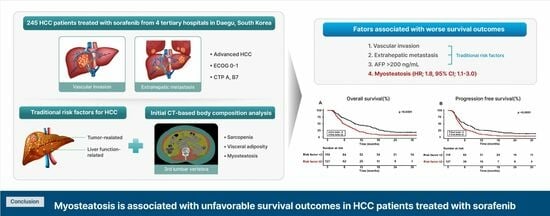
The Clinical Significance of Myosteatosis in Survival Outcomes in Patients with Hepatocellular Carcinoma Treated with Sorafenib
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Clinical Data
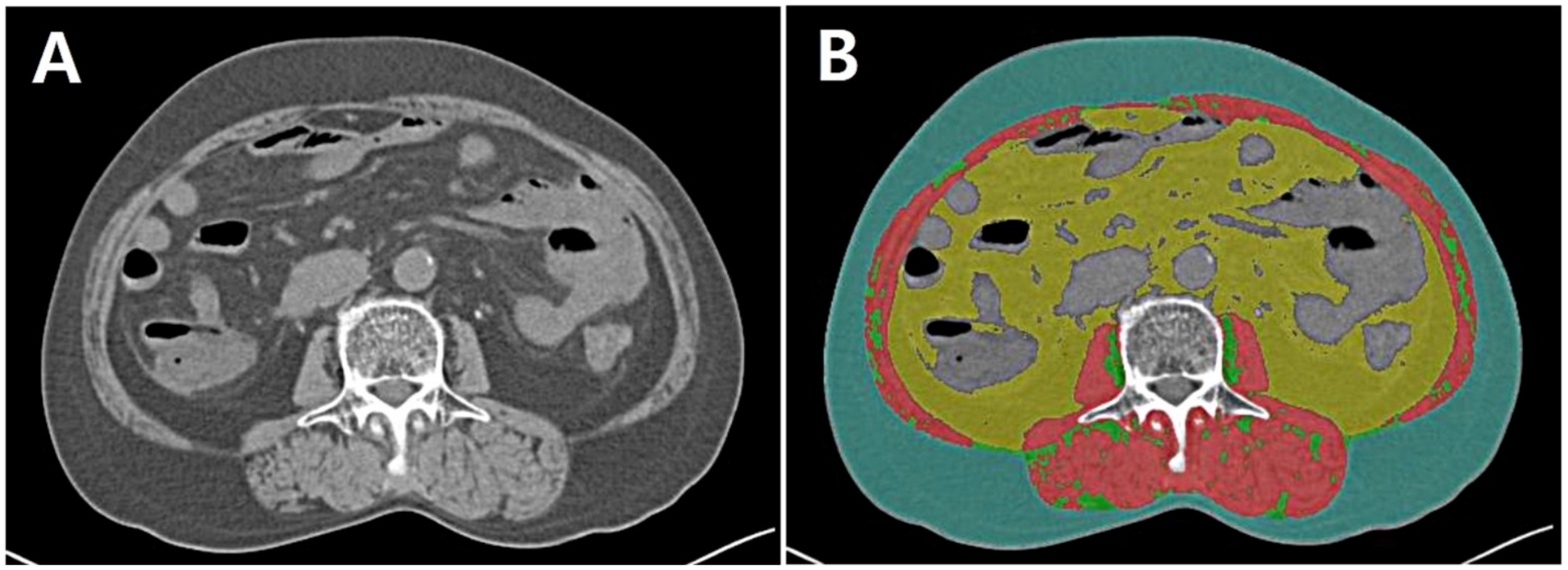
2.3. Assessment of Body Composition Data
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
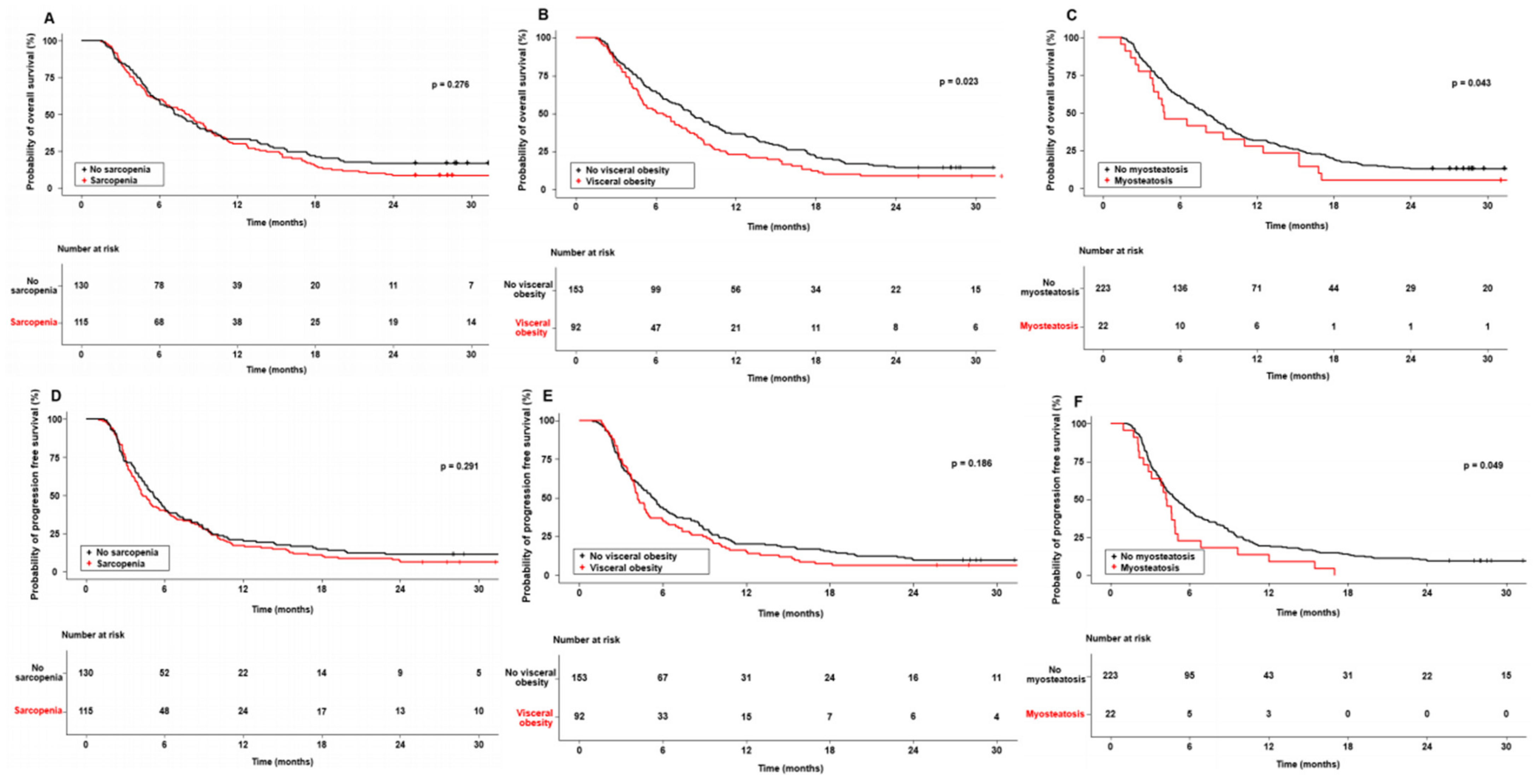
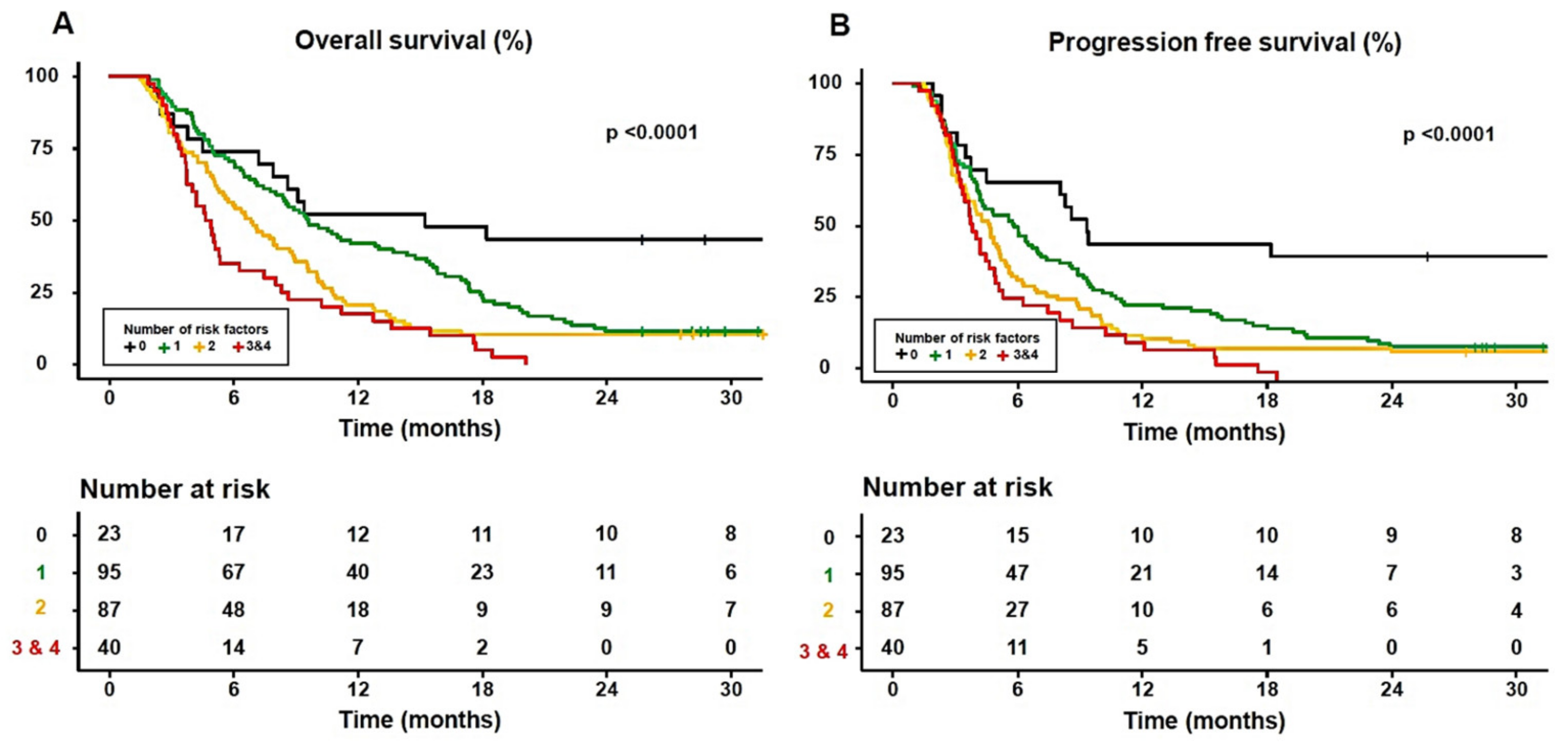
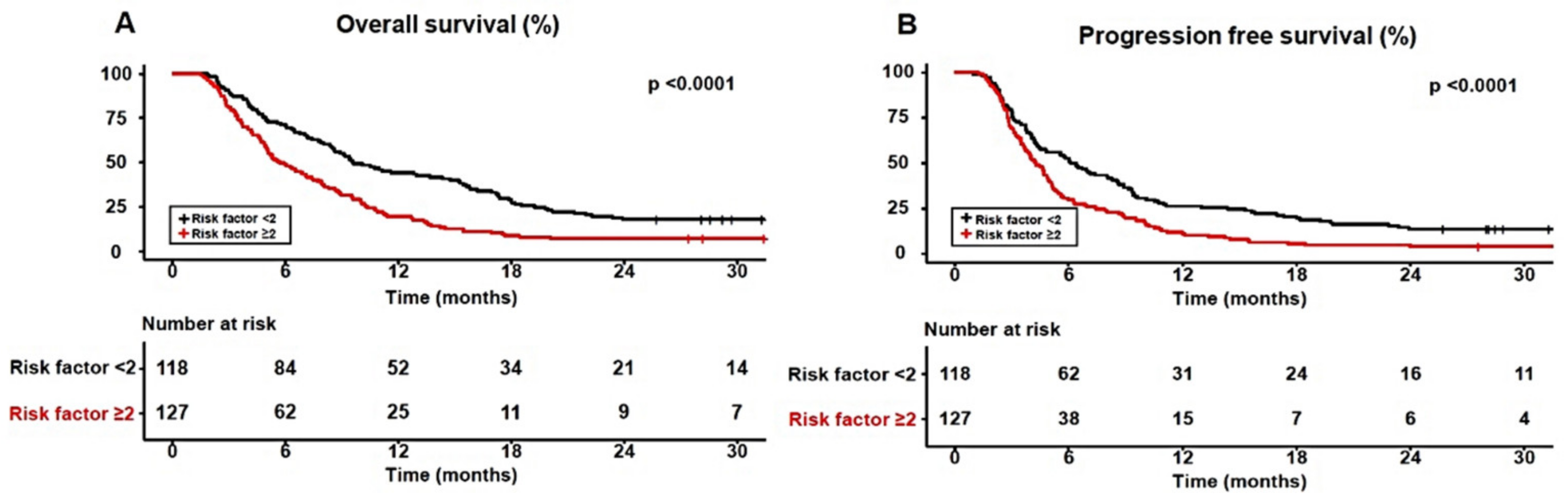
3.2. Impact of Body Composition on Survival in Sorafenib-Treated HCC Patients
3.3. Factors Associated with Survival in HCC Patients Treated with Sorafenib
3.4. The Adequate Number of Significant Prognostic Factors for Predicting Survival in Sorafenib-Treated HCC Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, M.; Cabibbo, G.; Piscaglia, F.; Zavaglia, C.; Grieco, A.; Villa, E.; Camma, C.; Colombo, M.; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology 2011, 54, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Kudo, M.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.F.; de Guevara, L.L.; Papandreou, C.; et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): Second interim analysis. Int. J. Clin. Pract. 2014, 68, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol. Lett. 2017, 14, 1637–1647. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Miwa, T.; Taguchi, D.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers 2019, 11, 1206. [Google Scholar] [CrossRef]
- Labeur, T.A.; van Vugt, J.L.A.; Ten Cate, D.W.G.; Takkenberg, R.B.; JNM, I.J.; Groot Koerkamp, B.; de Man, R.A.; van Delden, O.M.; Eskens, F.; Klumpen, H.J. Body Composition Is an Independent Predictor of Outcome in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Liver Cancer 2019, 8, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Pigneur, F.; Nelson, A.C.; Costentin, C.; Tselikas, L.; Katsahian, S.; Diao, G.; Laurent, A.; Mallat, A.; Duvoux, C.; et al. Visceral fat area predicts survival in patients with advanced hepatocellular carcinoma treated with tyrosine kinase inhibitors. Dig. Liver Dis. 2015, 47, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Kawano, R.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; et al. No Muscle Depletion with High Visceral Fat as a Novel Beneficial Biomarker of Sorafenib for Hepatocellular Carcinoma. Liver Cancer 2018, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Meister, F.A.; Lurje, G.; Verhoeven, S.; Wiltberger, G.; Heij, L.; Liu, W.-J.; Jiang, D.; Bruners, P.; Lang, S.A.; Ulmer, T.F. The role of sarcopenia and myosteatosis in short-and long-term outcomes following curative-intent surgery for hepatocellular carcinoma in a European cohort. Cancers 2022, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Seo, S.; Taura, K.; et al. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2019, 8, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Liver, E.A.F.T.S.O.T. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Paris, M.T.; Tandon, P.; Heyland, D.K.; Furberg, H.; Premji, T.; Low, G.; Mourtzakis, M. Automated body composition analysis of clinically acquired computed tomography scans using neural networks. Clin. Nutr. 2020, 39, 3049–3055. [Google Scholar] [CrossRef]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A.; Fitness, L.E.; Exercise in Liver Transplantation, C. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017, 23, 625–633. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Kaibori, M.; Ishizaki, M.; Iida, H.; Matsui, K.; Sakaguchi, T.; Inoue, K.; Mizuta, T.; Ide, Y.; Iwasaka, J.; Kimura, Y.; et al. Effect of Intramuscular Adipose Tissue Content on Prognosis in Patients Undergoing Hepatocellular Carcinoma Resection. J. Gastrointest. Surg. 2015, 19, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Fu, Y.; Long, Q.; Zhao, Y.; Li, S.; Zhou, C.; Lin, H.; Liu, X.; Liu, C.; Chen, C.; et al. Myosteatosis can Predict Unfavorable Outcomes in Advanced Hepatocellular Carcinoma Patients Treated With Hepatic Artery Infusion Chemotherapy and Anti-PD-1 Immunotherapy. Front. Oncol. 2022, 12, 892192. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Choi, G.H.; Hwang, S.H.; Jang, E.S.; Kim, J.W.; Ahn, J.M.; Choi, Y.; Cho, J.Y.; Han, H.S.; Lee, J.; et al. Sarcopenia and visceral adiposity predict poor overall survival in hepatocellular carcinoma patients after curative hepatic resection. Transl. Cancer Res. 2021, 10, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M. Interplay of adipokines and myokines in cancer pathophysiology: Emerging therapeutic implications. World J. Exp. Med. 2013, 3, 26. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer—Mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ghigliotti, G.; Barisione, C.; Garibaldi, S.; Fabbi, P.; Brunelli, C.; Spallarossa, P.; Altieri, P.; Rosa, G.; Spinella, G.; Palombo, D. Adipose tissue immune response: Novel triggers and consequences for chronic inflammatory conditions. Inflammation 2014, 37, 1337–1353. [Google Scholar] [CrossRef]
- Marra, F.; Bertolani, C. Adipokines in liver diseases. Hepatology 2009, 50, 957–969. [Google Scholar] [CrossRef]
- Vansaun, M.N. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin. Cancer Res. 2013, 19, 1926–1932. [Google Scholar] [CrossRef]
- Laurens, C.; Moro, C. Intramyocellular fat storage in metabolic diseases. Horm. Mol. Biol. Clin. Investig. 2016, 26, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Hirooka, M.; Koizumi, Y.; Izumoto, H.; Ueki, H.; Kaneto, M.; Kitahata, S.; Aibiki, T.; Tomida, H.; Miyamoto, Y.; et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. 2017, 47, 558–565. [Google Scholar] [CrossRef] [PubMed]
- March, C.; Omari, J.; Thormann, M.; Pech, M.; Wienke, A.; Surov, A. Prevalence and role of low skeletal muscle mass (LSMM) in hepatocellular carcinoma. A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 49, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef]
- Feng, H.; Wang, X.; Mao, L.; Yu, Z.; Cui, B.; Lin, L.; Hui, Y.; Zhao, X.; Xu, X.; Fan, X.; et al. Relationship between sarcopenia/myosteatosis and frailty in hospitalized patients with cirrhosis: A sex-stratified analysis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211026996. [Google Scholar] [CrossRef]
| Variable | Enrolled Patients n = 245 |
|---|---|
| Age, year | 67.0 [61.0–78.0] |
| Men, n (%) | 211 (86.1) |
| Body mass index, kg/m2 | 22.8 [20.9–25.3] |
| Etiology, n (%) | |
| HBV/HCV/Alcohol/other | 154(62.8)/26 (10.5)/33 (13.4)/32(13.3) |
| Comorbidities, n (%) | |
| Obesity | 66 (26.9) |
| Diabetes mellitus | 75 (30.6) |
| Hypertension | 75 (30.6) |
| Tumor profiles | |
| Single/multiple, n (%) | 75 (33.5)/149 (66.5) |
| Largest diameter, mm | 68.5 [33.0–110.0] |
| Vessel invasion, n (%) | 105 (42.9) |
| Extrahepatic metastasis, n (%) | 122 (49.8) |
| BCLC stage, n (%) | |
| B/C | 34 (13.8)/211 (86.2) |
| CTP classification, n (%) | |
| A/B | 231 (94.3)/14 (5.7) |
| Laboratory profiles | |
| Platelet count, ×109/µL | 137 [88.5–188.0] |
| Aspartate aminotransferase, IU/L | 61.0 [40.0–105.0] |
| Alanine aminotransferase, IU/L | 31.0 [20.0–50.0] |
| Serum albumin, g/dL | 3.7 [3.4–4.1] |
| Prothrombin time, INR | 1.1 [1.1–1.2] |
| Alpha-fetoprotein, >200 ng/mL, n (%) | 124 (53.0) |
| PIVKA >400 mAU/mL, n (%) | 111 (55.5) |
| Response at 2-/3-month follow-up, n (%) | |
| Complete response | 1 (0.4) |
| Partial response | 9 (3.7) |
| Stable disease | 113 (46.1) |
| Progressive disease | 122 (49.8) |
| Objective response rate, % | 4.1 |
| Disease control rate, % | 50.2 |
| Body composition analyses based on CT | |
| SMI, cm2/m2 | 49.4 [43.0–58.1] |
| VATI, cm2/m2 | 42.7 [23.5–62.7] |
| SATI., cm2/m2 | 36.7 [24.6–51.7] |
| HU | 53.7 [48.3–56.6] |
| VSR | 1.1 [0.8–1.5] |
| Sarcopenia, n (%) | 115 (46.9) |
| Presence of visceral obesity, n (%) | 92 (37.6) |
| Myosteatosis, n (%) | 22 (9.0) |
| Clinical outcomes | |
| Overall survival, months | 7.9 [4.1–15.3] |
| Progression-free survival, months | 4.8 [2.9–9.5] |
| Variables | Overall Survival | Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate p-Value | Multivariate | Univariate p-Value | Multivariate | |||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||
| Age, years | 0.677 | 0.729 | ||||||
| Male | 0.354 | 0.657 | ||||||
| Obesity | 0.534 | 0.909 | ||||||
| Diabetes mellitus | 0.878 | 0.397 | ||||||
| Hypertension | 0.928 | 0.777 | ||||||
| Tumor number, multiple vs. single | 0.249 | 0.064 | ||||||
| Tumor size > 70 mm | 0.001 | 0.029 | ||||||
| Vessel invasion | <0.001 | 1.727 | 1.258–2.371 | 0.001 | 0.011 | 1.480 | 1.104–1.982 | 0.009 |
| Extrahepatic metastasis | 0.081 | 1.401 | 1.028–1.908 | 0.033 | 0.009 | 1.456 | 1.092–1.940 | 0.011 |
| CTP classification, B vs. A | 0.045 | 0.336 | ||||||
| AFP > 200 ng/mL | 0.003 | 1.559 | 1.105–2.201 | 0.012 | 0.093 | 1.395 | 1.036–1.879 | 0.028 |
| PIVKA > 400 mAU/mL | 0.272 | 0.992 | ||||||
| Sarcopenia, yes/no | 0.276 | 0.291 | ||||||
| Visceral adiposity | 0.023 | 1.478 | 1.062–2.057 | 0.021 | 0.186 | |||
| Myosteatosis | 0.043 | 1.814 | 1.112–2.960 | 0.017 | 0.049 | 1.732 | 1.085–2.767 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.K.; Song, J.E.; Jang, S.Y.; Kim, B.S.; Chung, W.J.; Lee, C.; Park, S.Y.; Tak, W.Y.; Kweon, Y.O.; Hwang, J.S.; et al. The Clinical Significance of Myosteatosis in Survival Outcomes in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers 2024, 16, 454. https://doi.org/10.3390/cancers16020454
Kang MK, Song JE, Jang SY, Kim BS, Chung WJ, Lee C, Park SY, Tak WY, Kweon YO, Hwang JS, et al. The Clinical Significance of Myosteatosis in Survival Outcomes in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers. 2024; 16(2):454. https://doi.org/10.3390/cancers16020454
Chicago/Turabian StyleKang, Min Kyu, Jeong Eun Song, Se Young Jang, Byung Seok Kim, Woo Jin Chung, Changhyeong Lee, Soo Young Park, Won Young Tak, Young Oh Kweon, Jae Seok Hwang, and et al. 2024. "The Clinical Significance of Myosteatosis in Survival Outcomes in Patients with Hepatocellular Carcinoma Treated with Sorafenib" Cancers 16, no. 2: 454. https://doi.org/10.3390/cancers16020454
APA StyleKang, M. K., Song, J. E., Jang, S. Y., Kim, B. S., Chung, W. J., Lee, C., Park, S. Y., Tak, W. Y., Kweon, Y. O., Hwang, J. S., Jang, B. K., Lee, Y. R., Park, J. G., & on behalf of Daegu-Gyeongbuk Liver Study Group (DGLSG). (2024). The Clinical Significance of Myosteatosis in Survival Outcomes in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers, 16(2), 454. https://doi.org/10.3390/cancers16020454