Toll-like Receptor Agonists Are Unlikely to Provide Benefits in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
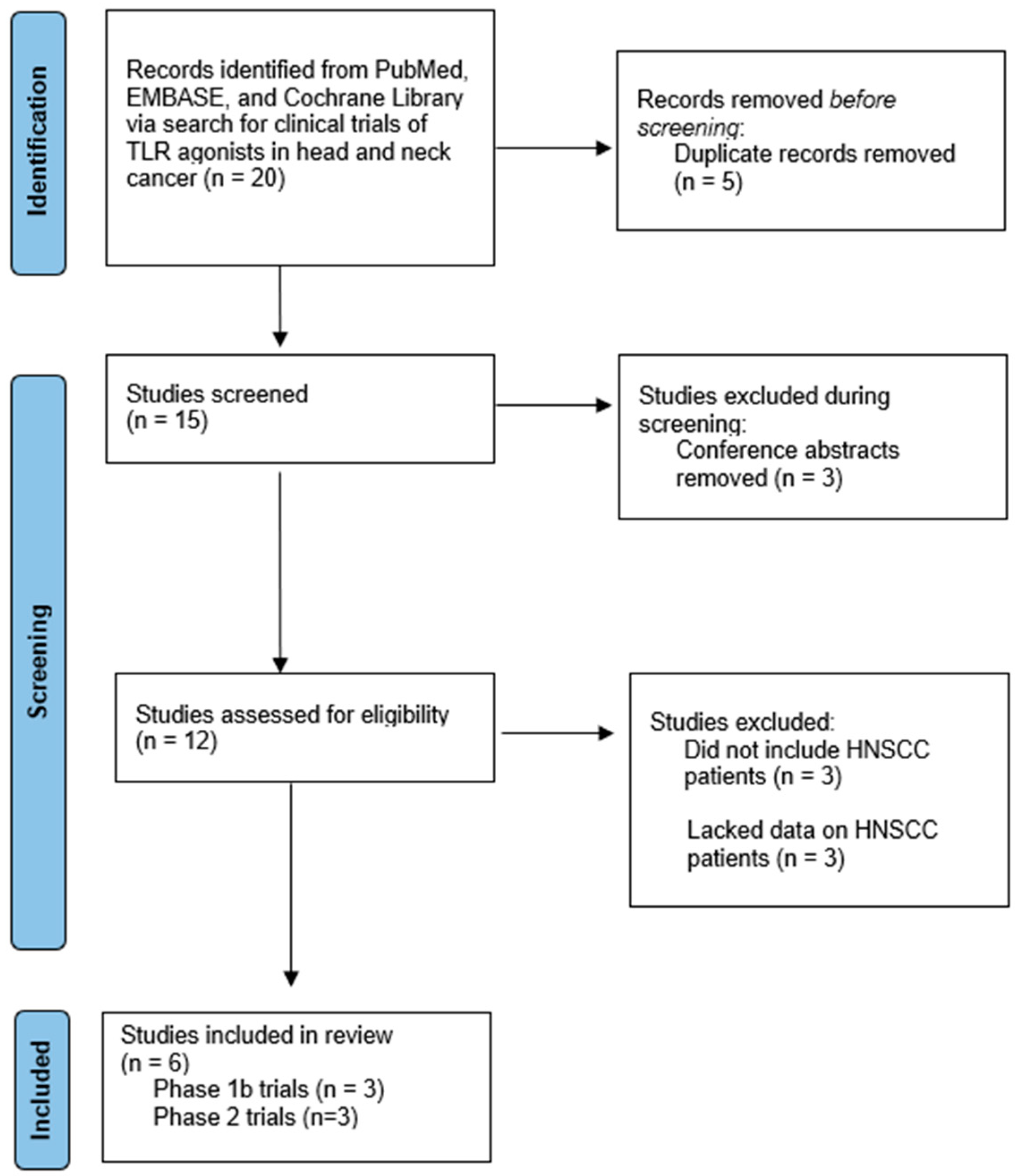
3.1. Study Selection
3.2. Phase 1b Trials
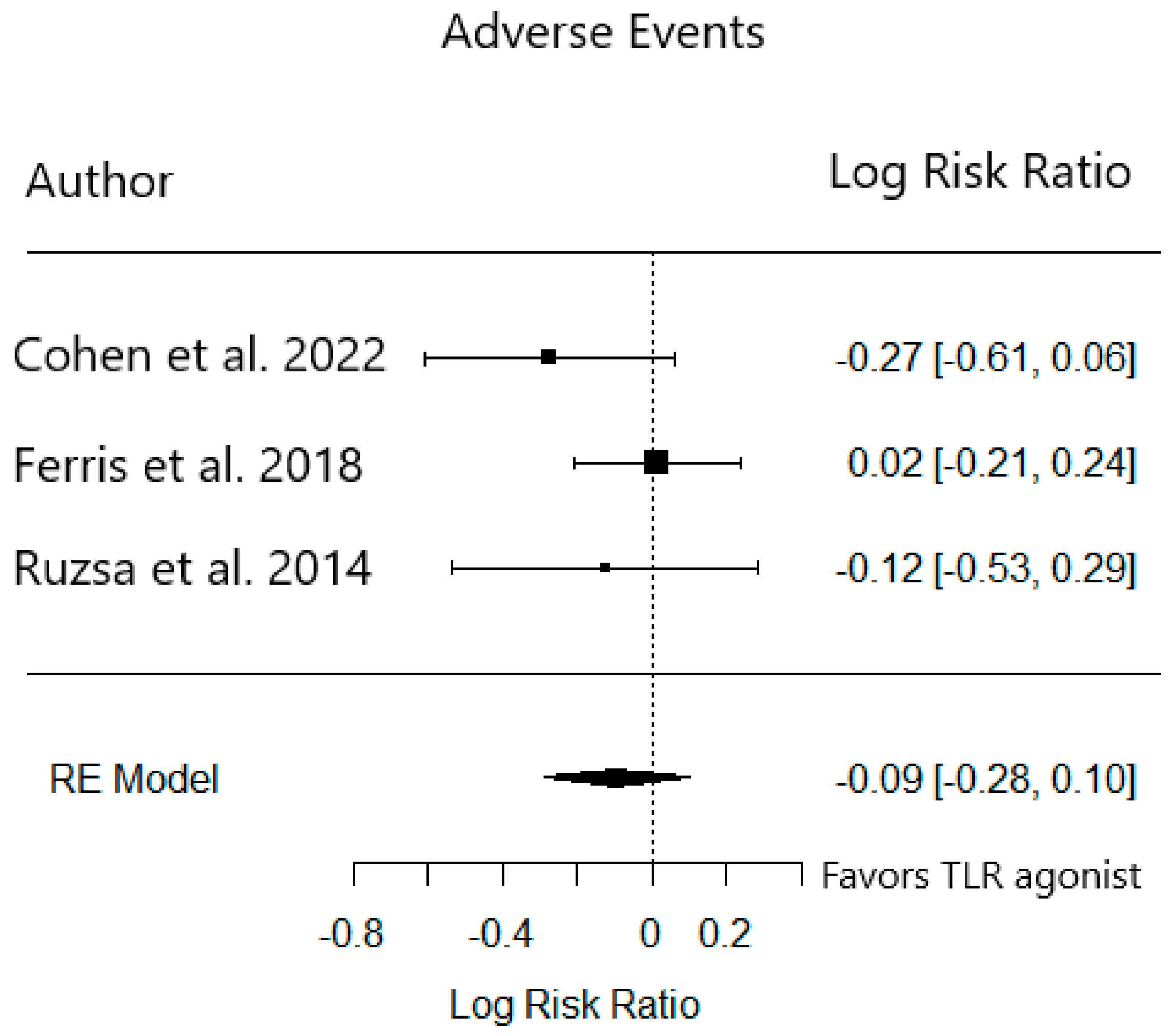
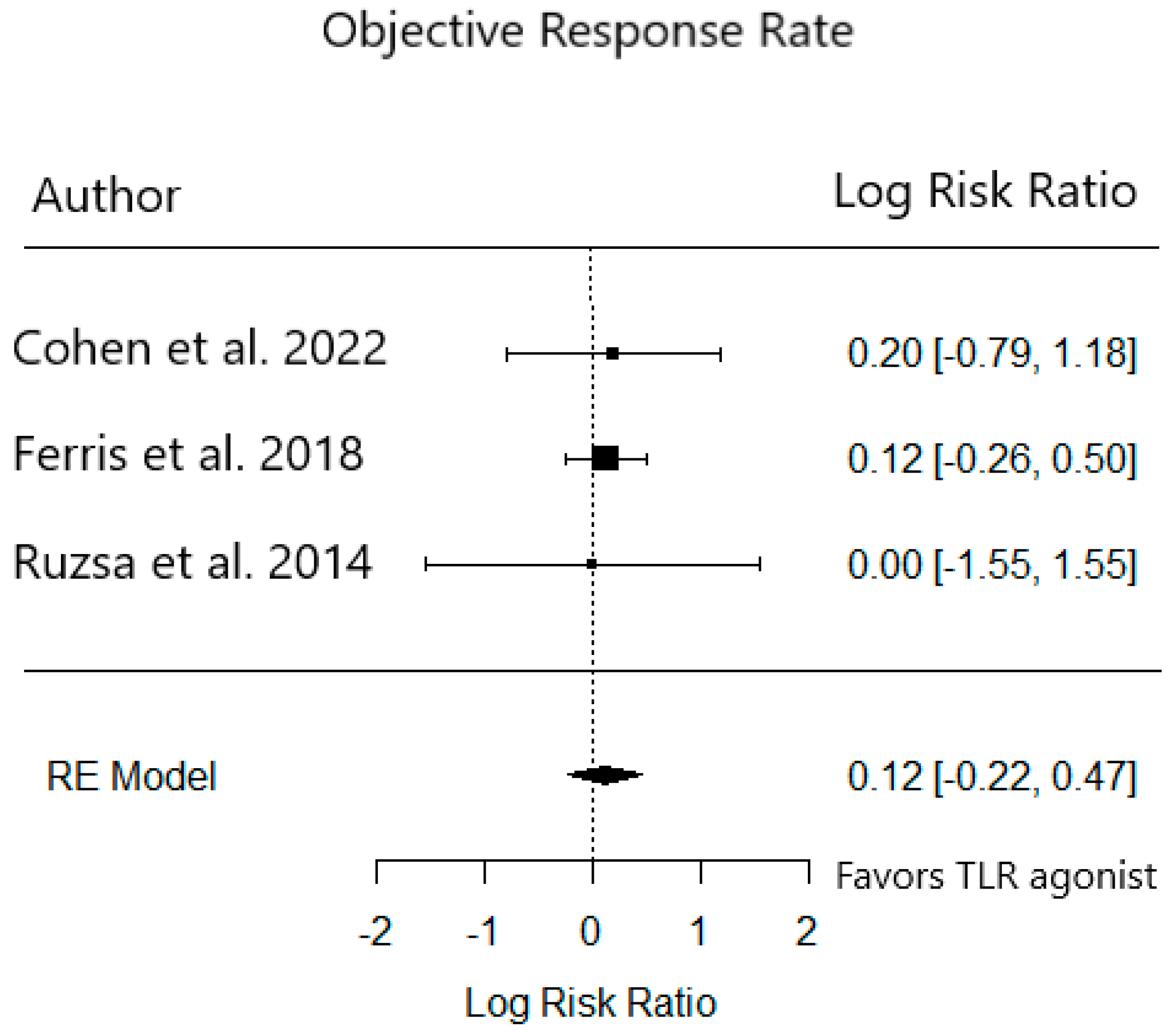
3.3. Phase 2 Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Larynx—Cancer Stat Facts. SEER. Available online: https://seer.cancer.gov/statfacts/html/laryn.html (accessed on 10 April 2023).
- Cancer of the Oral Cavity and Pharynx—Cancer Stat Facts. SEER. Available online: https://seer.cancer.gov/statfacts/html/oralcav.html (accessed on 10 April 2023).
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: An Open-Label, Multi-Arm, Non-Randomised, Multicentre, Phase 2 Trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Saito, S.; Uppaluri, R. Immunotherapy for Head and Neck Cancer: A Paradigm Shift From Induction Chemotherapy to Neoadjuvant Immunotherapy. Front. Oncol. 2021, 11, 727433. Available online: https://www.frontiersin.org/article/10.3389/fonc.2021.727433 (accessed on 15 March 2022). [CrossRef]
- Urban-Wojciuk, Z.; Khan, M.M.; Oyler, B.L.; Fåhraeus, R.; Marek-Trzonkowska, N.; Nita-Lazar, A.; Hupp, T.R.; Goodlett, D.R. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front. Immunol. 2019, 10, 2388. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chow, L.Q.; Morishima, C.; Eaton, K.D.; Baik, C.S.; Goulart, B.H.; Anderson, L.N.; Manjarrez, K.L.; Dietsch, G.N.; Bryan, J.K.; Hershberg, R.M.; et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin. Cancer Res. 2017, 23, 2442–2450. [Google Scholar] [CrossRef]
- Shayan, G.; Kansy, B.A.; Gibson, S.P.; Srivastava, R.M.; Bryan, J.K.; Bauman, J.E.; Ohr, J.; Kim, S.; Duvvuri, U.; Clump, D.A.; et al. Phase Ib Study of Immune Biomarker Modulation with Neoadjuvant Cetuximab and TLR8 stimulation in Head and Neck Cancer to Overcome Suppressive Myeloid Signals. Clin. Cancer Res. 2018, 24, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.-P.; Kaminsky, M.-C.; Keller, U.; Brümmendorf, T.H.; Goddemeier, T.; Forssmann, U.; Delord, J.-P. Phase Ib trial of the Toll-like receptor 9 agonist IMO-2055 in combination with 5-fluorouracil, cisplatin, and cetuximab as first-line palliative treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Investig. New Drugs 2013, 31, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Nabell, L.; Wong, D.J.; Day, T.; Daniels, G.A.; Milhem, M.; Deva, S.; Jameson, M.; Guntinas-Lichius, O.; Almubarak, M.; et al. Intralesional SD-101 in Combination with Pembrolizumab in Anti-PD-1 Treatment-Naïve Head and Neck Squamous Cell Carcinoma: Results from a Multicenter, Phase II Trial. Clin. Cancer Res. 2022, 28, 1157–1166. [Google Scholar] [CrossRef]
- Ferris, R.L.; Saba, N.F.; Gitlitz, B.J.; Haddad, R.; Sukari, A.; Neupane, P.; Morris, J.C.; Misiukiewicz, K.; Bauman, J.E.; Fenton, M.; et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients With Squamous Cell Carcinoma of the Head and Neck. JAMA Oncol. 2018, 4, 1583–1588. [Google Scholar] [CrossRef]
- Ruzsa, A.; Sen, M.; Evans, M.; Lee, L.W.; Hideghety, K.; Rottey, S.; Klimak, P.; Holeckova, P.; Fayette, J.; Csoszi, T.; et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naïve patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Investig. New Drugs 2014, 32, 1278–1284. [Google Scholar] [CrossRef]
- Owen, A.M.; Fults, J.B.; Patil, N.K.; Hernandez, A.; Bohannon, J.K. TLR Agonists as Mediators of Trained Immunity: Mechanistic Insight and Immunotherapeutic Potential to Combat Infection. Front. Immunol. 2021, 11, 622614. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2020.622614 (accessed on 28 April 2023). [CrossRef]
- Adams, S. Toll-like receptor agonists in cancer therapy. Immunotherapy 2009, 1, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef]
- Farooq, M.; Batool, M.; Kim, M.S.; Choi, S. Toll-Like Receptors as a Therapeutic Target in the Era of Immunotherapies. Front. Cell Dev. Biol. 2021, 9, 756315. Available online: https://www.frontiersin.org/articles/10.3389/fcell.2021.756315 (accessed on 28 April 2023). [CrossRef] [PubMed]
- Kaur, P.; Asea, A. Radiation-induced effects and the immune system in cancer. Front. Oncol. 2012, 2, 191. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2012.00191 (accessed on 28 April 2023). [CrossRef]
- Carvalho, H.d.A.; Villar, R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics 2018, 73 (Suppl. 1), e557s. [Google Scholar] [CrossRef]
- Smith, J.D.; Ludwig, M.L.; Bhangale, A.D.; Brummel, C.; Swiecicki, P.L.; Worden, F.P.; Chinn, S.B.; Stucken, C.L.; Rosko, A.J.; Prince, M.E.P.; et al. Tumor immune microenvironment alterations using induction cetuximab in a phase II trial of deintensified therapy for p16-positive oropharynx cancer. Head Neck 2023, 45, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Medina, T.; Kummar, S.; Amin, A.; Kalbasi, A.; Drabick, J.J.; Barve, M.; Daniels, G.A.; Wong, D.J.; Schmidt, E.V.; et al. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discov. 2018, 8, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Brady, M.F.; Aghajanian, C.; Lankes, H.A.; Rizack, T.; Leach, J.; Fowler, J.M.; Higgins, R.; Hanjani, P.; Morgan, M.; et al. A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: A Gynecologic Oncology Group partners study. Ann. Oncol. 2017, 28, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Conkling, P.; Richards, D.A.; Nemunaitis, J.J.; Boyd, T.E.; Mita, A.C.; de La Bourdonnaye, G.; Wages, D.; Bexon, A.S. Antitumor activity and safety of combination therapy with the Toll-like receptor 9 agonist IMO-2055, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol. Immunother. 2014, 63, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Shah, M.; Kim, J.; Choi, S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med. Res. Rev. 2019, 39, 1053–1090. [Google Scholar] [CrossRef]
- Rolfo, C.; Giovannetti, E.; Martinez, P.; McCue, S.; Naing, A. Applications and clinical trial landscape using Toll-like receptor agonists to reduce the toll of cancer. npj Precis. Oncol. 2023, 7, 26. [Google Scholar] [CrossRef] [PubMed]
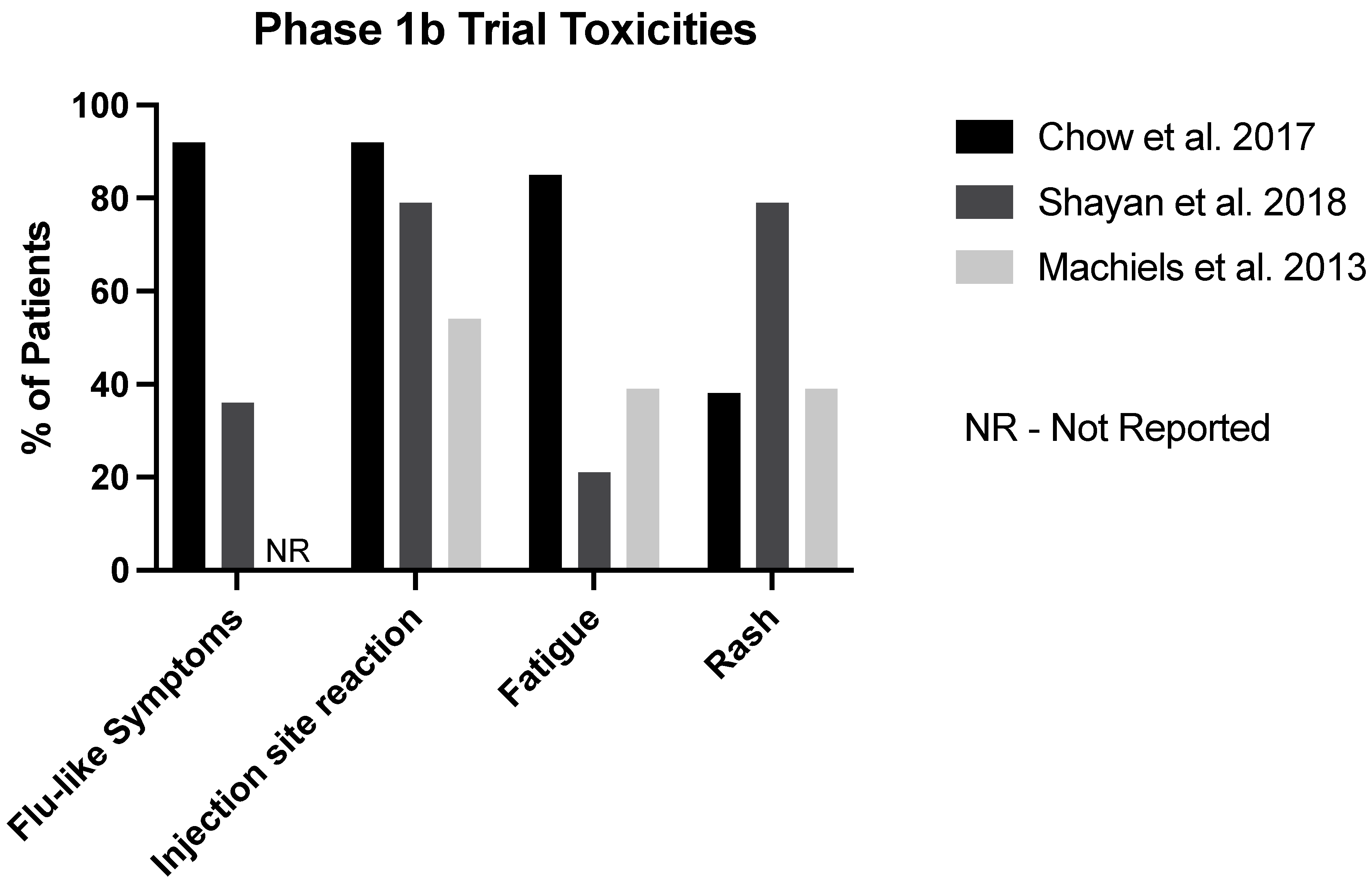
| Study | Chow et al. 2017 [12] | Shayan et al. 2018 [13] | Machiels et al. 2013 [14] * |
|---|---|---|---|
| Agent | Motolimod | Motolimod | IMO-2055 |
| Treatment | Motolimod + cetuximab | Motolimod + cetuximab | IMO-2055 + 5-fluorouracil, cisplatin, and cetuximab |
| Patient Population | R/M HNSCC | Untreated HNSCC | R/M HNSCC |
| Number of Patients | 13 | 14 | 13 |
| Median Age | 62 | 61 | 59 |
| ECOG | |||
| 0 (%) | 15 | - | 62 |
| 1 (%) | 70 | - | 38 |
| ≥2 (%) | 15 | - | 0 |
| Sex | |||
| Male (%) | 77 | 64 | 92 |
| Female (%) | 23 | 36 | 8 |
| Tumor Site | |||
| Oral cavity (%) | 15 | 71 | 38 |
| Oropharynx (%) | 46 | 7 | 38 |
| Larynx (%) | 23 | 14 | 8 |
| Hypopharynx (%) | 8 | 7 | 15 |
| Other (%) | 8 | 0 | 0 |
| HPV Status | |||
| Positive (%) | 23 | - | - |
| Negative (%) | 8 | - | - |
| Unknown (%) | 70 | - | - |
| Prior Treatment | |||
| Chemotherapy (%) | 77 | - | 69 |
| Radiation (%) | 92 | - | 100 |
| Surgery (%) | 70 | - | 77 |
| Cetuximab (%) | 77 | - | - |
| Recurrence Type | |||
| Locoregional (%) | 8 | - | 31 |
| Distant Metastasis (%) | 46 | - | 69 |
| Both (%) | 46 | - | 0 |
| Study | Chow et al. 2017 [12] | Shayan et al. 2018 [13] | Machiels et al. 2013 [14] * |
|---|---|---|---|
| Agent | Motolimod | Motolimod | IMO-2055 |
| Treatment | Motolimod + cetuximab | Motolimod + cetuximab | IMO-2055 + 5-fluorouracil, cisplatin, and cetuximab |
| Number of Patients | 13 | 14 | 13 |
| Dosage | Motolimod: 2.5 mg/m2, 3.0 mg/m2, or 3.5 mg/m2 Cetuximab: 250 mg/m2 | Motolimod: 2.5 mg/m2 Cetuximab: 400 mg/m2 loading then 250 mg/m2 | IMO-2055: 0.16 mg/kg or 0.32 mg/kg Cetuximab: 400 mg/m2 loading then 250 mg/m2 Cisplatin: 100 mg/m2/day 5-fluorouracil: 1000 mg/m2/day |
| Flu-Like Symptoms (%) | 92 | 36 | - |
| Injection Site Reaction (%) | 92 | 79 | 54 |
| Fatigue (%) | 85 | 21 | 39 |
| Rash (%) | 38 | 79 | 39 |
| Grade 3+ AEs (%) | 8 | - * | 92 |
| Fatal AEs (%) | 0 | 0 | 8 |
| Discontinued Due to AEs (%) | 0 | 0 | 31 |
| Trial Reference | Cohen et al. 2022 [15] | Ferris et al. 2018 [16] | Ruzsa et al. 2014 [17] | |||
|---|---|---|---|---|---|---|
| Agent | SD-101 | Motolimod | EMD 1201081 | |||
| Treatment Group | SD-101 8 mg + pembrolizumab | SD-101 2 mg + pembrolizumab | EXTREME regimen + motolimod | EXTREME regimen + placebo | EMD 1201081 + cetuximab | Cetuximab only |
| Number of Patients | 23 | 28 | 100 | 95 | 53 | 53 |
| Median Age | 65 | 63 | 58 | 60 | 58 | 57 |
| ECOG | ||||||
| 0 (%) | 26 | 18 | 38 | 39 | 23 | 23 |
| 1 (%) | 74 | 82 | 62 | 61 | 77 | 77 |
| ≥2 (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Sex | ||||||
| Male (%) | 91 | 68 | 85 | 85 | 85 | 85 |
| Female (%) | 9 | 32 | 15 | 15 | 15 | 15 |
| Tumor Site | ||||||
| Oral cavity (%) | 57 | 46 | 27 | 27 | - | - |
| Oropharynx (%) | 9 | 32 | 40 | 45 | - | - |
| Larynx (%) | 17 | 11 | 22 | 21 | - | - |
| Hypopharynx (%) | 0 | 7 | 4 | 5 | - | - |
| Other (%) | 17 | 4 | 7 | 1 | - | - |
| HPV Status | ||||||
| Positive (%) | 26 | 36 | 60 * | 65 * | - | - |
| Negative (%) | 26 | 39 | 33 * | 28 * | - | - |
| Unknown (%) | 48 | 25 | 8 * | 7 * | - | - |
| Prior Treatment | ||||||
| Chemotherapy (%) | - | - | 63 | 58 | 98 | 100 |
| Radiation (%) | 87 | 75 | 79 | 85 | 85 | 77 |
| Surgery (%) | 96 | 86 | 56 | 56 | 38 | 53 |
| Cetuximab (%) | - | - | 10 | 21 | ||
| Recurrence Type | ||||||
| Locoregional (%) | 9 | 7 | - | - | 70 | 57 |
| Distant Metastasis (%) | 61 | 57 | - | - | 30 | 43 |
| Both (%) | 30 | 36 | - | - | - | - |
| Trial Reference | Cohen et al. 2022 [15] | Ferris et al. 2018 [16] | Ruzsa et al. 2014 [17] | |||
|---|---|---|---|---|---|---|
| Agent | SD-101 | Motolimod | EMD 1201081 | |||
| Treatment Group | SD-101 8 mg + pembrolizumab | SD-101 2 mg + pembrolizumab | EXTREME regimen + motolimod | EXTREME regimen + placebo | EMD 1201081 + cetuximab | Cetuximab only |
| Number of Patients | 23 | 27 | 86 | 86 | 54 | 53 |
| Dosage | SD-101 8 mg in 1 lesion + pembrolizumab | SD-101 2 mg in 1–4 lesions + pembrolizumab | Motolimod 3 mg/m2 + cisplatin 100 mg/m2 + fluorouracil 1000 mg/m2 + cetuximab 400 mg/m2 loading then 250 mg/m2 | Placebo + cisplatin 100 mg/m2 + fluorouracil 1000 mg/m2 + cetuximab 400 mg/m2 loading then 250 mg/m2 | EMD 1201081 0.32 mg/kg + cetuximab 400 mg/m2 loading then 250 mg/m2 | Cetuximab 400 mg/m2 loading then 250 mg/m2 |
| Chills | 44 | 11 | 37 | 6 | - | - |
| Pyrexia | 26 | 22 | 43 | 12 | 19 | 6 |
| Injection Site Reaction | 17 * | 4 * | 39 | 0 | 20 | 0 |
| Fatigue | 74 | 56 | 43 | 45 | 15 | 23 |
| Rash | - | - | 19 | 27 | 30 | 32 |
| Grade 3+ AEs (%) | 35 | 15 | 39 | 40 | 56 | 51 |
| Fatal AEs (%) | 0 | 0 | 5 | 8 | 0 | 0 |
| Discontinued Due to AEs (%) | - | - | - | - | 19 | 15 |
| Trial Reference | Cohen et al. 2022 [15] | Ferris et al. 2018 [16] | Ruzsa et al. 2014 [17] | |||
|---|---|---|---|---|---|---|
| Agent | SD-101 | Motolimod | EMD 1201081 | |||
| Treatment Group | SD-101 8 mg + pembrolizumab | SD-101 2 mg + pembrolizumab | EXTREME regimen + motolimod | EXTREME regimen + placebo | EMD 1201081 + cetuximab | Cetuximab only |
| Number of Patients | 28 | 23 | 100 | 95 | 53 | 53 |
| Median Age | 63 | 65 | 58 | 60 | 58 | 57 |
| ORR (%) | 21 | 26 | 38 | 34 | 6 | 6 |
| CR (%) | 7 | 0 | 2 | 5 | 0 | 0 |
| PR (%) | 14 | 26 | 36 | 28 | 6 | 6 |
| SD (%) | 25 | 22 | 22 | 24 | 32 | 38 |
| PD (%) | 36 | 39 | 9 | 8 | 40 | 34 |
| Median PFS | 2.5 | 2.3 | 6.1 | 5.9 | 1.5 | 1.9 |
| Median OS | Not reached | 9 | 13.5 | 11.3 | 6.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddineni, S.; Chen, M.; Baik, F.; Divi, V.; Sunwoo, J.B.; Finegersh, A. Toll-like Receptor Agonists Are Unlikely to Provide Benefits in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 4386. https://doi.org/10.3390/cancers15174386
Maddineni S, Chen M, Baik F, Divi V, Sunwoo JB, Finegersh A. Toll-like Receptor Agonists Are Unlikely to Provide Benefits in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(17):4386. https://doi.org/10.3390/cancers15174386
Chicago/Turabian StyleMaddineni, Sainiteesh, Michelle Chen, Fred Baik, Vasu Divi, John B. Sunwoo, and Andrey Finegersh. 2023. "Toll-like Receptor Agonists Are Unlikely to Provide Benefits in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis" Cancers 15, no. 17: 4386. https://doi.org/10.3390/cancers15174386
APA StyleMaddineni, S., Chen, M., Baik, F., Divi, V., Sunwoo, J. B., & Finegersh, A. (2023). Toll-like Receptor Agonists Are Unlikely to Provide Benefits in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers, 15(17), 4386. https://doi.org/10.3390/cancers15174386