Cyclophilin A: An Independent Prognostic Factor for Survival in Patients with Metastatic Colorectal Cancer Treated with Bevacizumab and Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Specimen Characteristics
2.3. Assay Methods
2.4. Study Design
2.5. Statistical Analysis
3. Results
3.1. Patient and Disease Characteristics
3.2. Adverse Events
3.3. Serum Biomarkers
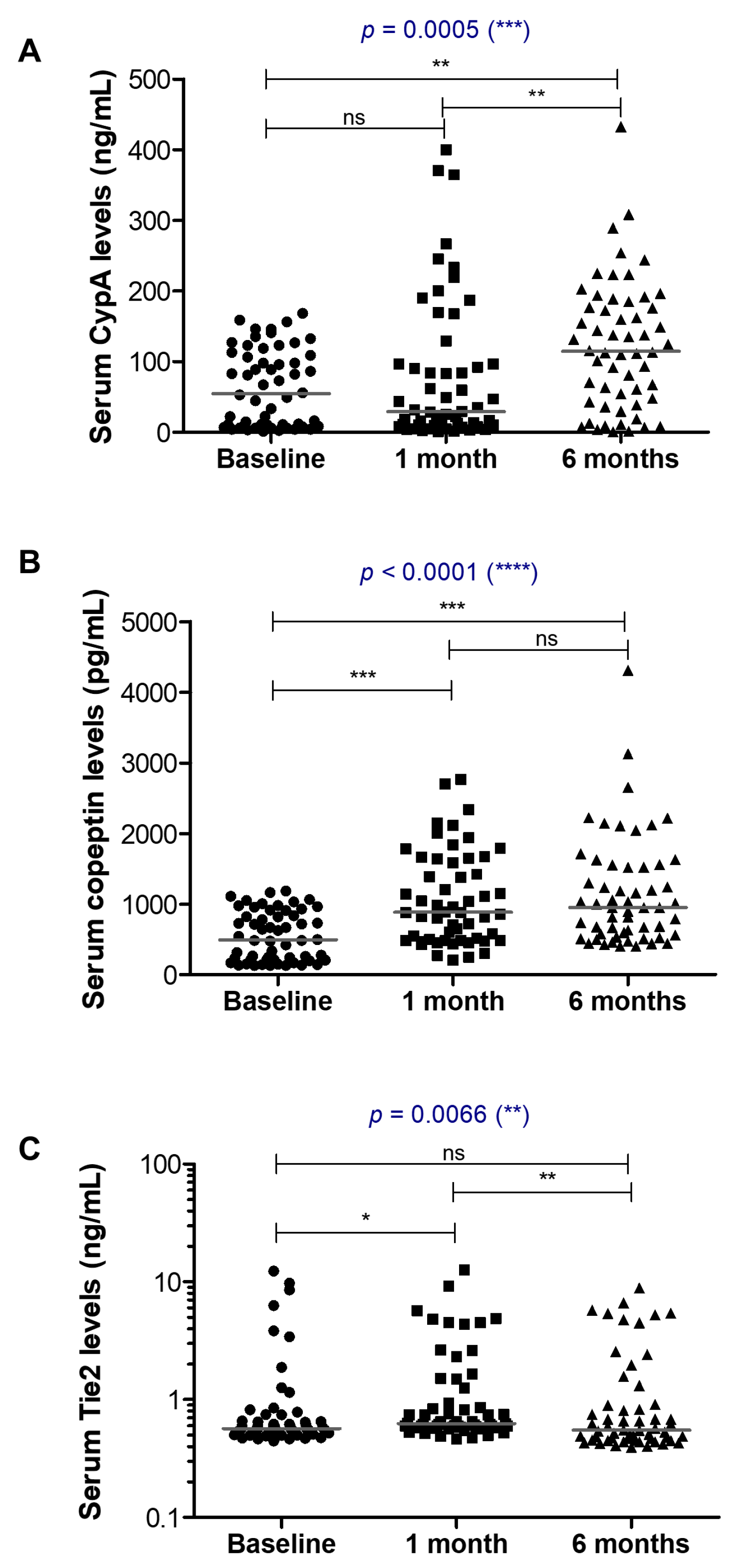
3.3.1. CypA
3.3.2. Copeptin and Tie2
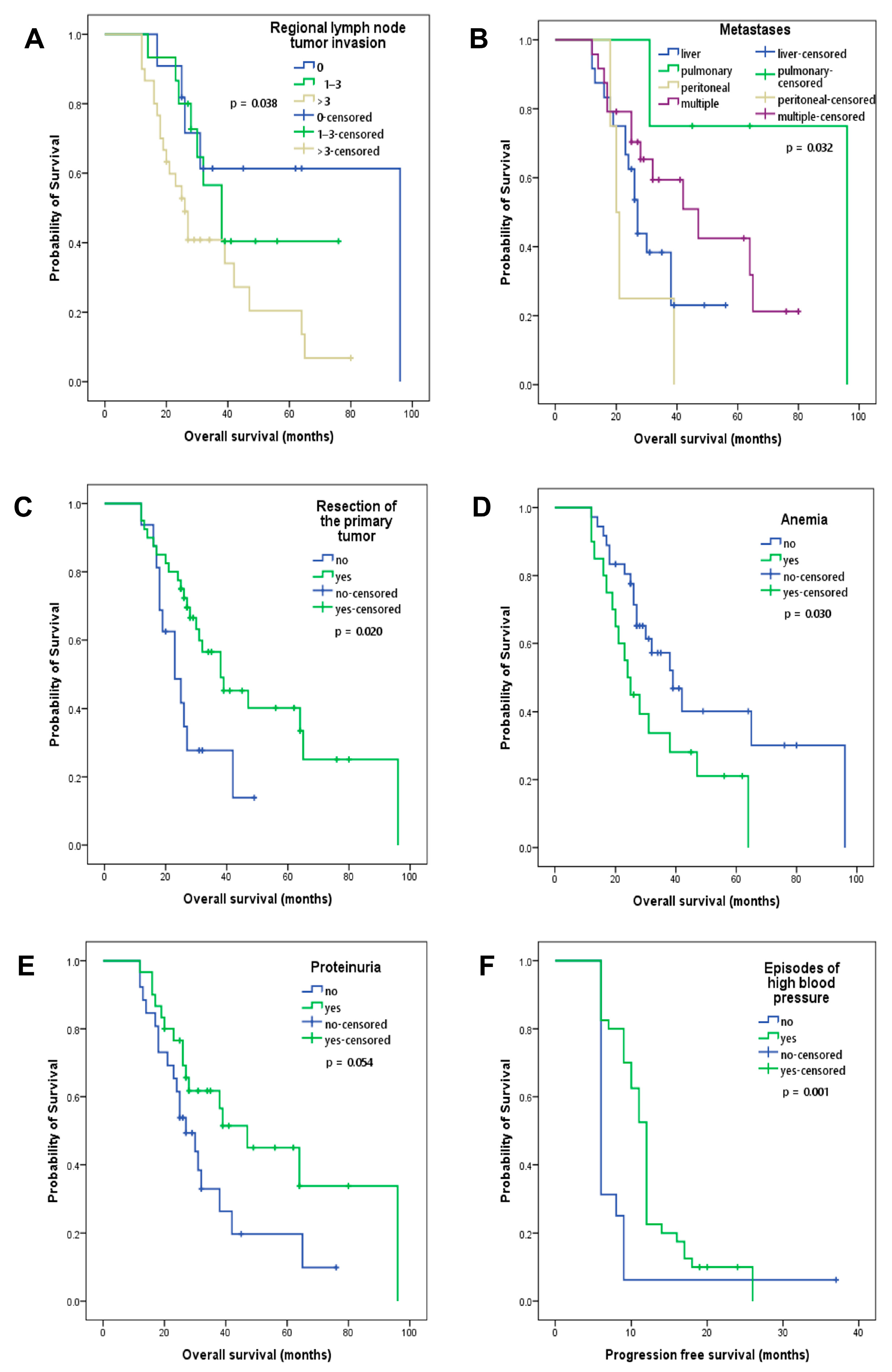
3.4. Clinical and Paraclinical Characteristics as Prognostic Factors
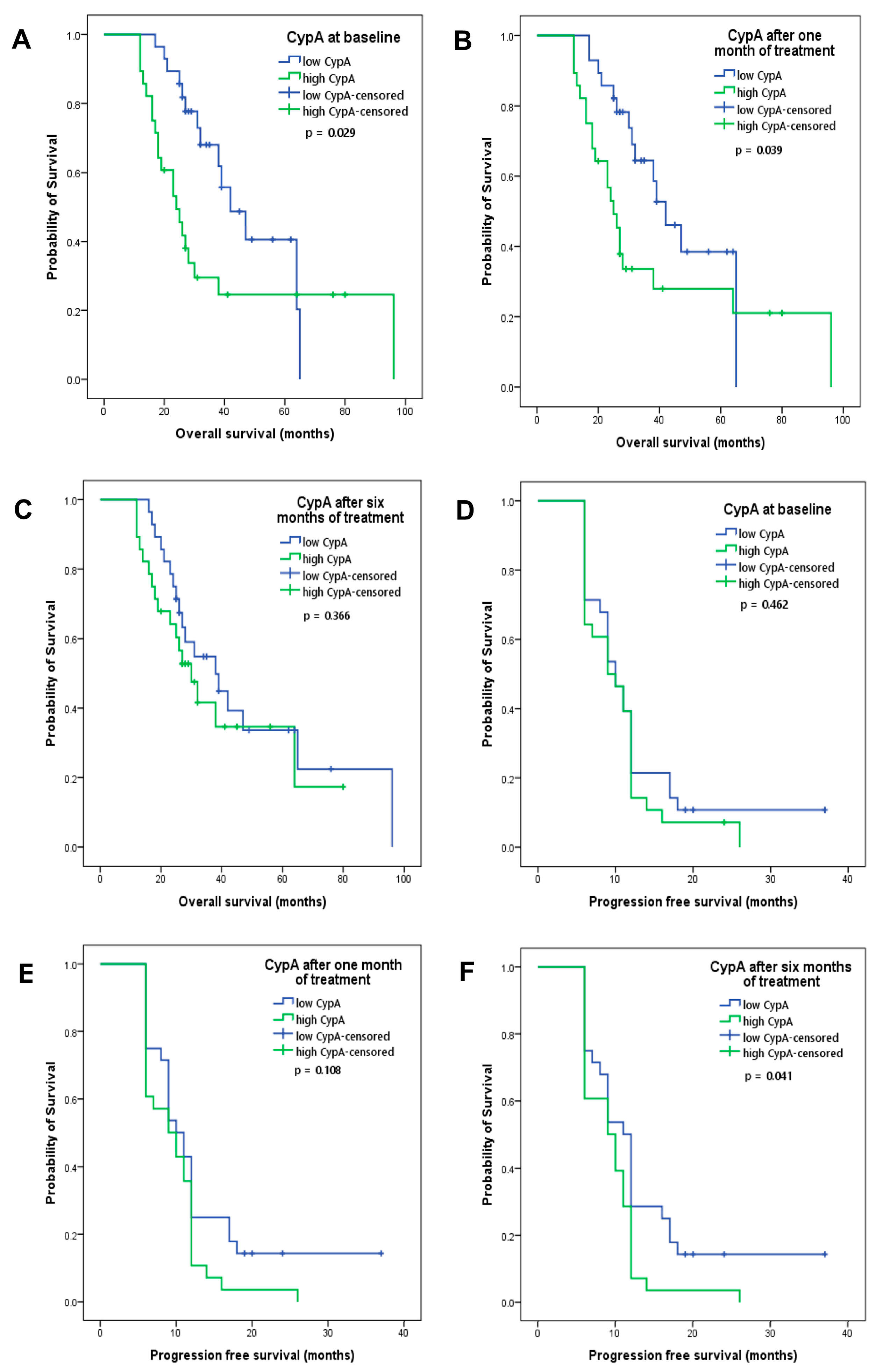
3.5. CypA at Baseline and after One Month of Treatment as Prognostic Factors
4. Discussion
Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ma, H.; Huang, F.; Zhu, D.; Bi, J.; Ke, Y.; Zhang, T. Correlation of bevacizumab-induced hypertension and outcomes of metastatic colorectal cancer patients treated with bevacizumab: A systematic review and meta-analysis. World J. Surg. Oncol. 2013, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; De Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, microRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef] [PubMed]
- Bernaards, C.; Hegde, P.; Chen, D.; Holmgren, E.; Zheng, M.; Jubb, A.M.; Koeppen, H.; Scherer, S.J.; Chen, D.S. Circulating vascular endothelial growth factor (VEGF) as a biomarker for bevacizumab-based therapy in metastatic colorectal, non-small cell lung, and renal cell cancers: Analysis of phase III studies. J. Clin. Oncol. 2010, 28, 10519. [Google Scholar] [CrossRef]
- Duda, D.G. Molecular Biomarkers of Response to Antiangiogenic Therapy for Cancer. ISRN Cell Biol. 2012, 2012, 587259. [Google Scholar] [CrossRef]
- Nigro, P.; Pompilio, G.; Capogrossi, M.C. Cyclophilin A: A key player for human disease. Cell Death Dis. 2013, 4, e888. [Google Scholar] [CrossRef]
- Calhoun, C.C.; Lu, Y.C.; Song, J.; Chiu, R. Knockdown endogenous CypA with siRNA in U2OS cells results in disruption of F-actin structure and alters tumor phenotype. Mol. Cell Biochem. 2009, 320, 35–43. [Google Scholar] [CrossRef]
- Han, J.M.; Jung, H.J. Cyclophilin A/CD147 Interaction: A Promising Target for Anticancer Therapy. Int. J. Mol. Sci. 2022, 23, 9341. [Google Scholar] [CrossRef]
- Lee, J. Role of cyclophilin a during oncogenesis. Arch. Pharm. Res. 2010, 33, 181–187. [Google Scholar] [CrossRef]
- Kim, S.H.; Lessner, S.M.; Sakurai, Y.; Galis, Z.S. Cyclophilin A as a novel biphasic mediator of endothelial activation and dysfunction. Am. J. Pathol. 2004, 164, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J.; Liu, F.; Gao, C.; Wang, X.; Zhao, T.; Liu, J.; Gao, S.; Zhao, X.; Ren, H.; et al. CypA, a gene downstream of HIF-1α, promotes the development of PDAC. PLoS ONE 2014, 9, e92824. [Google Scholar] [CrossRef] [PubMed]
- Tasevska, I.; Enhörning, S.; Christensson, A.; Persson, M.; Nilsson, P.M.; Melander, O. Increased Levels of Copeptin, a Surrogate Marker of Arginine Vasopressin, Are Associated with an Increased Risk of Chronic Kidney Disease in a General Population. Am. J. Nephrol. 2016, 44, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Welsh, P.; Papacosta, O.; Lennon, L.; Whincup, P.H.; Sattar, N. Copeptin, Insulin Resistance, and Risk of Incident Diabetes in Older Men. J. Clin. Endocrinol. Metab. 2015, 100, 3332–3339. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, S.M.; Ghandehari, M.; Parizadeh, M.R.; Ferns, G.A.; Ghayour-Mobarhan, M.; Avan, A.; Hassanian, S.M. The diagnostic and prognostic value of copeptin in cardiovascular disease, current status, and prospective. J. Cell Biochem. 2018, 119, 7913–7923. [Google Scholar] [CrossRef]
- Hagman, H.; Bendahl, P.O.; Melander, O.; Sundberg, J.; Johnsson, A.; Belting, M. Vasoactive peptides associate with treatment outcome ofbevacizumab-containing therapy in metastatic colorectal cancer. Acta Oncol. 2017, 56, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Humar, R.; Zimmerli, L.; Battegay, E. Angiogenesis and hypertension: An update. J. Hum. Hypertens. 2009, 23, 773–782. [Google Scholar] [CrossRef]
- Tahara, A.; Tsukada, J.; Tomura, Y.; Yatsu, T.; Shibasaki, M. Vasopressin induces human mesangial cell growth via induction of vascular endothelial growth factor secretion. Neuropeptides 2011, 45, 105–111. [Google Scholar] [CrossRef]
- Duran, C.L.; Borriello, L.; Karagiannis, G.S.; Entenberg, D.; Oktay, M.H.; Condeelis, J.S. Targeting Tie2 in the Tumor Microenvironment: From Angiogenesis to Dissemination. Cancers 2021, 13, 5730. [Google Scholar] [CrossRef]
- Tan, S.; Chen, Y.; Du, S.; Li, W.; Liu, P.; Zhao, J.; Yang, P.; Cai, J.; Gao, R.; Wang, Z. TIE2-high cervical cancer cells promote tumor angiogenesis by upregulating TIE2 and VEGFR2 in endothelial cells. Transl. Oncol. 2022, 26, 101539. [Google Scholar] [CrossRef] [PubMed]
- Backen, A.; Renehan, A.G.; Clamp, A.R.; Berzuini, C.; Zhou, C.; Oza, A.; Bannoo, S.; Scherer, S.J.; Banks, R.E.; Dive, C.; et al. The combination of circulating Ang1 and Tie2 levels predicts progression-free survival advantage in bevacizumab-treated patients with ovarian cancer. Clin. Cancer Res. 2014, 20, 4549–4558. [Google Scholar] [CrossRef] [PubMed]
- Engin, H.; Üstündağ, Y.; Tekin, İ.Ö.; Gökmen, A.; Ertop, Ş.; İlikhan, S.U. Plasma concentrations of angiopoietin-1, angiopoietin-2 and Tie-2 in colon cancer. Eur. Cytokine Netw. 2012, 23, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhou, M.; Peng, L.; Kong, S.; Miao, R.; Shi, Y.; Sheng, H.; Li, L. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7432–7441. [Google Scholar]
- Peng, L.; Jiang, J.; Chen, H.N.; Zhou, L.; Huang, Z.; Qin, S.; Jin, P.; Luo, M.; Li, B.; Shi, J.; et al. Redox-sensitive cyclophilin A elicits chemoresistance through realigning cellular oxidative status in colorectal cancer. Cell Rep. 2021, 37, 110069. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, J.; Yang, J.; Qiao, S.; Zhao, S.; Yu, L. Cyclophilin A is upregulated in small cell lung cancer and activates ERK1/2 signal. Biochem. Biophys. Res. Commun. 2007, 361, 763–767. [Google Scholar] [CrossRef]
- Gong, Z.; Chi, C.; Huang, X.; Chu, H.; Wang, J.; Du, F.; Jiang, L.; Chen, J. Cyclophilin A Is Overexpressed in Hepatocellular Carcinoma and Is Associated with the Cell Cycle. Anticancer Res. 2017, 37, 4443–4447. [Google Scholar]
- Yamamoto, T.; Takakura, H.; Mitamura, K.; Taga, A. Cyclophilin a knokdown inhibits cell migration and invasion through the suppression of epithelial-mesenchymal transition in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2020, 526, 55–61. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, M.; Zhao, X.; Gu, M.; Wang, Z.; Xu, S.; Yue, W. Cyclophilin A promotes non-small cell lung cancer metastasis via p38 MAPK. Thorac. Cancer 2018, 9, 120–128. [Google Scholar] [CrossRef]
- Grigoryeva, E.S.; Cherdyntseva, N.V.; Karbyshev, M.S.; Volkomorov, V.V.; Stepanov, I.V.; Zavyalova, M.V.; Perelmuter, V.M.; Buldakov, M.A.; Afanasjev, S.G.; Tuzikov, S.A.; et al. Expression of cyclophilin A in gastric adenocarcinoma patients and its inverse association with local relapses and distant metastasis. Pathol. Oncol. Res. 2014, 20, 467–473. [Google Scholar] [CrossRef]
- Fu, J.; Fu, J.; Chen, X.; Zhang, Y.; Gu, H.; Bai, Y. CD147 and VEGF co-expression predicts prognosis in patients with acute myeloid leukemia. Jpn. J. Clin. Oncol. 2010, 40, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Miyagaki, T.; Kamijo, H.; Oka, T.; Boki, H.; Takahashi-Shishido, N.; Suga, H.; Sugaya, M.; Sato, S. CD147-Cyclophilin a Interactions Promote Proliferation and Survival of Cutaneous T-Cell Lymphoma. Int. J. Mol. Sci. 2021, 22, 7889. [Google Scholar] [CrossRef]
- Su, H.; Wan, C.; Wang, Z.D.; Gao, Y.; Li, Y.C.; Tang, F.; Zhu, H.Y.; Yi, L.X.; Zhang, C. Expression of CD147 and Cyclophilin A in Kidneys of Patients with COVID-19. Clin. J. Am. Soc. Nephrol. 2021, 16, 618–619. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kudo, M.; Peng, W.X.; Takata, H.; Takakura, H.; Teduka, K.; Fujii, T.; Mitamura, K.; Taga, A.; Uchida, E.; et al. Identification of aldolase A as a potential diagnostic biomarker for colorectal cancer based on proteomic analysis using formalin-fixed paraffin-embedded tissue. Tumour. Biol. 2016, 37, 13595–13606. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Piao, Y.J.; Lim, M.J.; Kim, J.H.; Ha, J.; Choe, W.; Kim, S.S. Overexpressed cyclophilin A in cancer cells renders resistance to hypoxia- and cisplatin-induced cell death. Cancer Res. 2007, 67, 3654–3662. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Ma, H.; Saiyin, H.; Shen, S.; Xi, J.; Wan, B.; Yu, L. Oligo-microarray analysis reveals the role of cyclophilin A in drug resistance. Cancer Chemother. Pharmacol. 2008, 61, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; Dang, Y.Z.; Gao, H.X.; Jiang, J.L.; Chen, Z.N. HAb18G/CD147 promotes radioresistance in hepatocellular carcinoma cells: A potential role for integrin β1 signaling. Mol. Cancer Ther. 2015, 14, 553–563. [Google Scholar] [CrossRef]
- Valeri, N.; Gasparini, P.; Braconi, C.; Paone, A.; Lovat, F.; Fabbri, M.; Sumani, K.M.; Alder, H.; Amadori, D.; Patel, T.; et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2). Proc. Natl. Acad. Sci. USA 2010, 107, 21098–21103. [Google Scholar] [CrossRef]
- Dyhl-Polk, A.; Schou, M.; Vistisen, K.K.; Sillesen, A.S.; Serup-Hansen, E.; Faber, J.; Klausen, T.W.; Bojesen, S.E.; Vaage-Nilsen, M.; Nielsen, D.L. Myocardial Ischemia Induced by 5-Fluorouracil: A Prospective Electrocardiographic and Cardiac Biomarker Study. Oncologist 2021, 26, e403–e413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, W.; Yue, D.; Chen, C.; Li, C.; Zhang, Z.; Wang, C. Bevacizumab-Induced Mitochondrial Dysfunction, Endoplasmic Reticulum Stress, and ERK Inactivation Contribute to Cardiotoxicity. Oxid. Med. Cell Longev. 2021, 2021, 5548130. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Schuler, M.; Stuschke, M.; Heusch, G.; Rassaf, T. Cardio-oncology-strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019, 280, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Jakab, M.; Rostalski, T.; Lee, K.H.; Mogler, C.; Augustin, H.G. Tie2 Receptor in Tumor-Infiltrating Macrophages Is Dispensable for Tumor Angiogenesis and Tumor Relapse after Chemotherapy. Cancer Res. 2022, 82, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Jayson, G.C.; Zhou, C.; Backen, A.; Horsley, L.; Marti-Marti, K.; Shaw, D.; Mescallado, N.; Clamp, A.; Saunders, M.P.; Valle, J.W.; et al. Plasma Tie2 is a tumor vascular response biomarker for VEGF inhibitors in metastatic colorectal cancer. Nat. Commun. 2018, 9, 4672. [Google Scholar] [CrossRef] [PubMed]
- Moisuc, D.C.; Marinca, M.V.; Gafton, B.; Alexa-Stratulat, T.; Pavel-Tanasa, M.; Cianga, P. Antiangiogenic Drug-Induced Proteinuria as a Prognostic Factor in Metastatic Colorectal Cancer. Curr. Oncol. 2022, 29, 3996–4011. [Google Scholar] [CrossRef]
- Dionísio de Sousa, I.J.; Ferreira, J.; Rodrigues, J.; Bonito, N.; Jacinto, P.; Marques, M.; Ribeiro, J.; Pais, A.; Gervásio, H. Association between bevacizumab-related hypertension and response to treatment in patients with metastatic colorectal cancer. ESMO Open 2016, 1, e000045. [Google Scholar] [CrossRef]
- Österlund, P.; Soveri, L.M.; Isoniemi, H.; Poussa, T.; Alanko, T.; Bono, P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br. J. Cancer 2011, 104, 599–604. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 2005, 97, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Frequency | Percent |
|---|---|---|
| Median age, years (range) | 61 (37–82) | |
| Gender | ||
| Male | 30 | 54 |
| Female | 26 | 46 |
| Primary tumor location | ||
| Descending colon | 41 | 73 |
| Ascending colon | 15 | 27 |
| Stage at diagnosis | ||
| Metastatic | 44 | 79 |
| Non-metastatic | 12 | 21 |
| Primary tumor resection | 40 | 71 |
| RAS status | ||
| Wild type | 17 | 31 |
| Mutant | 36 | 64 |
| Not tested | 3 | 5 |
| Associated chemotherapy | ||
| Oxaliplatin-based | 37 | 66 |
| Irinotecan-based | 16 | 29 |
| Fluorouracil/Capecitabine-based | 3 | 5 |
| Preexisting arterial hypertension | 27 | 48 |
| High blood pressure during treatment | 40 | 71 |
| Number and location of metastases | ||
| Liver | 24 | 43 |
| Lung | 4 | 7 |
| Peritoneal | 4 | 7 |
| Multiple | 24 | 43 |
| Tumor response | ||
| CR 1 | 2 | 3.6 |
| PR 2 | 11 | 19.6 |
| SD 3 | 25 | 44.6 |
| PD 4 | 18 | 32.1 |
| Event | All Grades n (%) | Grades ≥ 3 n (%) |
|---|---|---|
| Any | 54 (96.4) | 13 (23.2) |
| Proteinuria | 30 (53.6) | * |
| Anemia | 20 (35.7) | 5 (9) |
| Neutropenia | 26 (46.4) | 7 (12.5) |
| Thrombocytopenia | 19 (34) | 0 |
| Neurological toxicity 2 | 25 (44.7) | 1 (1.8) |
| Liver toxicity 3 | 17 (30.4) | 0 |
| Biomarker | Baseline Levels (Median, Min–Max) | After One Month Treatment Levels (Median, Min–Max) | After Six Months Treatment Levels (Median, Min–Max) |
|---|---|---|---|
| Cyclophilin A (ng/mL) | 54.65 | 29.38 | 114.92 |
| (1.54–168.61) | (1.05–400.03) | (0.72–432.71) | |
| copeptin (pg/mL) | 492.54 | 884.35 | 950.42 |
| (129.67–1183.92) | (206.15–6740.80) | (402.93–4308.60) | |
| Tie2 (ng/mL) | 0.56 | 0.62 | 0.55 |
| (0.44–12.34) | (0.46–12.54) | (0.39–8.88) |
| Factor | p | HR | 95% Confidence Interval |
|---|---|---|---|
| CypA at baseline adjusted for baseline factors: | |||
| CEA | 0.929 | - | - |
| CA 19-9 | 0.643 | - | - |
| LDH | 0.711 | - | - |
| Number of metastases | 0.224 | - | - |
| Resection of the primary tumor | 0.181 | - | - |
| Number of lymph node invasion | 0.010 | 1.981 | 1.182–3.321 |
| CypA at baseline | 0.016 | 0.424 | 0.210–0.855 |
| CypA at baseline adjusted for post-treatment factors: | |||
| Thrombocytopenia | 0.568 | - | - |
| Neutropenia | 0.755 | - | - |
| Liver toxicity | 0.991 | - | - |
| Anemia | 0.002 | 0.317 | 0.151–0.666 |
| Proteinuria | 0.026 | 2.197 | 1.100–4.390 |
| CypA at baseline | 0.004 | 0.330 | 0.156–0.694 |
| CypA after one month of treatment adjusted for baseline factors: | |||
| CEA | 0.096 | - | - |
| CA 19-9 | 0.184 | - | - |
| LDH | 0.735 | - | - |
| Number of metastases | 0.109 | - | - |
| Resection of the primary tumor | 0.068 | - | - |
| Number of lymph node invasion | 0.017 | 1.829 | 1.113–3.005 |
| CypA after one month of treatment | 0.101 | - | - |
| CypA after one month of treatment adjusted for post-treatment factors: | |||
| Thrombocytopenia | 0.521 | - | - |
| Neutropenia | 0.900 | - | - |
| Liver toxicity | 0.923 | - | - |
| Anemia | 0.008 | 0.386 | 0.190–0.782 |
| Proteinuria | 0.019 | 2.312 | 1.146–4.665 |
| CypA after one month of treatment | 0.013 | 0.406 | 0.200–0.828 |
| Factor | p | HR | 95% Confidence Interval |
|---|---|---|---|
| CypA after 6 months of treatment | 0.011 | 0.461 | 0.253–0.840 |
| Treatment-induced hypertension | 0.001 | 3.315 | 1.636–6.716 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisuc, D.C.; Constantinescu, D.; Marinca, M.V.; Gafton, B.; Pavel-Tanasa, M.; Cianga, P. Cyclophilin A: An Independent Prognostic Factor for Survival in Patients with Metastatic Colorectal Cancer Treated with Bevacizumab and Chemotherapy. Cancers 2024, 16, 385. https://doi.org/10.3390/cancers16020385
Moisuc DC, Constantinescu D, Marinca MV, Gafton B, Pavel-Tanasa M, Cianga P. Cyclophilin A: An Independent Prognostic Factor for Survival in Patients with Metastatic Colorectal Cancer Treated with Bevacizumab and Chemotherapy. Cancers. 2024; 16(2):385. https://doi.org/10.3390/cancers16020385
Chicago/Turabian StyleMoisuc, Diana Cornelia, Daniela Constantinescu, Mihai Vasile Marinca, Bogdan Gafton, Mariana Pavel-Tanasa, and Petru Cianga. 2024. "Cyclophilin A: An Independent Prognostic Factor for Survival in Patients with Metastatic Colorectal Cancer Treated with Bevacizumab and Chemotherapy" Cancers 16, no. 2: 385. https://doi.org/10.3390/cancers16020385
APA StyleMoisuc, D. C., Constantinescu, D., Marinca, M. V., Gafton, B., Pavel-Tanasa, M., & Cianga, P. (2024). Cyclophilin A: An Independent Prognostic Factor for Survival in Patients with Metastatic Colorectal Cancer Treated with Bevacizumab and Chemotherapy. Cancers, 16(2), 385. https://doi.org/10.3390/cancers16020385







