Why Is Surgery Still Done after Concurrent Chemoradiotherapy in Locally Advanced Cervical Cancer in Romania?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
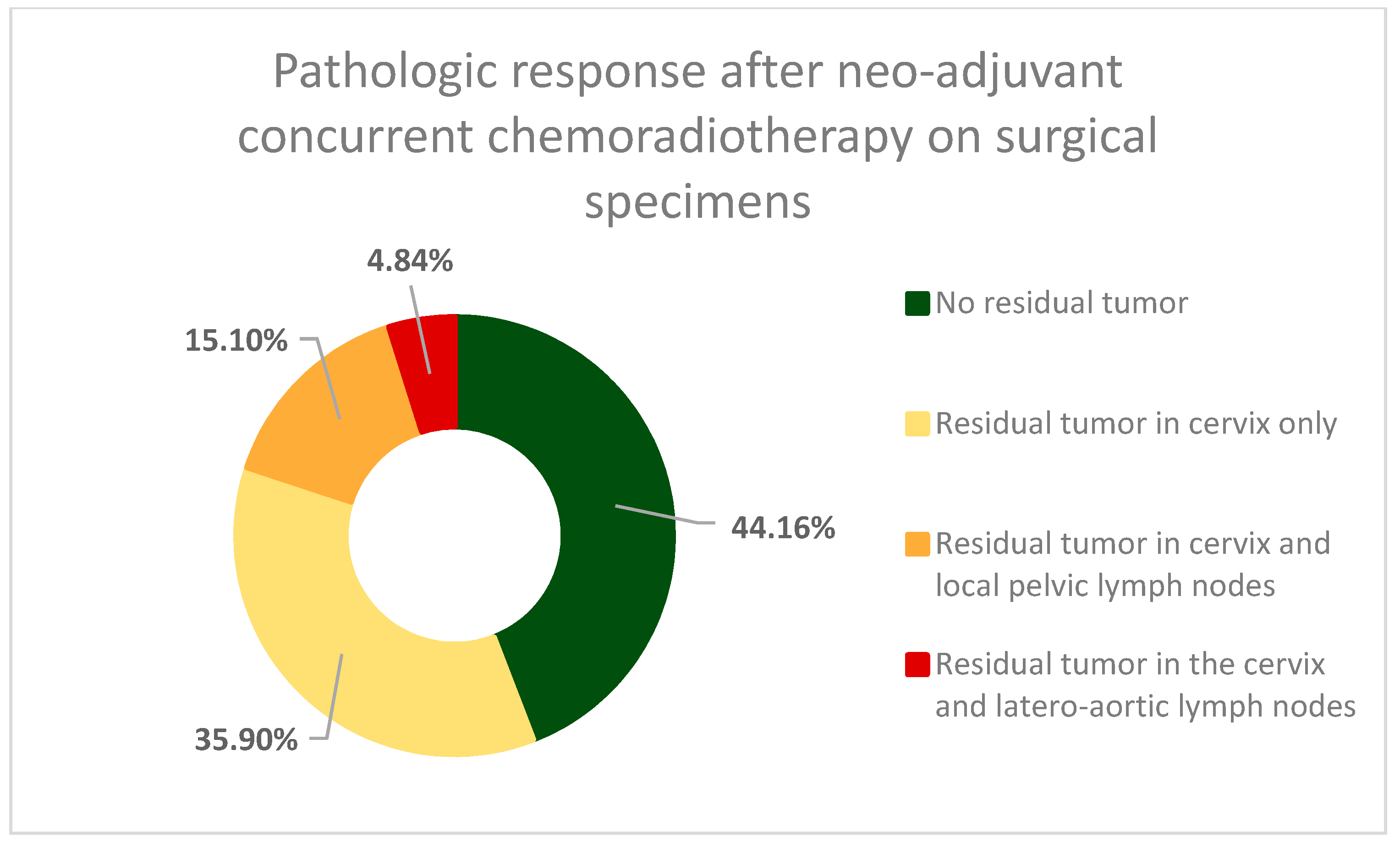
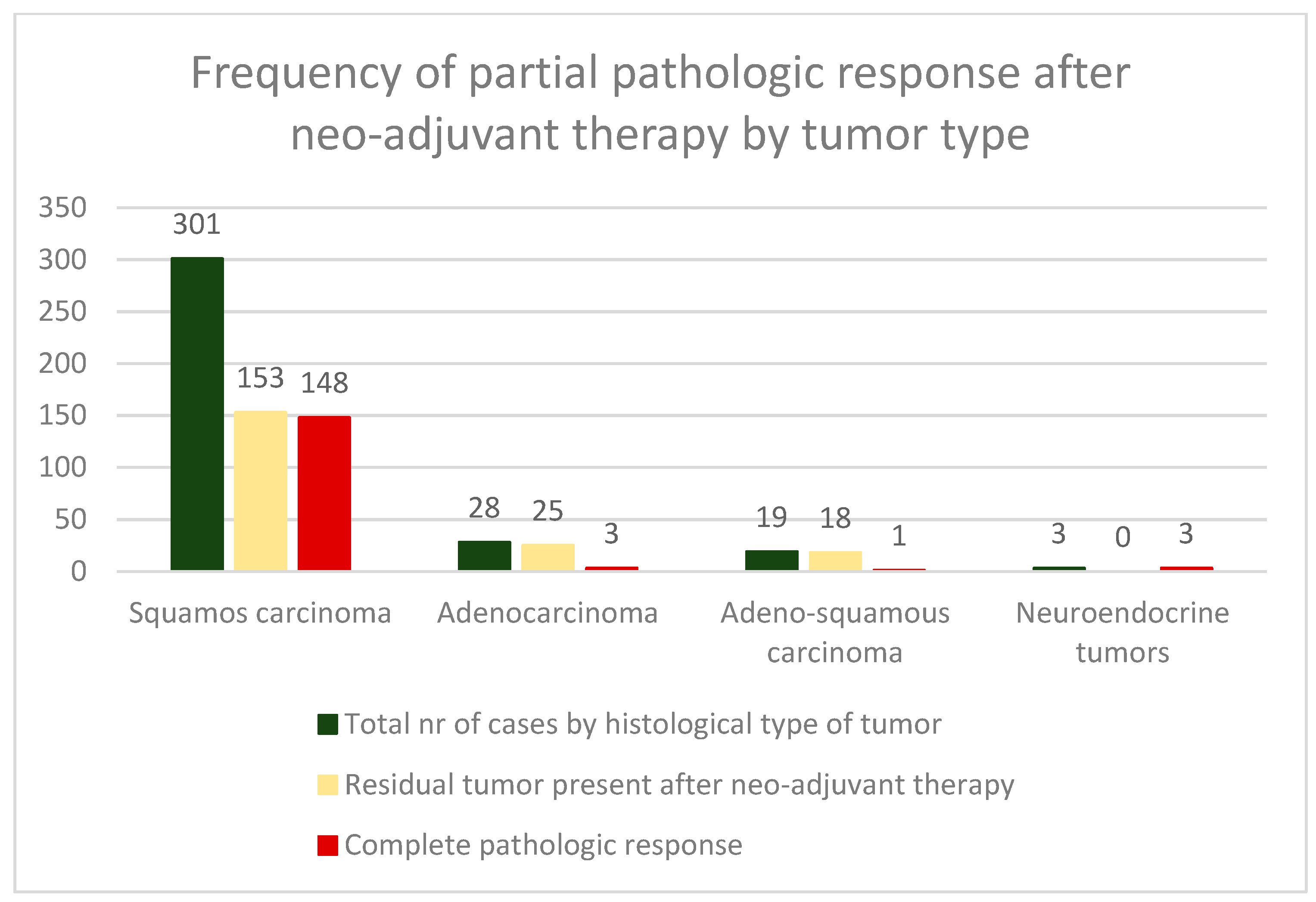
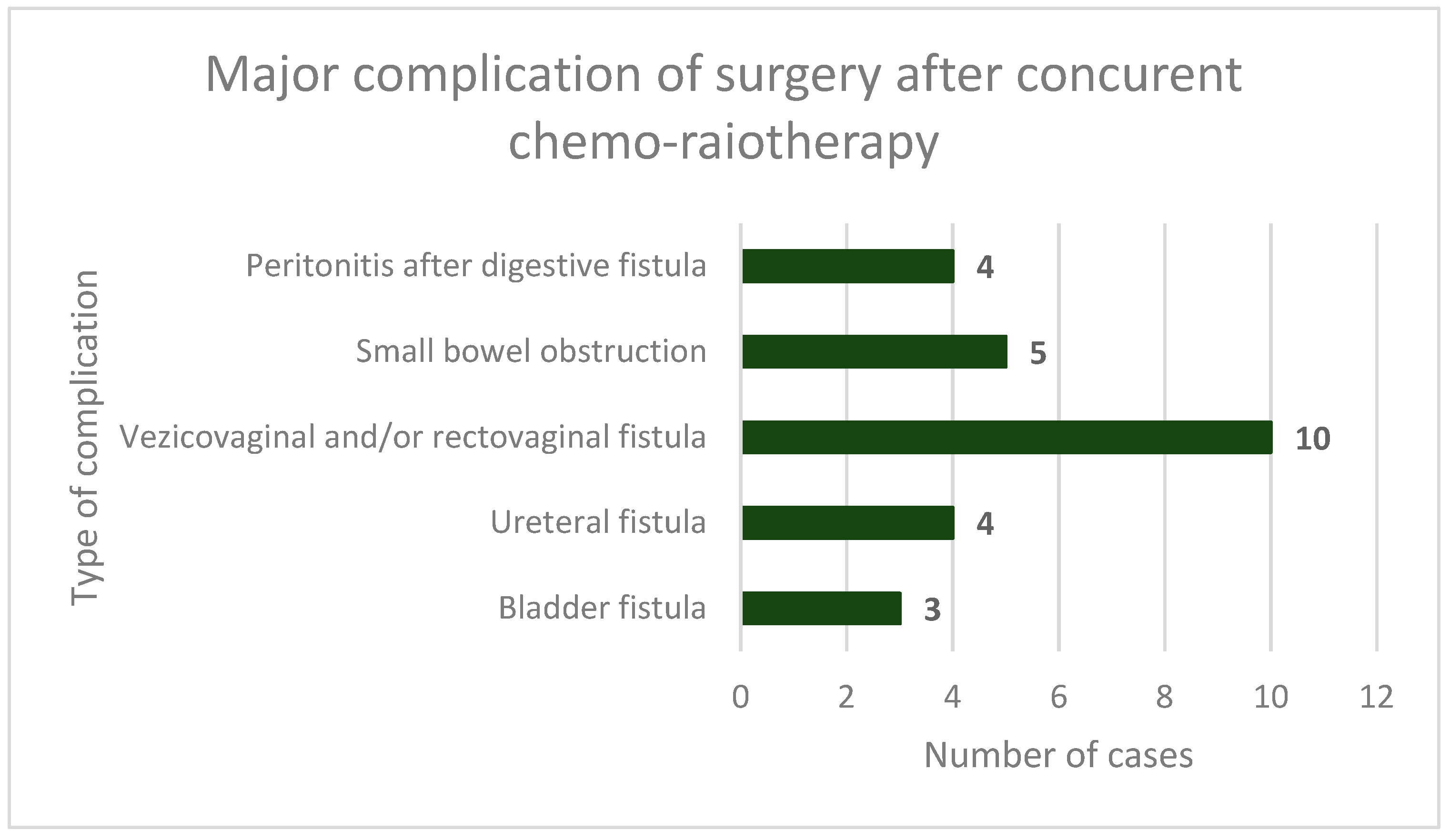
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer; World Health Organization; Global Cancer Observatory—Cancer Today. Estimated Age-Standardized Incidence Rates in 2020, Cervix Uteri, Females, All Ages. Available online: https://gco.iarc.fr/today (accessed on 10 January 2024).
- Simion, L.; Rotaru, V.; Cirimbei, C.; Gales, L.; Stefan, D.-C.; Ionescu, S.-O.; Luca, D.; Doran, H.; Chitoran, E. Inequities in Screening and HPV Vaccination Programs and Their Impact on Cervical Cancer Statistics in Romania. Diagnostics 2023, 13, 2776. [Google Scholar] [CrossRef] [PubMed]
- Simion, L.; Rotaru, V.; Cirimbei, C.; Stefan, D.C.; Gherghe, M.; Ionescu, S.; Tanase, B.C.; Luca, D.C.; Gales, L.N.; Chitoran, E. Analysis of Efficacy-To-Safety Ratio of Angiogenesis-Inhibitors Based Therapies in Ovarian Cancer: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 1040. [Google Scholar] [CrossRef]
- Blidaru, A.; Bordea, C.; Burcoæ, T.; Duduæ, L.; Eniu, D.; Ioanid, N.; Kacso, G.; Minciuna, C.; Savu, M.; Scripcariu, V.; et al. Mind the Gap Between Scientific Literature Recommendations and Effective Implementation. Is There Still a Role for Surgery in the Treatment of Locally Advanced Cervical Carcinoma? Chirurgia 2019, 114, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Cho, O.; Chun, M. Management for Locally Advanced Cervical Cancer: New Trends and Controversial Issues. Radiat. Oncol. J. 2018, 36, 254–264. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mukherjee, U.; Ghosh, S.; Ghosh, S.; Sarkar, S.K. Pattern of Failure with Locally Advanced Cervical Cancer—A Retrospective Audit and Analysis of Contributory Factors. Asian Pac. J. Cancer Prev. 2018, 19, 73–79. [Google Scholar] [CrossRef]
- Simion, L.; Chitoran, E.; Cirimbei, C.; Stefan, D.-C.; Neicu, A.; Tanase, B.; Ionescu, S.O.; Luca, D.C.; Gales, L.; Gheorghe, A.S.; et al. A Decade of Therapeutic Challenges in Synchronous Gynecological Cancers from the Bucharest Oncological Institute. Diagnostics 2023, 13, 2069. [Google Scholar] [CrossRef]
- NCCN Guidelines Cervical Cancer Version 1.2023—April 28, 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1426 (accessed on 24 August 2023).
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Cervical Cancer—Update 2023. Virchows Arch. 2023, 482, 935–966. [Google Scholar] [CrossRef]
- Voinea, S.; Herghelegiu, C.; Sandru, A.; Ioan, R.; Bohilțea, R.; Bacalbașa, N.; Chivu, L.; Furtunescu, F.; Stanica, D.; Neacșu, A. Impact of Histological Subtype on the Response to Chemoradiation in Locally Advanced Cervical Cancer and the Possible Role of Surgery. Exp. Ther. Med. 2021, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Cirimbei, C.; Rotaru, V.; Chitoran, E.; Cirimbei, S. Laparoscopic Approach in Abdominal Oncologic Pathology. In Proceedings of the 35th Balkan Medical Week, Athens, Greece, 25–27 September 2018; Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000471903700043 (accessed on 20 July 2023).
- Rotaru, V.; Chitoran, E.; Cirimbei, C.; Cirimbei, S.; Simion, L. Preservation of Sensory Nerves During Axillary Lymphadenectomy. In Proceedings of the 35th Balkan Medical Week, Athens, Greece, 25–27 September 2018; Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000471903700045 (accessed on 20 July 2023).
- Simion, L.; Ionescu, S.; Chitoran, E.; Rotaru, V.; Cirimbei, C.; Madge, O.-L.; Nicolescu, A.C.; Tanase, B.; Dicu-Andreescu, I.-G.; Dinu, D.M.; et al. Indocyanine Green (ICG) and Colorectal Surgery: A Literature Review on Qualitative and Quantitative Methods of Usage. Medicina 2023, 59, 1530. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.T.; Kim, S.; Lee, B.; Lim, M.C.; Kim, J.W.; Won, Y.J. Prognosis of Cervical Cancer in the Era of Concurrent Chemoradiation from National Database in Korea: A Comparison between Squamous Cell Carcinoma and Adenocarcinoma. PLoS ONE 2015, 10, e0144887. [Google Scholar] [CrossRef]
- Classe, J.M.; Rauch, P.; Rodier, J.F.; Morice, P.; Stoeckle, E.; Lasry, S.; Houvenaeghel, G. Surgery after Concurrent Chemoradiotherapy and Brachytherapy for the Treatment of Advanced Cervical Cancer: Morbidity and Outcome: Results of a Multicenter Study of the GCCLCC (Groupe Des Chirurgiens de Centre de Lutte Contre Le Cancer). Gynecol. Oncol. 2006, 102, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lu, C.; Yu, Z.; Gao, L. Chemoradiotherapy Alone vs. Chemoradiotherapy and Hysterectomy for Locally Advanced Cervical Cancer: A Systematic Review and Updated Meta-Analysis. Oncol. Lett. 2021, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, S.N.; Chae, S.H.; Kim, J.E.; Lee, S.J. Impact of Adjuvant Hysterectomy on Prognosis in Patients with Locally Advanced Cervical Cancer Treated with Concurrent Chemoradiotherapy: A Meta-Analysis. J. Gynecol. Oncol. 2018, 29, e25. [Google Scholar] [CrossRef] [PubMed]
- Motton, S.P.; Houvenaeghel, G.; Delannes, M.; Querleu, D.; Soulé-Tholy, M.; Hoff, J.; Guevaque, P.L. Results of Surgery after Concurrent Chemoradiotherapy in Advanced Cervical Cancer: Comparison of Extended Hysterectomy and Extrafascial Hysterectomy. Int. J. Gynecol. Cancer 2010, 20, 268–275. [Google Scholar] [CrossRef]
- Chereau, E.; de la Hosseraye, C.; Ballester, M.; Monnier, L.; Rouzier, R.; Touboul, E.; Darai, E. The Role of Completion Surgery after Concurrent Radiochemotherapy in Locally Advanced Stages IB2-IIB Cervical Cancer. Anticancer Res. 2013, 33, 1661–1666. [Google Scholar] [PubMed]
- Darus, C.J.; Callahan, M.B.; Nguyen, Q.N.; Pastore, L.M.; Schneider, B.F.; Rice, L.W.; Jazaeri, A.A. Chemoradiation with and without Adjuvant Extrafascial Hysterectomy for IB2 Cervical Carcinoma. Int. J. Gynecol. Cancer 2008, 18, 730–735. [Google Scholar] [CrossRef]
- Green, J.A.; Kirwan, J.M.; Tierney, J.F.; Symonds, P.; Fresco, L.; Collingwood, M.; Williams, C.J. Survival and Recurrence after Concomitant Chemotherapy and Radiotherapy for Cancer of the Uterine Cervix: A Systematic Review and Meta-Analysis. Lancet 2001, 358, 781–786. [Google Scholar] [CrossRef]
- Maghous, A.; Elmarjany, M.; Marnouche, E.; Andaloussi, K.; Bazine, A.; Lalya, I.; Zaghba, N.; Hadadi, K.; Sifat, H.; Habib, M.A.B.; et al. Surgical Resection after Concurrent Chemoradiotherapy for Locally Advanced Cervical Carcinoma. J. Oncol. Med. Pract. 2016, 1, 1000107. [Google Scholar] [CrossRef]
- Lèguevaque, P.; Motton, S.; Delannes, M.; Querleu, D.; Soulé-Tholy, M.; Tap, G.; Houvenaeghel, G. Completion Surgery or Not after Concurrent Chemoradiotherapy for Locally Advanced Cervical Cancer? Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 188–192. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Lelievre, L.; Gonzague-Casabianca, L.; Buttarelli, M.; Moutardier, V.; Goncalves, A.; Resbeut, M. Long-Term Survival after Concomitant Chemoradiotherapy Prior to Surgery in Advanced Cervical Carcinoma. Gynecol. Oncol. 2006, 100, 338–343. [Google Scholar] [CrossRef]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Okagaki, T.; Gallup, D.G.; Burnett, A.F.; Rotman, M.Z.; Fowler, W.C. Radiation Therapy with and without Extrafascial Hysterectomy for Bulky Stage IB Cervical Carcinoma: A Randomized Trial of the Gynecologic Oncology Group. Gynecol. Oncol. 2003, 89, 343–353. [Google Scholar] [CrossRef]
- Vízkeleti, J.; Vereczkey, I.; Fröhlich, G.; Varga, S.; Horváth, K.; Pulay, T.; Pete, I.; Nemeskéri, C.; Mayer, Á.; Sipos, N.; et al. Pathologic Complete Remission after Preoperative High-Dose-Rate Brachytherapy in Patients with Operable Cervical Cancer: Preliminary Results of a Prospective Randomized Multicenter Study. Pathol. Oncol. Res. 2015, 21, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Margariti, P.A.; Smaniotto, D.; Petrillo, M.; Salerno, M.G.; Fagotti, A.; MacChia, G.; Morganti, A.G.; Cellini, N.; Scambia, G. Long-Term Analysis of Clinical Outcome and Complications in Locally Advanced Cervical Cancer Patients Administered Concomitant Chemoradiation Followed by Radical Surgery. Gynecol. Oncol. 2010, 119, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Sanei Sistani, S.; Parooie, F.; Salarzaei, M. Diagnostic Accuracy of 18F-FDG-PET/CT and MRI in Predicting the Tumor Response in Locally Advanced Cervical Carcinoma Treated by Chemoradiotherapy: A Meta-Analysis. Contrast Media Mol. Imaging 2021, 2021, 8874990. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Chatzikonstantinou, G.; Fokas, E.; Wichmann, J.; Christiansen, H.; Strebhardt, K.; Rödel, C.; Tselis, N.; Rödel, F. Molecular Markers to Predict Prognosis and Treatment Response in Uterine Cervical Cancer. Cancers 2021, 13, 5748. [Google Scholar] [CrossRef]
- Du, P.; Li, G.; Wu, L.; Huang, M. Perspectives of ERCC1 in Early-Stage and Advanced Cervical Cancer: From Experiments to Clinical Applications. Front. Immunol. 2023, 13, 1065379. [Google Scholar] [CrossRef]
- Fu, J.; Wang, W.; Wang, Y.; Liu, C.; Wang, P. The Role of Squamous Cell Carcinoma Antigen (SCC Ag) in Outcome Prediction after Concurrent Chemoradiotherapy and Treatment Decisions for Patients with Cervical Cancer. Radiat. Oncol. 2019, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Rosolen, D.; Nunes-Souza, E.; Marchi, R.; Tofolo, M.V.; Antunes, V.C.; Berti, F.C.B.; Fonseca, A.S.; Cavalli, L.R. MiRNAs Action and Impact on Mitochondria Function, Metabolic Reprogramming and Chemoresistance of Cancer Cells: A Systematic Review. Biomedicines 2023, 11, 693. [Google Scholar] [CrossRef]
- Karimi, A.; Jafari-Koshki, T.; Zehtabi, M.; Kargar, F.; Gheit, T. Predictive Impact of Human Papillomavirus Circulating Tumor DNA in Treatment Response Monitoring of HPV-associated Cancers; a Meta-analysis on Recurrent Event Endpoints. Cancer Med. 2023, 12, 17592–17602. [Google Scholar] [CrossRef]
- Kong, X.; Xiong, Y.; Xue, M.; He, J.; Lu, Q.; Chen, M.; Li, L. Identification of Cuproptosis-Related LncRNA for Predicting Prognosis and Immunotherapeutic Response in Cervical Cancer. Sci. Rep. 2023, 13, 10697. [Google Scholar] [CrossRef]
- Lakomy, D.S.; Wu, J.; Lombe, D.; Papasavvas, E.; Msadabwe, S.C.; Geng, Y.; Montaner, L.J.; Chiao, E.; Lin, L.L. Immune Correlates of Therapy Outcomes in Women with Cervical Cancer Treated with Chemoradiotherapy: A Systematic Review. Cancer Med. 2021, 10, 4206–4220. [Google Scholar] [CrossRef]
- Shah, S.; Xu, M.; Mehta, P.; Zetola, N.M.; Grover, S. Differences in Outcomes of Chemoradiation in Women with Invasive Cervical Cancer by Human Immunodeficiency Virus Status: A Systematic Review. Pract. Radiat. Oncol. 2021, 11, 53–65. [Google Scholar] [CrossRef]
- Schernberg, A.; Kumar, T.; Achkar, S.; Espenel, S.; Bockel, S.; Majer, M.; Escande, A.; Mignot, F.; Annede, P.; Monnier, L.; et al. Incorporating Magnetic Resonance Imaging (MRI) Based Radiation Therapy Response Prediction into Clinical Practice for Locally Advanced Cervical Cancer Patients. Semin. Radiat. Oncol. 2020, 30, 291–299. [Google Scholar] [CrossRef]
- Jha, A.K.; Mithun, S.; Sherkhane, U.B.; Jaiswar, V.; Osong, B.; Purandare, N.; Kannan, S.; Prabhash, K.; Gupta, S.; Vanneste, B.; et al. Systematic Review and Meta-Analysis of Prediction Models Used in Cervical Cancer. Artif. Intell. Med. 2023, 139, 102549. [Google Scholar] [CrossRef]
- IAEA—International Atomic Energy Agency; DIRAC—Directory of Radiotherapy Centers. Available online: https://dirac.iaea.org/Query/Map2?mapId=0 (accessed on 10 January 2024).
- Ota, T.; Takeshima, N.; Tabata, T.; Hasumi, K.; Takizawa, K. Adjuvant Hysterectomy for Treatment of Residual Disease in Patients with Cervical Cancer Treated with Radiation Therapy. Br. J. Cancer 2008, 99, 1216–1220. [Google Scholar] [CrossRef]
- Simion, L.; Rotaru, V.; Cirimbei, S.; Chitoran, E.; Gales, L.; Luca, D.C.; Ionescu, S.; Tanase, B.; Ginghina, O.; Alecu, M.; et al. Simultaneous Approach of Colo-Rectal And Hepatic Lesions In Colo-Rectal Cancers with Liver Metastasis—A Single Oncological Center Overview. Chirurgia 2023, 118, 237–249. [Google Scholar] [CrossRef]
- Gui, B.; Valentini, A.L.; Miccò, M.; D’Agostino, G.R.; Tagliaferri, L.; Zannoni, G.F.; Fanfani, F.; Manfredi, R.; Bonomo, L. Cervical Cancer Response to Neoadjuvant Chemoradiotherapy: MRI Assessment Compared with Surgery. Acta Radiol. 2016, 57, 1123–1131. [Google Scholar] [CrossRef]
- Manfredi, R.; Maresca, G.; Smaniotto, D.; Greggi, S.; Andrulli, D.; Rabitti, C.; Summaria, V.; Valentini, A.L.; Panici, P.B.; Cellini, N.; et al. Cervical Cancer Response to Neoadjuvant Therapy: MR Imaging Assessment. Radiology 1998, 209, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Rouanet, P.; Rey, A.; Romestaing, P.; Houvenaeghel, G.; Boulanger, J.C.; Leveque, J.; Cowen, D.; Mathevet, P.; Malhaire, J.P.; et al. Results of the GYNECO 02 Study, an FNCLCC Phase III Trial Comparing Hysterectomy with No Hysterectomy in Patients with a (Clinical and Radiological) Complete Response after Chemoradiation Therapy for Stage IB2 or II Cervical Cancer. Oncologist 2012, 17, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.G.M.; Solomon, M.J.; Koh, C.E. Pelvic Exenteration Surgery: The Evolution of Radical Surgical Techniques for Advanced and Recurrent Pelvic Malignancy. Dis. Colon Rectum 2017, 60, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.J. Redefining the Boundaries of Advanced Pelvic Oncology Surgery. Br. J. Surg. 2021, 108, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Abstracts from 18th International Meeting of the European Society of Gynaecological Oncology (ESGO), 19–22 October 2013, Liverpool, UK. Int. J. Gynecol. Cancer 2013, 23, 1–1281. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voinea, S.C.; Bordea, C.I.; Chitoran, E.; Rotaru, V.; Andrei, R.I.; Ionescu, S.-O.; Luca, D.; Savu, N.M.; Capsa, C.M.; Alecu, M.; et al. Why Is Surgery Still Done after Concurrent Chemoradiotherapy in Locally Advanced Cervical Cancer in Romania? Cancers 2024, 16, 425. https://doi.org/10.3390/cancers16020425
Voinea SC, Bordea CI, Chitoran E, Rotaru V, Andrei RI, Ionescu S-O, Luca D, Savu NM, Capsa CM, Alecu M, et al. Why Is Surgery Still Done after Concurrent Chemoradiotherapy in Locally Advanced Cervical Cancer in Romania? Cancers. 2024; 16(2):425. https://doi.org/10.3390/cancers16020425
Chicago/Turabian StyleVoinea, Silviu Cristian, Cristian Ioan Bordea, Elena Chitoran, Vlad Rotaru, Razvan Ioan Andrei, Sinziana-Octavia Ionescu, Dan Luca, Nicolae Mircea Savu, Cristina Mirela Capsa, Mihnea Alecu, and et al. 2024. "Why Is Surgery Still Done after Concurrent Chemoradiotherapy in Locally Advanced Cervical Cancer in Romania?" Cancers 16, no. 2: 425. https://doi.org/10.3390/cancers16020425
APA StyleVoinea, S. C., Bordea, C. I., Chitoran, E., Rotaru, V., Andrei, R. I., Ionescu, S.-O., Luca, D., Savu, N. M., Capsa, C. M., Alecu, M., & Simion, L. (2024). Why Is Surgery Still Done after Concurrent Chemoradiotherapy in Locally Advanced Cervical Cancer in Romania? Cancers, 16(2), 425. https://doi.org/10.3390/cancers16020425









