadi, D.; Scholten, L.; Lu, S.; Castiglia, D.; Zambruno, G.; Volz, A.; Arnold, A.; Saleva, M.; Martin, L.; Technau-Hafsi, K.; et al. Epidermolysis-Bullosa-Associated Squamous Cell Carcinomas Support an Immunosuppressive Tumor Microenvironment: Prospects for Immunotherapy. Cancers 2024, 16, 471. https://doi.org/10.3390/cancers16020471
Rafei-Shamsabadi D, Scholten L, Lu S, Castiglia D, Zambruno G, Volz A, Arnold A, Saleva M, Martin L, Technau-Hafsi K, et al. Epidermolysis-Bullosa-Associated Squamous Cell Carcinomas Support an Immunosuppressive Tumor Microenvironment: Prospects for Immunotherapy. Cancers. 2024; 16(2):471. https://doi.org/10.3390/cancers16020471
Chicago/Turabian StyleRafei-Shamsabadi, David, Lena Scholten, Sisi Lu, Daniele Castiglia, Giovanna Zambruno, Andreas Volz, Andreas Arnold, Mina Saleva, Ludovic Martin, Kristin Technau-Hafsi, and et al. 2024. "Epidermolysis-Bullosa-Associated Squamous Cell Carcinomas Support an Immunosuppressive Tumor Microenvironment: Prospects for Immunotherapy" Cancers 16, no. 2: 471. https://doi.org/10.3390/cancers16020471
APA StyleRafei-Shamsabadi, D., Scholten, L., Lu, S., Castiglia, D., Zambruno, G., Volz, A., Arnold, A., Saleva, M., Martin, L., Technau-Hafsi, K., Meiss, F., von Bubnoff, D., & Has, C. (2024). Epidermolysis-Bullosa-Associated Squamous Cell Carcinomas Support an Immunosuppressive Tumor Microenvironment: Prospects for Immunotherapy. Cancers, 16(2), 471. https://doi.org/10.3390/cancers16020471





