Adapting the Fitness Criteria for Non-Intensive Treatments in Older Patients with Acute Myeloid Leukemia to the Use of Venetoclax-Hypomethylating Agents Combination—Practical Considerations from the Real-Life Experience of the Hematologists of the Rete Ematologica Lombarda
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
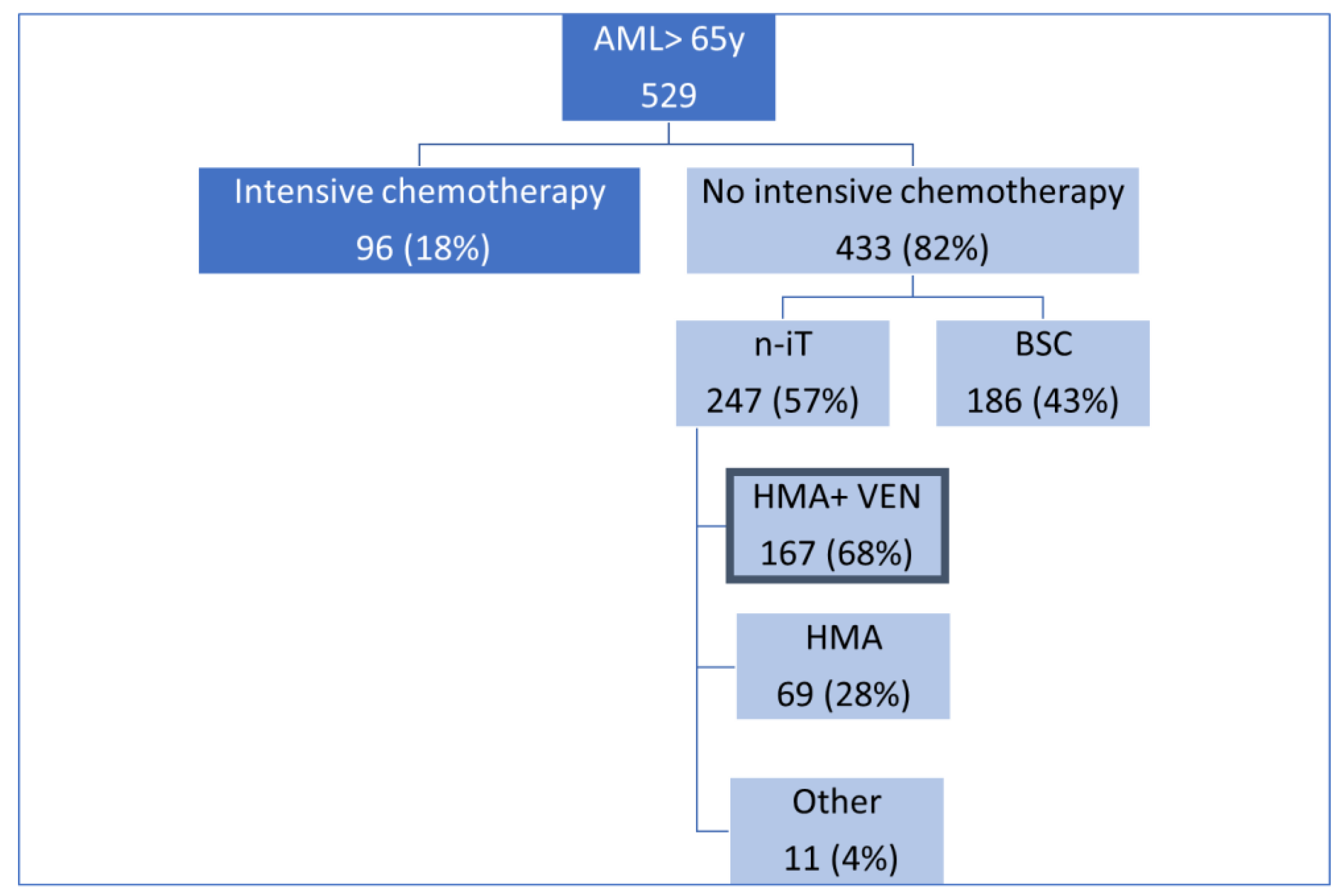
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Yi, M.; Li, A.; Zhou, L.; Chu, Q.; Song, Y.; Wu, K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: Estimates based on the global burden of disease study 2017. J. Hematol. Oncol. 2020, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.A.; Pratz, K.W. Acute myeloid leukemia in the elderly: Therapeutic options and choice. Leuk Lymphoma 2018, 59, 274–287. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Stat Facts: Acute Myeloid Leukemia; National Cancer Institute: Bethesda, MD, USA, 2017. Available online: http://seer.cancer.gov/statfacts/html/amyl.html (accessed on 1 January 2022).
- Kantarjian, H.M.; Short, N.J.; Fathi, A.T.; Marcucci, G.; Ravandi, F.; Tallman, M.; Wang, E.S.; Wei, A.H. Acute myeloid leukemia: Historical perspective and progress in research and therapy over 5 decades. Clin. Lymphoma Myeloma Leuk 2021, 21, 580–597. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; DiNardo, C.D.; Loghavi, S. Molecularly Targeted Therapy in Acute Myeloid Leukemia: Current Treatment Landscape and Mechanisms of Response and Resistance. Cancers 2023, 15, 1617. [Google Scholar] [CrossRef] [PubMed]
- Min, G.J.; Cho, B.S.; Park, S.S.; Jeon, Y.-W.; Shin, S.H.; Yahng, S.A.; Yoon, J.H.; Lee, S.E.; Eom, K.S.; Kim, Y.J. Geriatric assessment predict non fatal toxicities and survival for intensively treated older adults with AML. Blood 2022, 139, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Cairoli, R.; Caira, M.; Finsinger, P.; Finocchiaro, F.; Neri, B.; De Benedittis, D.; Rossi, G.; Ferrara, F. Assessing eligibility for treatment in acute myeloid leukemia in 2023. Expert Rev. Hematol. 2023, 16, 181–1908. [Google Scholar] [CrossRef] [PubMed]
- Deschler, B.; Ihorst, G.; Platzbecker, U.; Germing, U.; Marz, E.; de Figuerido, M.; Fritzsche, K.; Haas, P.; Salih, H.R.; Giagounidis, A.; et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica 2013, 98, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, E.; Klepin, H.; Storrick, E.; Major, B.; Le-Rademacher, J.; Wadleigh, M.; Walker, A.; Larson, R.A.; Roboz, G.J. Geriatric assessment for older adults receiving less-intensive therapy for acute myeloid leukaemia: Report of CALGB 361101. Blood Adv. 2022, 6, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Barosi, G.; Venditti, A.; Angelucci, E.; Gobbi, M.; Pane, F.; Tosi, P.; Zinzani, P.; Tura, S. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: A project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia 2013, 27, 997–999. [Google Scholar] [CrossRef]
- Palmieri, R.; Othus, M.; Halpern, A.B.; Percival, M.M.; Godwin, C.D.; Becker, P.S.; Walter, R.B. Accuracy of SIE/SIES/GITMO Consensus Criteria for Unfitness to Predict Early Mortality After Intensive Chemotherapy in Adults With AML or Other High-Grade Myeloid Neoplasm. J. Clin. Oncol. 2020, 38, 4163–4174. [Google Scholar] [CrossRef]
- Borlenghi, E.; Pagani, C.; Zappasodi, P.; Bernardi, M.; Basilico, C.; Cairoli, R.; Fracchiolla, N.; Todisco, E.; Turrini, M.; Cattaneo, C.; et al. Validation of the “fitness criteria” for the treatment of older patients with acute myeloid leukemia: A multicenter study on a series of 699 patients by the Network Rete Ematologica Lombarda (REL). J. Geriatr. Oncol. 2021, 12, 550–556. [Google Scholar] [CrossRef]
- Heuser, M.; Ofran, Y.; Boissel, N.; Brunet Mauri, S.; Craddock, C.; Janssen, J.; Dummer, R.; Keilholz, U.; ESMO Guidelines Committee. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 697–712. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Stone, R.M.; Stahl, M. Venetoclax combination therapy in acute myeloid leukemia and myelodysplastic syndromes. Curr. Opin. Hematol. 2022, 29, 63–73. [Google Scholar] [CrossRef]
- Candoni, A.; Lazzarotto, D.; Papayannidis, C.; Piccini, M.; Nadali, G.; Dargenio, M.; Riva, M.; Fracchiolla, N.; Mellillo, L.; Dragonetti, G.; et al. Prospective multicenter study on infectious complications and clinical outcome of 230 unfit acute myeloid leukemia patients receiving first-line therapy with hypomethylating agents alone or in combination with Venetoclax. Am. J. Hematol. 2023, 98, E80–E83. [Google Scholar] [CrossRef]
- Diekmann, B.; Timmerman, M.; Hempenius, L.; van Roon, E.; Franken, B.; Hoogendoorn, M. New treatment opportunities for older patients with acute myeloid leukemia and the increasing importance of frailty assessment—An oncogeriatric perspective. J. Geriatr. Oncol. 2023, 30, 101631. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.M.; Bezerra, E.D.; Farrukh, F.; McCullough, K.; Al-Kali, A.; Alkhateeb, H.B.; Begna, K.; Litzow, M.R.; Hogan, W.J.; Shah, M.V.; et al. Cardiac events in patients with acute myeloid leukemia treated with venetoclax combined with hypomethylating agents. Blood Adv. 2022, 6, 5227–5231. [Google Scholar] [CrossRef] [PubMed]
- Hernlund, E.; Redig, J.B.; Pausson, B.; Derolf, A.R.; Höglund, M.; Vertuani, S.; Juliusson, G. Socioeconomic cost of AML in Sweden. A population-based study using multiple nation-wide registers. Eur. J. Haem. 2021, 2, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Dharmani, C.; Wang, E.; Tu, N.; Fofah, O.; Cueto, J.; Salas, M.; Kamel, Y.M. Elderly patients with acute myeloid leukemia who only receive supportive care in the Surveillance, Epidemiology and End Results-Medicare database: Demographics, treatment patterns and outcomes. Future Oncol. 2023, 19, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
| Fitness Criteria | SIE/SIES/GITMO Criteria | REL (Rete Ematologica Lombarda) CENTERS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Age Limit | >75 y | 80 y | 80–85 y | 80 y | 85 y | No Cut Off | 80–85 y | 85 y | 85 y | 80–85 y | 85 y | 80 y | 80 y |
| PS (ECOG) | PS > 2 not related to leukemia | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° |
| Cardiac Comorbidity | Cardiac comorbidity (EF ≤ 50%) * | EF 40% ** | EF 40% *** | EF 40% | EF 40% | EF 40% | EF 40% | EF 50% | EF 50% | EF 40% | EF 45% | EF 50% | EF 40% |
| Pulmonary | Severe pulmonary comorbidity ^ | Recurrent infections | Bronchiectasis | COPD: >2 exacerbations/y | Bronchiectasis; MR colonization | Oxygen need | No infection at lung CT scan | COPD: frequent exacerbations | No limits by pulmonary function tests | COPD; Bronchiectasis | SO2 < 92%: >3 infections by y | COPD | Documented recurrent infections |
| Renal | On dialysis and age > 60 y ^^ | NC ° | eGFR > 30 mL/min | eGFR > 30 mL/min | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° |
| Liver | Severe hepatic comorbidity ^^^ | NC ° | Child C or AST/ALT > 3 N | Child C; Child B: TBE °° | Child C | Child C | NC ° | NC ° | Child C; Child B: TBE °° | Child C | Child C | NC ° | Child C |
| Cognitive impairment | Current mental illness ^^^^ | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° |
| Neoplasia uncontrolled | Neoplasia uncontrolled | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° |
| Further Comorbidities | Any other Comorbidities ^^^^^ | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° | NC ° |
| Social domains | not mentioned | Absence adequate caregiver | Absence adequate caregiver | Absence adequate caregiver | Absence adequate caregiver | Absence adequate caregiver | Absence adequate caregiver | Absence adequate caregiver | Jehovah’s Witness | Absence adequate caregiver | |||
| Social domains | not mentioned | Difficult accessibility to center § | Difficult accessibility to center § | Difficult accessibility to center § | Difficult accessibility to center § | Difficult accessibility to center § | |||||||
| Geriatric domains | not mentioned | ADL < 3 and/or IADL * < 4 | Low ADL/IADL; impairment in SPPB | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, G.; Borlenghi, E.; Zappasodi, P.; Lussana, F.; Bernardi, M.; Basilico, C.; Molteni, A.; Lotesoriere, I.; Turrini, M.; Frigeni, M.; et al. Adapting the Fitness Criteria for Non-Intensive Treatments in Older Patients with Acute Myeloid Leukemia to the Use of Venetoclax-Hypomethylating Agents Combination—Practical Considerations from the Real-Life Experience of the Hematologists of the Rete Ematologica Lombarda. Cancers 2024, 16, 386. https://doi.org/10.3390/cancers16020386
Rossi G, Borlenghi E, Zappasodi P, Lussana F, Bernardi M, Basilico C, Molteni A, Lotesoriere I, Turrini M, Frigeni M, et al. Adapting the Fitness Criteria for Non-Intensive Treatments in Older Patients with Acute Myeloid Leukemia to the Use of Venetoclax-Hypomethylating Agents Combination—Practical Considerations from the Real-Life Experience of the Hematologists of the Rete Ematologica Lombarda. Cancers. 2024; 16(2):386. https://doi.org/10.3390/cancers16020386
Chicago/Turabian StyleRossi, Giuseppe, Erika Borlenghi, Patrizia Zappasodi, Federico Lussana, Massimo Bernardi, Claudia Basilico, Alfredo Molteni, Ivana Lotesoriere, Mauro Turrini, Marco Frigeni, and et al. 2024. "Adapting the Fitness Criteria for Non-Intensive Treatments in Older Patients with Acute Myeloid Leukemia to the Use of Venetoclax-Hypomethylating Agents Combination—Practical Considerations from the Real-Life Experience of the Hematologists of the Rete Ematologica Lombarda" Cancers 16, no. 2: 386. https://doi.org/10.3390/cancers16020386
APA StyleRossi, G., Borlenghi, E., Zappasodi, P., Lussana, F., Bernardi, M., Basilico, C., Molteni, A., Lotesoriere, I., Turrini, M., Frigeni, M., Fumagalli, M., Cozzi, P., Gigli, F., Cattaneo, C., Fracchiolla, N. S., Riva, M., Martini, G., Mancini, V., Cairoli, R., & Todisco, E. (2024). Adapting the Fitness Criteria for Non-Intensive Treatments in Older Patients with Acute Myeloid Leukemia to the Use of Venetoclax-Hypomethylating Agents Combination—Practical Considerations from the Real-Life Experience of the Hematologists of the Rete Ematologica Lombarda. Cancers, 16(2), 386. https://doi.org/10.3390/cancers16020386







