Primary Tumor Resection in Synchronous Metastatic Colorectal Cancer Patients Treated with Upfront Chemotherapy plus Bevacizumab: A Pooled Analysis of TRIBE and TRIBE2 Studies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
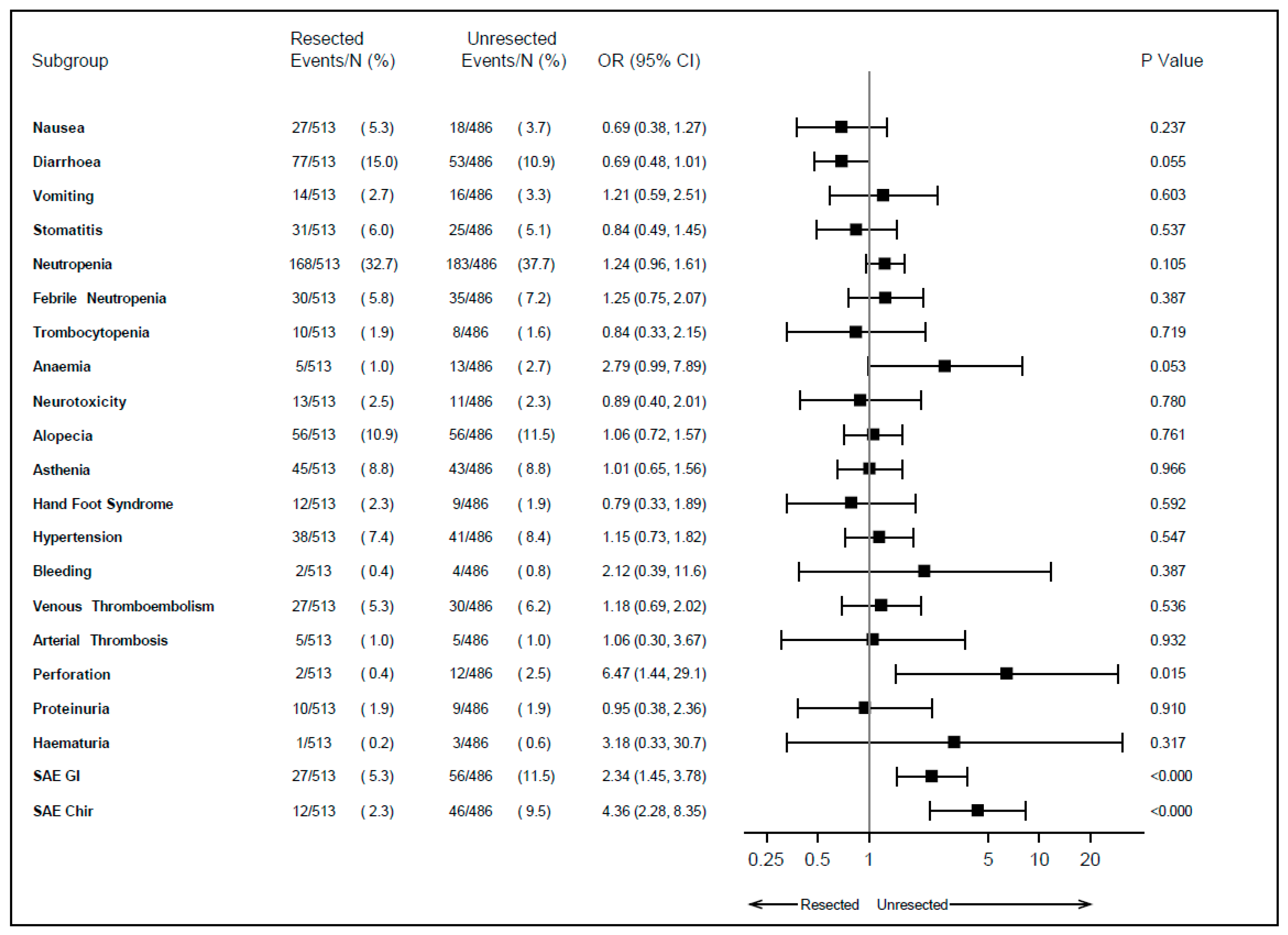
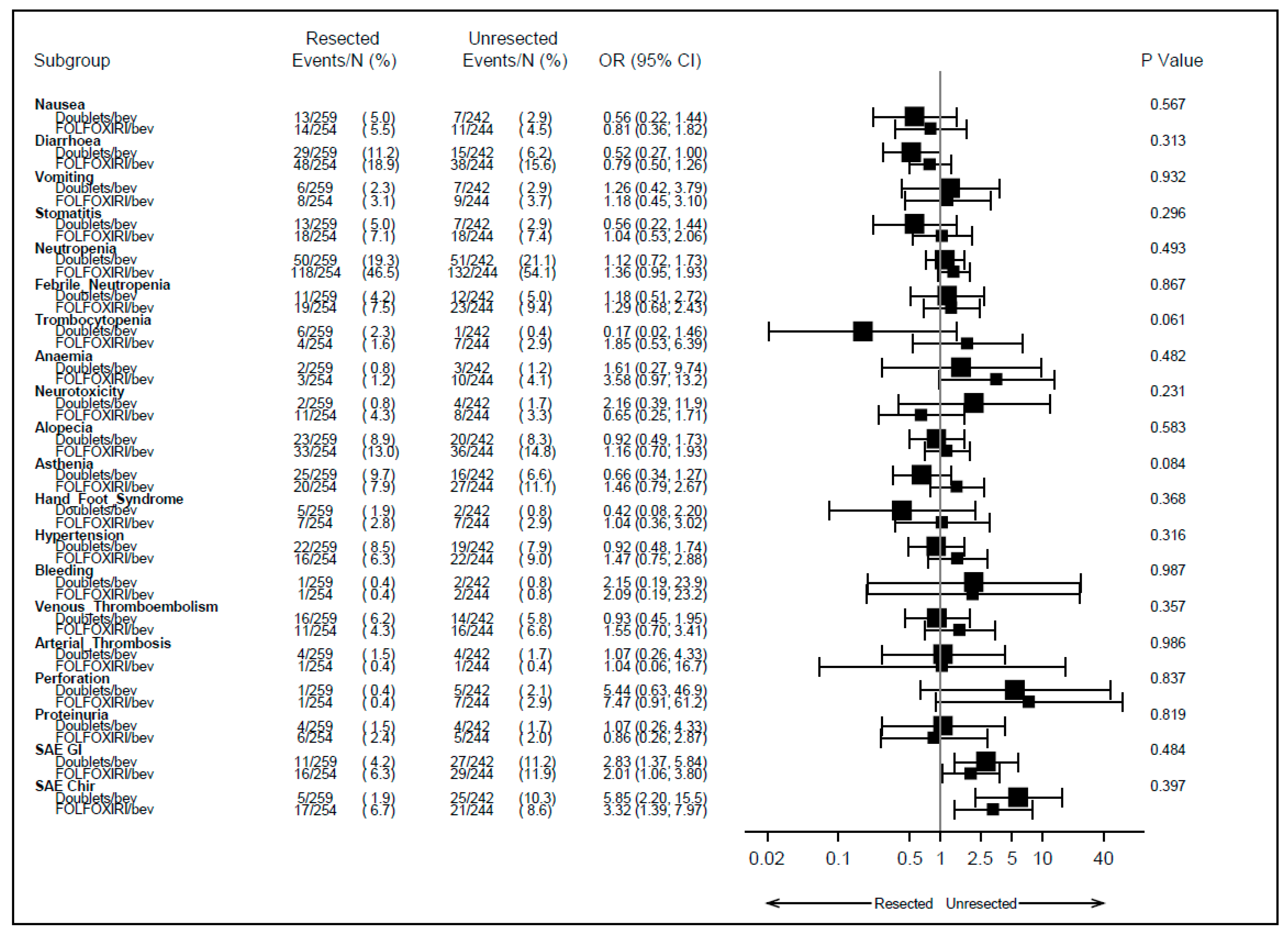
3.2. Safety
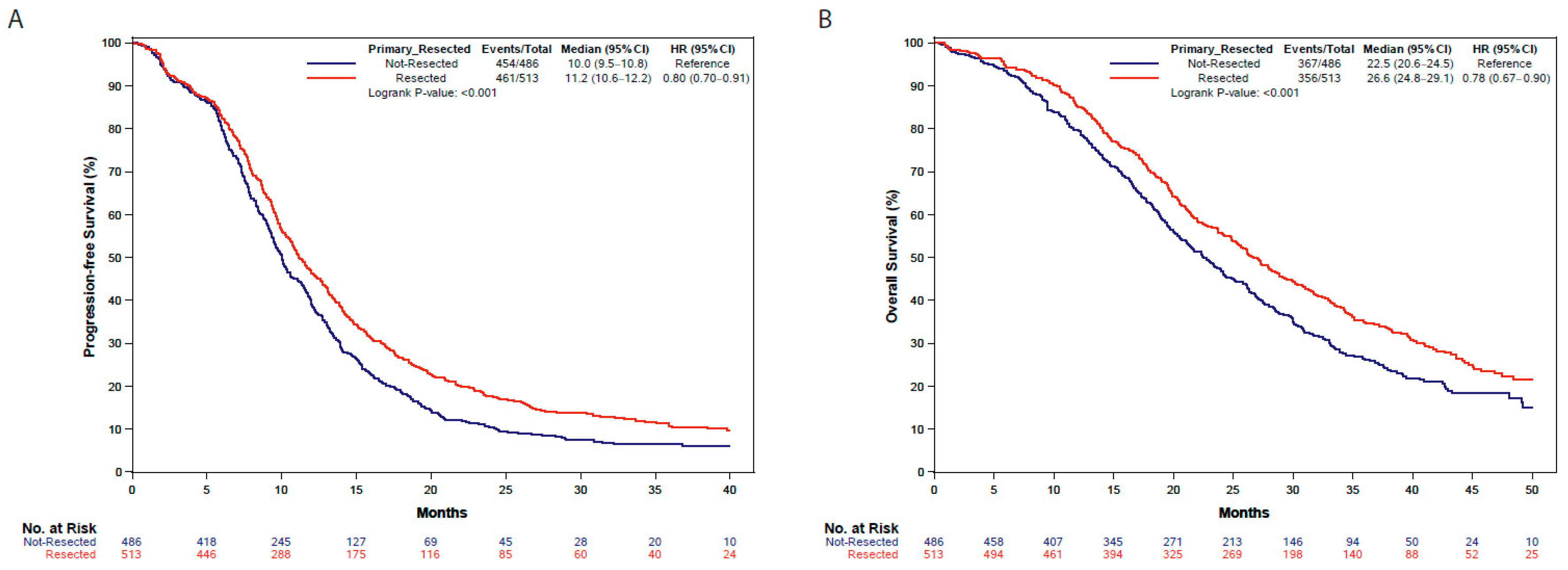
3.3. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Hompes, D.; Ruers, T. Review: Incidence and clinical significance of Bevacizumab-related non-surgical and surgical serious adverse events in metastatic colorectal cancer. Eur. J. Surg. Oncol. 2011, 37, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Tran, B.; Tran, P.V.; Sinnathamby, M.; Wong, H.L.; Jones, I.; Croxford, M.; Desai, J.; Tie, J.; Field, K.M.; et al. Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival. Clin. Color. Cancer 2015, 14, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ruo, L.; Gougoutas, C.; Paty, P.B.; Guillem, J.G.; Cohen, A.M.; Wong, W.D. Elective bowel resection for incurable stage IV colorectal cancer: Prognostic variables for asymptomatic patients. J. Am. Coll. Surg. 2003, 196, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Galizia, G.; Lieto, E.; Orditura, M.; Castellano, P.; Imperatore, V.; Pinto, M.; Zamboli, A. First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch. Surg. 2008, 143, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef]
- Peeters, C.F.; Westphal, J.R.; de Waal, R.M.; Ruiter, D.J.; Wobbes, T.; Ruers, T.J. Vascular density in colorectal liver metastases increases after removal of the primary tumor in human cancer patients. Int. J. Cancer 2004, 112, 554–559. [Google Scholar] [CrossRef]
- van der Wal, G.E.; Gouw, A.S.; Kamps, J.A.; Moorlag, H.E.; Bulthuis, M.L.; Molema, G.; de Jong, K.P. Angiogenesis in synchronous and metachronous colorectal liver metastases: The liver as a permissive soil. Ann. Surg. 2012, 255, 86–94. [Google Scholar] [CrossRef]
- Peeters, C.F.; de Waal, R.M.; Wobbes, T.; Westphal, J.R.; Ruers, T.J. Outgrowth of human liver metastases after resection of the primary colorectal tumor: A shift in the balance between apoptosis and proliferation. Int. J. Cancer 2006, 119, 1249–1253. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology, Colon Cancer, Version 3. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428 (accessed on 1 October 2023).
- NCCN Clinical Practice Guidelines in Oncology, Rectal Cancer, Version 5. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1461 (accessed on 1 October 2023).
- Fanotto, V.; Salani, F.; Vivaldi, C.; Scartozzi, M.; Ribero, D.; Puzzoni, M.; Montagnani, F.; Leone, F.; Vasile, E.; Bencivenga, M.; et al. Primary Tumor Resection for Metastatic Colorectal, Gastric and Pancreatic Cancer Patients: In Search of Scientific Evidence to Inform Clinical Practice. Cancers 2023, 15, 900. [Google Scholar] [CrossRef]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Cirocchi, R.; Trastulli, S.; Abraha, I.; Vettoretto, N.; Boselli, C.; Montedori, A.; Parisi, A.; Noya, G.; Platell, C. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst. Rev. 2012, 8, CD008997. [Google Scholar] [CrossRef] [PubMed]
- Faron, M.; Pignon, J.P.; Malka, D.; Bourredjem, A.; Douillard, J.Y.; Adenis, A.; Elias, D.; Bouché, O.; Ducreux, M. Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur. J. Cancer 2015, 51, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Bailey, C.E.; You, Y.N.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Feig, B.W.; Chang, G.J. Time trend analysis of primary tumor resection for stage IV colorectal cancer: Less surgery, improved survival. JAMA Surg. 2015, 150, 245–251. [Google Scholar] [CrossRef]
- Tarantino, I.; Warschkow, R.; Worni, M.; Cerny, T.; Ulrich, A.; Schmied, B.M.; Güller, U. Prognostic Relevance of Palliative Primary Tumor Removal in 37,793 Metastatic Colorectal Cancer Patients: A Population-Based, Propensity Score-Adjusted Trend Analysis. Ann. Surg. 2015, 262, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Xu, L.; Yang, W.; Xu, X.; Zheng, S. Asymptomatic Primary Tumor Resection in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 836404. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, C.; Liu, L.; Lv, Y.; Li, J.; Lu, J.; Wang, S.; Du, B.; Yang, X. Effect of primary tumor resection on survival in patients with asymptomatic unresectable metastatic colorectal cancer: A systematic review and meta-analysis. Expert. Rev. Anticancer Ther. 2023, 23, 107–115. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients With Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 1098–1107. [Google Scholar] [CrossRef]
- Koopman, M.; van der Kruijssen, D.E.W.; Elias, S.G.; van de Ven, P.M.; Mol, L.; Punt, C.J.A.; Tanis, P.J.; Nielsen, J.D.; Yilmaz, M.K.; Loosveld, O.; et al. Upfront palliative resection of primary tumor versus no resection in patients with synchronous metastatic colorectal cancer: The randomized phase 3 CAIRO4 study of the Dutch Colorectal Cancer Group (DCCG). J. Clin. Oncol. 2023, 41 (Suppl. S16), 3517. [Google Scholar] [CrossRef]
- van der Kruijssen, D.E.W.; Elias, S.G.; Vink, G.R.; van Rooijen, K.L.; Lam-Boer, J.‘T.; Mol, L.; Punt, C.J.A.; de Wilt, J.H.W.; Koopman, M.; CAIRO4 Working Group. Sixty-Day Mortality of Patients with Metastatic Colorectal Cancer Randomized to Systemic Treatment vs Primary Tumor Resection Followed by Systemic Treatment: The CAIRO4 Phase 3 Randomized Clinical Trial. JAMA Surg. 2021, 156, 1093–1101. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Resected Primary Tumor | |||
|---|---|---|---|---|
| No (n = 486) | Yes (n = 513) | Total (n = 999) | p-Value | |
| Age (years) | 0.7309 1 | |||
| N | 486 | 513 | 999 | |
| Mean (SD) | 59.0 (9.4) | 58.7 (9.9) | 58.8 (9.7) | |
| Median | 60.0 | 60.0 | 60.0 | |
| Range | 33.0, 75.0 | 29.0, 75.0 | 29.0, 75.0 | |
| Age, n (%) | 0.9412 2 | |||
| <70 years | 417 (85.8%) | 441 (86.0%) | 858 (85.9%) | |
| ≥70 years | 69 (14.2%) | 72 (14.0%) | 141 (14.1%) | |
| Treatment arm, n (%) | 0.8267 2 | |||
| Doublets CT + bevacizumab | 242 (49.8%) | 259 (50.5%) | 501 (50.2%) | |
| FOLFOXIRI + bevacizumab | 244 (50.2%) | 254 (49.5%) | 498 (49.8%) | |
| Gender, n (%) | 0.0087 2 | |||
| Female | 181 (37.2%) | 233 (45.4%) | 414 (41.4%) | |
| Male | 305 (62.8%) | 280 (54.6%) | 585 (58.6%) | |
| ECOG PS, n (%) | 0.0821 2 | |||
| 0 | 413 (85.0%) | 455 (88.7%) | 868 (86.9%) | |
| 1–2 | 73 (15.0%) | 58 (11.3%) | 131 (13.1%) | |
| Site of primary tumor, n (%) | <0.001 2 | |||
| Left rectum | 325 (69.4%) | 292 (57.4%) | 617 (63.2%) | |
| Right rectum | 143 (30.6%) | 217 (42.6%) | 360 (36.8%) | |
| Missing | 18 | 4 | 22 | |
| RAS/BRAF mutational status, n (%) | 0.007 2 | |||
| BRAF mutated | 25 (6.4%) | 59 (12.8%) | 84 (9.8%) | |
| RAS mutated | 275 (70.0%) | 301 (65.4%) | 576 (67.5%) | |
| RAS/BRAF wild type | 93 (23.7%) | 100 (21.7%) | 193 (22.6%) | |
| Missing | 93 | 53 | 146 | |
| Number of metastatic sites, n (%) | <0.001 2 | |||
| 1 | 136 (28.1%) | 216 (42.1%) | 352 (35.3%) | |
| >1 | 348 (71.9%) | 297 (57.9%) | 645 (64.7%) | |
| Missing | 2 | 0 | 2 | |
| Liver-only disease, n (%) | <0.001 2 | |||
| No | 379 (78.3%) | 342 (66.7%) | 721 (72.3%) | |
| Yes | 105 (21.7%) | 171 (33.3%) | 276 (27.7%) | |
| Missing | 2 | 0 | 2 | |
| Characteristic | Subsequent PTR | |||
|---|---|---|---|---|
| No (n = 280) | Yes (n = 206) | Total (n = 486) | p-Value | |
| Age (years) | 0.0043 1 | |||
| N | 280 | 206 | 486 | |
| Mean (SD) | 59.9 (9.7) | 57.8 (8.9) | 59.0 (9.4) | |
| Median | 62.0 | 58.0 | 60.0 | |
| Range | 34.0, 75.0 | 33.0, 75.0 | 33.0, 75.0 | |
| Age, n (%) | 0.0031 2 | |||
| <70 years | 229 (81.8%) | 188 (91.3%) | 417 (85.8%) | |
| ≥70 years | 51 (18.2%) | 18 (8.7%) | 69 (14.2%) | |
| Treatment arm, n (%) | 0.1154 2 | |||
| Doublets CT + bevacizumab | 148 (52.9%) | 94 (45.6%) | 242 (49.8%) | |
| FOLFOXIRI + bevacizumab | 132 (47.1%) | 112 (54.4%) | 244 (50.2%) | |
| Gender, n (%) | 0.9576 2 | |||
| Female | 104 (37.1%) | 77 (37.4%) | 181 (37.2%) | |
| Male | 176 (62.9%) | 129 (62.6%) | 305 (62.8%) | |
| ECOG PS, n (%) | 0.0022 2 | |||
| 0 | 226 (80.7%) | 187 (90.8%) | 413 (85.0%) | |
| 1–2 | 54 (19.3%) | 19 (9.2%) | 74 (15.0%) | |
| Site of primary tumor, n (%) | 0.0880 2 | |||
| Left rectum | 177 (66.3%) | 148 (73.6%) | 325 (69.4%) | |
| Right rectum | 90 (33.7%) | 53 (26.4%) | 143 (30.6%) | |
| Missing | 13 | 5 | 18 | |
| RAS/BRAF mutational status, n (%) | 0.4153 2 | |||
| BRAF mutated | 16 (7.1%) | 9 (5.3%) | 25 (6.4%) | |
| RAS mutated | 160 (71.4%) | 115 (68.0%) | 275 (70.0%) | |
| RAS/BRAF wild type | 48 (21.4%) | 45 (26.6%) | 93 (23.7%) | |
| Missing | 56 | 37 | 93 | |
| Number of metastatic sites, n (%) | <0.001 2 | |||
| 1 | 52 (18.6%) | 84 (41.0%) | 136 (28.1%) | |
| >1 | 227 (81.4%) | 121 (59.0%) | 348 (71.9%) | |
| Missing | 1 | 1 | 2 | |
| Liver-only disease, n (%) | <0.001 2 | |||
| No | 243 (87.1%) | 136 (66.3%) | 379 (78.3%) | |
| Yes | 36 (12.9%) | 69 (33.7%) | 105 (21.7%) | |
| Missing | 1 | 1 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanotto, V.; Rossini, D.; Casagrande, M.; Bergamo, F.; Spagnoletti, A.; Santini, D.; Antoniotti, C.; Cupini, S.; Daniel, F.; Nasca, V.; et al. Primary Tumor Resection in Synchronous Metastatic Colorectal Cancer Patients Treated with Upfront Chemotherapy plus Bevacizumab: A Pooled Analysis of TRIBE and TRIBE2 Studies. Cancers 2023, 15, 5451. https://doi.org/10.3390/cancers15225451
Fanotto V, Rossini D, Casagrande M, Bergamo F, Spagnoletti A, Santini D, Antoniotti C, Cupini S, Daniel F, Nasca V, et al. Primary Tumor Resection in Synchronous Metastatic Colorectal Cancer Patients Treated with Upfront Chemotherapy plus Bevacizumab: A Pooled Analysis of TRIBE and TRIBE2 Studies. Cancers. 2023; 15(22):5451. https://doi.org/10.3390/cancers15225451
Chicago/Turabian StyleFanotto, Valentina, Daniele Rossini, Mariaelena Casagrande, Francesca Bergamo, Andrea Spagnoletti, Daniele Santini, Carlotta Antoniotti, Samanta Cupini, Francesca Daniel, Vincenzo Nasca, and et al. 2023. "Primary Tumor Resection in Synchronous Metastatic Colorectal Cancer Patients Treated with Upfront Chemotherapy plus Bevacizumab: A Pooled Analysis of TRIBE and TRIBE2 Studies" Cancers 15, no. 22: 5451. https://doi.org/10.3390/cancers15225451
APA StyleFanotto, V., Rossini, D., Casagrande, M., Bergamo, F., Spagnoletti, A., Santini, D., Antoniotti, C., Cupini, S., Daniel, F., Nasca, V., Vetere, G., Zaniboni, A., Borelli, B., Carullo, M., Conca, V., Passardi, A., Tamburini, E., Masi, G., Pella, N., & Cremolini, C. (2023). Primary Tumor Resection in Synchronous Metastatic Colorectal Cancer Patients Treated with Upfront Chemotherapy plus Bevacizumab: A Pooled Analysis of TRIBE and TRIBE2 Studies. Cancers, 15(22), 5451. https://doi.org/10.3390/cancers15225451










