Transarterial Bleomycin–Lipiodol Chemoembolization for the Treatment of Giant Hepatic Hemangiomas: An Assessment of Effectiveness
Abstract
Simple Summary
Abstract
1. Introduction
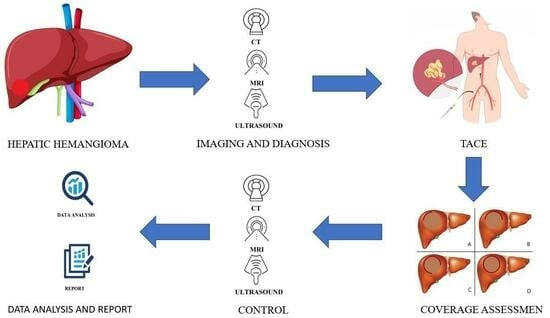
2. Materials and Methods
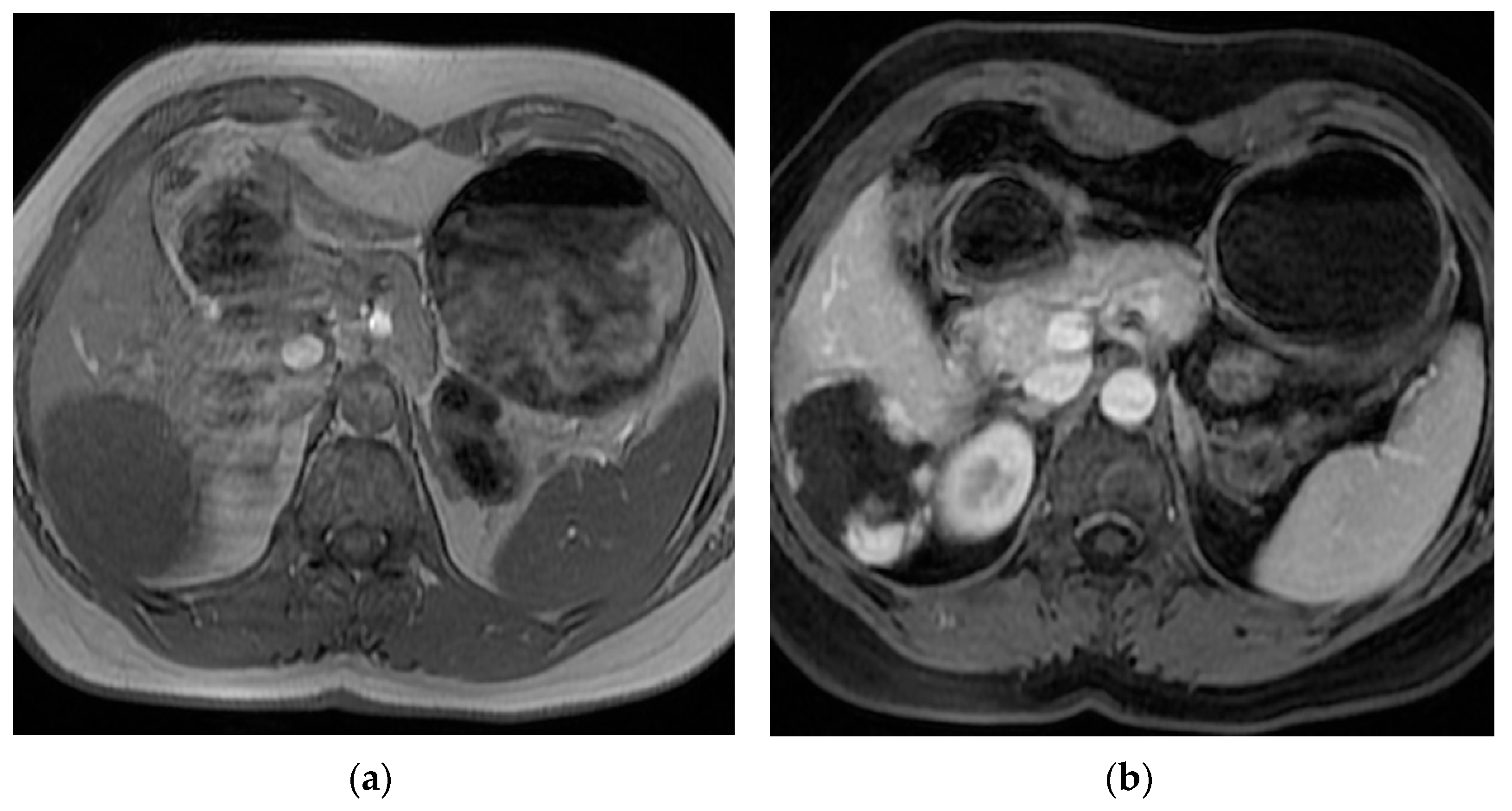
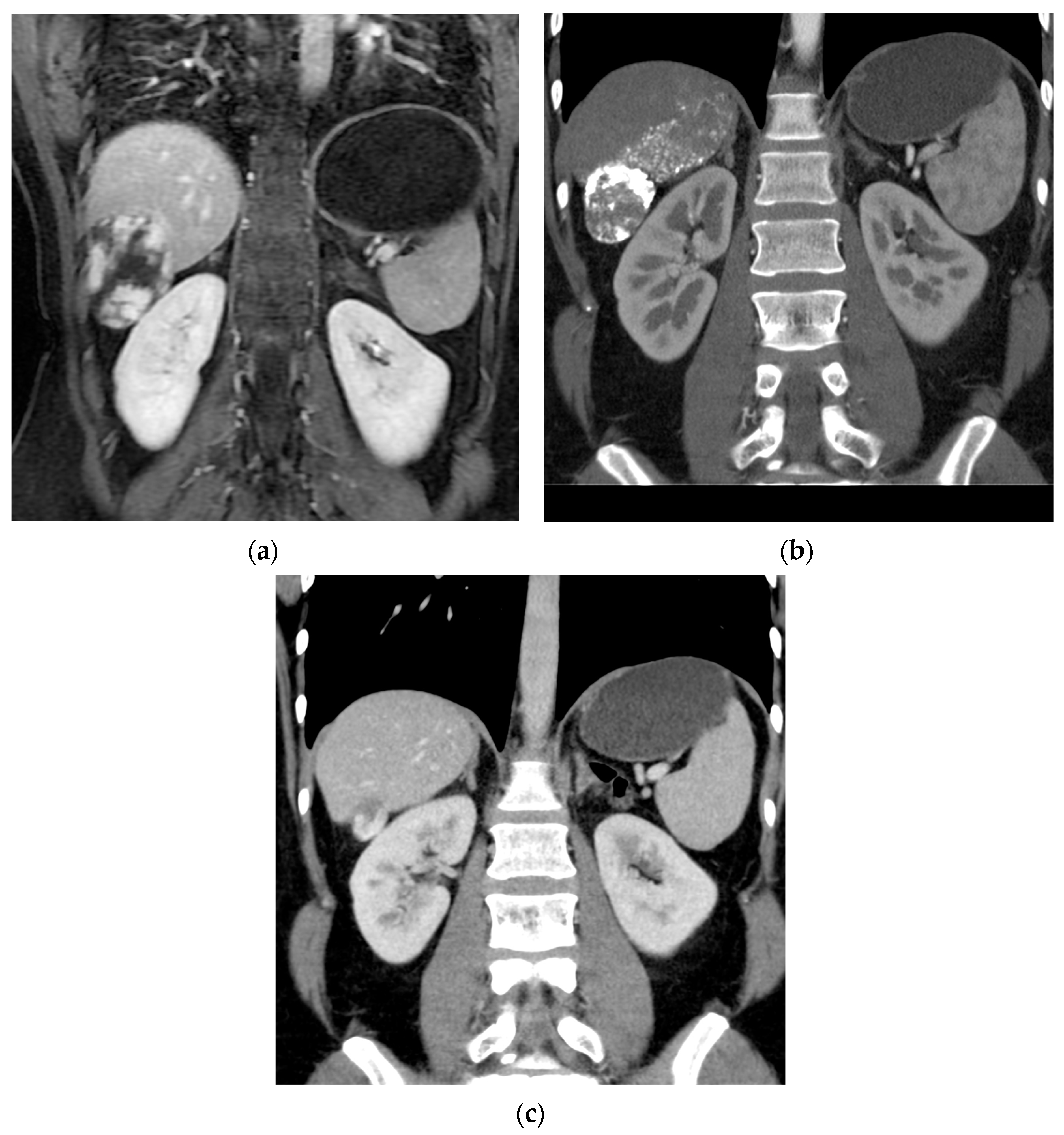
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishak, K.G.; Rabin, L. Benign tumors of the liver. Med. Clin. N. Am. 1975, 59, 995–1013. [Google Scholar] [CrossRef]
- Karhunen, P.J. Benign hepatic tumours and tumour like conditions in men. J. Clin. Pathol. 1986, 39, 183–188. [Google Scholar] [CrossRef]
- Bozkaya, H.; Cinar, C.; Besir, F.H.; Parildar, M.; Oran, I. Minimally invasive treatment of giant haemangiomas of the liver: Embolisation with bleomycin. Cardiovasc. Interv. Radiol. 2014, 37, 101–107. [Google Scholar] [CrossRef]
- Bajenaru, N.; Balaban, V.; Savulescu, F.; Campeanu, I.; Patrascu, T. Hepatic hemangioma—Review. J. Med. Life 2015, 8, 4–11. [Google Scholar] [PubMed]
- Hoekstra, L.T.; Bieze, M.; Erdogan, D.; Roelofs, J.J.; Beuers, U.H.; van Gulik, T.M. Management of giant liver hemangiomas: An update. Expert. Rev. Gastroenterol. Hepatol. 2013, 7, 263–268. [Google Scholar] [CrossRef]
- Stankiewicz, R.; Kobryn, K.; Patkowski, W.; Krawczyk, M. Management of giant hepatic hemangioma in atypical localization; report of a case and literature review. Pol. J. Surg. 2015, 87, 139–142. [Google Scholar] [CrossRef][Green Version]
- Vagefi, P.A.; Klein, I.; Gelb, B.; Hameed, B.; Moff, S.L.; Simko, J.P.; Fix, O.K.; Eilers, H.; Feiner, J.R.; Ascher, N.L.; et al. Emergent orthotopic liver transplantation for hemorrhage from a giant cavernous hepatic hemangioma: Case report and review. J. Gastrointest. Surg. 2011, 15, 209–214. [Google Scholar] [CrossRef]
- Vassiou, K.; Rountas, H.; Liakou, P.; Arvanitis, D.; Fezoulidis, I.; Tepetes, K. Embolization of a giant hepatic hemangioma prior to urgent liver resection. Case report and review of the literature. Cardiovasc. Interv. Radiol. 2007, 30, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.W.; Cheung, Y.S.; Lee, K.F.; Wong, J.; Yu, S.C.; Lee, P.S.; Lai, P.B. Is regular follow-up scan for giant liver haemangioma necessary? Hong Kong Med. J. 2007, 13, 353–358. [Google Scholar]
- Yamagata, M.; Kanematsu, T.; Matsumata, T.; Utsunomiya, T.; Ikeda, Y.; Sugimachi, K. Management of haemangioma of the liver: Comparison of results between surgery and observation. Br. J. Surg. 1991, 78, 1223–1225. [Google Scholar] [CrossRef]
- Giavroglou, C.; Economou, H.; Ioannidis, I. Arterial Embolization of Giant Hepatic Hemangiomas. Cardiovasc. Interv. Radiol. 2003, 26, 92–96. [Google Scholar] [CrossRef]
- Toro, A.; Mahfouz, A.E.; Ardiri, A.; Malaguarnera, M.; Malaguarnera, G.; Loria, F.; Bertino, G.; Di Carlo, I. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann. Hepatol. 2014, 13, 327–339. [Google Scholar] [CrossRef]
- Srivastava, D.N.; Gandhi, D.; Seith, A.; Pande, G.K.; Sahni, P. Transcatheter arterial embolization in the treatment of symptomatic cavernous hemangiomas of the liver: A prospective study. Abdom. Imaging 2001, 26, 510–514. [Google Scholar] [CrossRef]
- Erdogan, D.; Busch, O.R.; van Delden, O.M.; Bennink, R.J.; ten Kate, F.J.; Gouma, D.J.; van Gulik, T.M. Management of liver hemangiomas according to size and symptoms. J. Gastroenterol. Hepatol. 2007, 22, 1953–1958. [Google Scholar] [CrossRef]
- Kacała, A.; Dorochowicz, M.; Patrzałek, D.; Janczak, D.; Guziński, M. Safety and Feasibility of Transarterial Bleomycin–Lipiodol Embolization in Patients with Giant Hepatic Hemangiomas. Medicina 2023, 59, 1358. [Google Scholar] [CrossRef]
- Sharma, V.; Aggarwal, A.; Singla, R.; Kalra, N.; Chawla, Y.K. Giant hemangioma causing budd-Chiari syndrome. J. Clin. Exp. Hepatol. 2014, 4, 380–381. [Google Scholar] [CrossRef][Green Version]
- Sun, J.H.; Nie, C.H.; Zhang, Y.L.; Zhou, G.H.; Ai, J.; Zhou, T.Y.; Zhu, T.Y.; Zhang, A.B.; Wang, W.L.; Zheng, S.S. Transcatheter Arterial Embolization Alone for Giant Hepatic Hemangioma. PLoS ONE 2015, 10, e0135158. [Google Scholar] [CrossRef]
- Gandolfi, L.; Leo, P.; Solmi, L.; Vitelli, E.; Verros, G.; Colecchia, A. Natural history of hepatic haemangiomas: Clinical and ultrasound study. Gut 1991, 32, 677–680. [Google Scholar] [CrossRef]
- Pietrabissa, A.; Giulianotti, P.; Campatelli, A.; Di Candio, G.; Farina, F.; Signori, S.; Mosca, F. Management and follow-up of 78 giant haemangiomas of the liver. Br. J. Surg. 1996, 83, 915–918. [Google Scholar] [CrossRef]
- Mezhir, J.J.; Fourman, L.T.; Do, R.K.; Denton, B.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Fong, Y.; Jarnagin, W.R. Changes in the management of benign liver tumours: An analysis of 285 patients. HPB 2013, 15, 156–163. [Google Scholar] [CrossRef]
- Herman, P.; Costa, M.L.; Machado, M.A.; Pugliese, V.; D’Albuquerque, L.A.; Machado, M.C.; Gama-Rodrigues, J.J.; Saad, W.A. Management of hepatic hemangiomas: A 14-year experience. J. Gastrointest. Surg. 2005, 9, 853–859. [Google Scholar] [CrossRef]
- Yoon, S.S.; Charny, C.K.; Fong, Y.; Jarnagin, W.R.; Schwartz, L.H.; Blumgart, L.H.; DeMatteo, R.P. Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J. Am. Coll. Surg. 2003, 197, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Terkivatan, T.; de Wilt, J.H.; de Man, R.A.; van Rijn, R.R.; Zondervan, P.E.; Tilanus, H.W.; IJzermans, J.N. Indications and long-term outcome of treatment for benign hepatic tumors: A critical appraisal. Arch. Surg. 2001, 136, 1033–1038. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of benign liver tumours. J. Hepatol. 2016, 65, 386–398. [Google Scholar] [CrossRef]
- Martínez-González, M.N.; Mondragón-Sánchez, R.; Mondragón-Sánchez, A.; Gómez-Gómez, E.; Garduño-López, A.L.; Bernal-Maldonado, R.; Oñate-Ocaña, L.F.; Ruiz-Molina, J.M. Hemangioma cavernoso de hígado y hemangiomatosis hepática. Indicaciones y resultados de la resección quirúrgica [Cavernous hemangioma of the liver and hepatic hemangiomatosis. Indications and results of the surgical resection]. Rev. Gastroenterol. Mex 2003, 68, 277–282. (In Spanish) [Google Scholar]
- Ozden, I.; Emre, A.; Alper, A.; Tunaci, M.; Acarli, K.; Bilge, O.; Tekant, Y.; Ariogul, O. Long-term results of surgery for liver hemangiomas. Arch. Surg. 2000, 135, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Wu, T.H.; Yu, M.C.; Lee, W.C.; Chao, T.C.; Chen, M.F. Surgical management of giant hepatic hemangiomas: Complications and review of the literature. Chang. Gung Med. J. 2012, 35, 70–78. [Google Scholar] [PubMed]
- Brouwers, M.A.; Peeters, P.M.; de Jong, K.P.; Haagsma, E.B.; Klompmaker, I.J.; Bijleveld, C.M.; Zwaveling, J.H.; Slooff, M.J. Surgical treatment of giant haemangioma of the liver. Br. J. Surg. 1997, 84, 314–316. [Google Scholar] [PubMed]
- Duxbury, M.S.; Garden, O.J. Giant haemangioma of the liver: Observation or resection? Dig. Surg. 2010, 27, 7–11. [Google Scholar] [CrossRef]
- Torkian, P.; Li, J.; Kaufman, J.A.; Jahangiri, Y. Effectiveness of Transarterial Embolization in Treatment of Symptomatic Hepatic Hemangiomas: Systematic Review and Meta-analysis. Cardiovasc. Interv. Radiol. 2021, 44, 80–91. [Google Scholar] [CrossRef]
- Akhlaghpoor, S.; Torkian, P.; Golzarian, J. Transarterial Bleomycin-Lipiodol Embolization (B/LE) for Symptomatic Giant Hepatic Hemangioma. Cardiovasc. Interv. Radiol. 2018, 41, 1674–1682. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Yamashita, Y.; Ogata, I.; Urata, J.; Takahashi, M. Cavernous hemangioma of the liver: Pathologic correlation with dynamic CT findings. Radiology 1997, 203, 121–125. [Google Scholar] [CrossRef]
- Aziz, H.; Brown, Z.J.; Baghdadi, A.; Kamel, I.R.; Pawlik, T.M. A Comprehensive Review of Hepatic Hemangioma Management. J. Gastrointest. Surg. 2022, 26, 1998–2007. [Google Scholar] [CrossRef]
- Oldhafer, K.J.; Habbel, V.; Horling, K.; Makridis, G.; Wagner, K.C. Benign Liver Tumors. Visc. Med. 2020, 36, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Ammar, H.; Said, M.A.; Mizouni, A.; Ghabry, L.; Hammami, E.; Gupta, R.; Habiba, B.H.; Mabrouk, M.B.; Ali, A.B. Surgical management of giant hepatic hemangioma: A 10-year single center experience. Ann. Med. Surg. 2021, 69, 102542. [Google Scholar] [CrossRef] [PubMed]
- Glinkova, V.; Shevah, O.; Boaz, M.; Levine, A.; Shirin, H. Hepatic haemangiomas: Possible association with female sex hormones. Gut 2004, 53, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Mungovan, J.A.; Cronan, J.J.; Vacarro, J. Hepatic cavernous hemangiomas: Lack of enlargement over time. Radiology 1994, 191, 111–113. [Google Scholar] [CrossRef]
- Kim, G.E.; Thung, S.N.; Tsui, W.M.; Ferrell, L.D. Hepatic cavernous hemangioma: Underrecognized associated histologic features. Liver Int. 2006, 26, 334–338. [Google Scholar] [CrossRef]
- Ribeiro, M.A., Jr.; Papaiordanou, F.; Gonçalves, J.M.; Chaib, E. Spontaneous rupture of hepatic hemangiomas: A review of the literature. World J. Hepatol. 2010, 2, 428–433. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawarada, Y.; Yano, T.; Noguchi, T.; Mizumoto, R. Spontaneous rupture of hemangioma of the liver: Treatment with transcatheter hepatic arterial embolization. Am. J. Gastroenterol. 1991, 86, 1645–1649. [Google Scholar]
- Schnelldorfer, T.; Ware, A.L.; Smoot, R.; Schleck, C.D.; Harmsen, W.S.; Nagorney, D.M. Management of giant hemangioma of the liver: Resection versus observation. J. Am. Coll. Surg. 2010, 211, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Yedibela, S.; Alibek, S.; Müller, V.; Aydin, U.; Langheinrich, M.; Lohmüller, C.; Hohenberger, W.; Perrakis, A. Management of hemangioma of the liver: Surgical therapy or observation? World J. Surg. 2013, 37, 1303–1312. [Google Scholar] [CrossRef]
- Abdel, W.M.; El Nakeeb, A.; Ali, M.A.; Mahdy, Y.; Shehta, A.; Abdulrazek, M.; El Desoky, M.; Abdel, W.R. Surgical Management of Giant Hepatic Hemangioma: Single Center’s Experience with 144 Patients. J. Gastrointest. Surg. 2018, 22, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.S.; Chen, Z.X.; Zhao, Y.J.; Gu, H.; Geng, X.P.; Liu, F.B. Outcomes of surgery for giant hepatic hemangioma. BMC Surg. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Gao, R.; Wu, S.; Shi, Y.; Yin, T.; Guo, S.; Xin, Z.; Li, A.; Kong, X.; Ma, D.; et al. Safety and efficacy of microwave versus radiofrequency ablation for large hepatic hemangioma: A multicenter retrospective study with propensity score matching. Eur. Radiol. 2022, 32, 3309–3318, Erratum in Eur. Radiol. 2022, 32, 3615. [Google Scholar] [CrossRef]
- Ayoobi, Y.N.; Mehrabinejad, M.M.; Dashti, H.; Pourghorban, R.; Nassiri, T.M.; Rokni, Y.H. Percutaneous Sclerotherapy with bleomycin and Ethiodized Oil: A Promising Treatment in Symptomatic Giant Liver Hemangioma. Radiology 2021, 301, 464–471. [Google Scholar] [CrossRef]
- Lin, Z.; Zhu, X.; Zhou, J. Ultrasound-guided percutaneous sclerotherapy versus surgical resection in the treatment of large hepatic hemangiomas: A retrospective study. BMC Surg. 2022, 22, 130. [Google Scholar] [CrossRef]
- Wu, S.; Gao, R.; Yin, T.; Zhu, R.; Guo, S.; Xin, Z.; Li, A.; Kong, X.; Gao, J.; Sun, W. Complications of Radiofrequency Ablation for Hepatic Hemangioma: A Multicenter Retrospective Analysis on 291 Cases. Front. Oncol. 2021, 11, 706619. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Yang, X.; Xu, L.; Ke, S.; Ding, X.; Sun, W.; Gao, J. Endothelial pyroptosis underlies systemic inflammatory response following radiofrequency ablation of hepatic hemangiomas. Scand. J. Clin. Lab. Investig. 2019, 79, 619–628. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Li, S.; Wang, W.; Wang, Z.; Wang, Y.; Liu, B.; Wang, W.; Chang, H.; Li, Z. Transarterial Chemoembolization of Giant Liver Haemangioma: A Multi-center Study with 836 Cases. Cell Biochem. Biophys. 2015, 73, 469–472. [Google Scholar] [CrossRef]
- Furumaya, A.; van Rosmalen, B.V.; Takkenberg, R.B.; van Delden, O.M.; Dejong, C.H.C.; Verheij, J.; van Gulik, T.M. Transarterial(Chemo-)Embolization and Lipiodolization for Hepatic Haemangioma. Cardiovasc. Interv. Radiol. 2019, 42, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Özgür, Ö.; Sindel, H.T. Giant hepatic hemangioma treatment with transcatheter arterial embolisation and transcatheter arterial chemoembolization: Comparative results. Turk. J. Med. Sci. 2021, 51, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Tan, H.; Huang, J.; Xu, L.; Liu, L.; Si, S.; Sun, Y. Long-term result of transcatheter arterial embolization for liver hemangioma. Medicine 2017, 96, e9029. [Google Scholar] [CrossRef]
- Özden, İ.; Poyanlı, A.; Önal, Y.; Demir, A.A.; Hoş, G.; Acunaş, B. Superselective Transarterial Chemoembolization as an Alternative to Surgery in Symptomatic/Enlarging Liver Hemangiomas. World J. Surg. 2017, 41, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, A.; Marino, R.; Ratti, F.; Palumbo, D.; Guazzarotti, G.; Gusmini, S.; Augello, L.; Cipriani, F.; Fiorentini, G.; Venturini, M.; et al. The Two-Step Treatment for Giant Hepatic Hemangiomas. J. Clin. Med. 2021, 10, 4381. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhang, J.L.; Duan, F.; Wang, M.Q. Medium and Long-Term Outcome of Superselective Transcatheter Arterial Embolization with Lipiodol–Bleomycin Emulsion for Giant Hepatic Hemangiomas: Results in 241 Patients. J. Clin. Med. 2022, 11, 4762. [Google Scholar] [CrossRef] [PubMed]
- Bozkaya, H.; Cinar, C.; Unalp, O.V.; Parildar, M.; Oran, I. Unusual treatment of Kasabach–Merritt syndrome secondary to hepatic hemangioma: Embolization with bleomycin. Wien Klin Wochenschr 2015, 127, 488–490. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reich, S.D. Bleomycin. Ann. Intern. Med. 1979, 90, 945–948. [Google Scholar] [CrossRef]
- Sainsbury, D.C.; Kessell, G.; Fall, A.J.; Hampton, F.J.; Guhan, A.; Muir, T. Intralesional bleomycin injection treatment for vascular birthmarks: A 5-year experience at a single United Kingdom unit. Plast. Reconstr. Surg. 2011, 127, 2031–2044. [Google Scholar] [CrossRef]
- Oikawa, T.; Hirotani, K.; Ogasawara, H.; Katayama, T.; Ashino-Fuse, H.; Shimamura, M.; Iwaguchi, T.; Nakamura, O. Inhibition of angiogenesis by bleomycin and its copper complex. Chem. Pharm. Bull. 1990, 38, 1790–1792. [Google Scholar] [CrossRef]
- Ohishi, H.; Uchida, H.; Yoshimura, H.; Ohue, S.; Ueda, J.; Katsuragi, M.; Matsuo, N.; Hosogi, Y. Hepatocellular carcinoma detected by iodized oil. Use of anticancer agents. Radiology 1985, 154, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Shi, X.J.; Sun, X.D.; Wang, G. Sclerosing cholangitis secondary to bleomycin-iodinated embolization for liver hemangioma. World J. Gastroenterol. 2014, 20, 17680–17685. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, X.; Hu, J. The Risk Assessment and Clinical Research of Bile Duct Injury After Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma. Cancer Manag. Res. 2021, 13, 5039–5052. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Okuda, M.; Yoshie, Y.; Nakashima, Y.; Ikeno, H.; Orito, N.; Notsumata, K.; Watanabe, H.; Toya, D.; et al. Main bile duct stricture occurring after transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2010, 33, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, E.; Paul, S.B.; Gamanagatti, S.R.; Acharya, S.K. Biliary complications of arterial chemoembolization of hepatocellular carcinoma. Diagn. Interv. Imaging 2015, 96, 1169–1175. [Google Scholar] [CrossRef]
- Chen, W.; Ying, D.; Liu, Z.; He, Z. Analysis of the arterial supply of the extrahepatic bile ducts and its clinical significance. Clin. Anatomy 1999, 12, 245–249. [Google Scholar] [CrossRef]
- Cazejust, J.; Bessoud, B.; Colignon, N.; Garcia-Alba, C.; Planch, O.; Menu, Y. Hepatocellular carcinoma vascularization: From the most common to the lesser known arteries. Diagn. Interv. Imaging 2014, 95, 27–36. [Google Scholar] [CrossRef]
- Boulin, M.; Adam, H.; Guiu, B.; Aho, L.S.; Cercueil, J.P.; Di Martino, C.; Fagnoni, P.; Minello, A.; Jouve, J.L.; Hillon, P.; et al. Predictive factors of transarterial chemoembolisation toxicity in unresectable hepatocellular carcinoma. Dig. Liver Dis. 2014, 46, 358–362. [Google Scholar] [CrossRef]
- Thompson, C.M.; Saad, N.E.; Quazi, R.R.; Darcy, M.D.; Picus, D.D.; Menias, C.O. Management of iatrogenic bile duct injuries: Role of the interventional radiologist. Radiographics 2013, 33, 117–134. [Google Scholar] [CrossRef]
- O’Sullivan, J.M.; Huddart, R.A.; Norman, A.R.; Nicholls, J.; Dearnaley, D.P.; Horwich, A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann. Oncol. 2003, 14, 91–96. [Google Scholar] [CrossRef]
- Cahill, B.A. Management of patients who have undergone hepatic artery chemoembolization. Clin. J. Oncol. Nurs. 2005, 9, 69–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fiorentini, G.; Aliberti, C.; Tilli, M.; Mulazzani, L.; Graziano, F.; Giordani, P.; Mambrini, A.; Montagnani, F.; Alessandroni, P.; Catalano, V.; et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: Final results of a phase III study. Anticancer Res. 2012, 32, 1387–1395, Erratum in Anticancer Res. 2013, 33, 5211. [Google Scholar]
- Leung, D.A.; Goin, J.E.; Sickles, C.; Raskay, B.J.; Soulen, M.C. Determinants of postembolization syndrome after hepatic chemoembolization. J. Vasc. Interv. Radiol. 2001, 12, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Park, J.W.; Choi, J.I.; Kim, H.B.; Lee, W.J.; Kim, C.M. Does postembolization fever after chemoembolization have prognostic significance for survival in patients with unresectable hepatocellular carcinoma? J. Vasc. Interv. Radiol. 2009, 20, 209–216. [Google Scholar] [CrossRef]
| Variables | Group | Frequency | Percent |
|---|---|---|---|
| Sex | Male | 6 | 80.6 |
| Female | 25 | 19.4 | |
| Age | <40 years | 2 | 6.5 |
| 40–49 years | 11 | 35.5 | |
| 50–60 years | 10 | 32.3 | |
| >60 years | 8 | 25.8 | |
| Location | Left lobe | 13 | 41.9 |
| Right lobe | 17 | 54.8 | |
| Both lobes | 1 | 3.2 | |
| Drug coverage grade | <25% | 5 | 8.8 |
| 25–50% | 4 | 7 | |
| 50–75% | 5 | 8.8 | |
| >75% | 43 | 75.4 | |
| Number of procedures | 1 | 14 | 45.2 |
| 2 | 10 | 32.3 | |
| 3 | 5 | 16.1 | |
| 4 | 2 | 6.5 | |
| Preoperative volume | <100 cm3 | 5 | 16.1 |
| 100–500 cm3 | 17 | 54.8 | |
| 500–1000 cm3 | 7 | 22.6 | |
| >1000 cm3 | 2 | 6.5 | |
| Post-embolization syndrome | Yes | 29 | 50.9 |
| No | 28 | 49.1 |
| Group | Frequency | Percent |
|---|---|---|
| 30–40% | 2 | 6.45 |
| 40–50% | 4 | 12.9 |
| 50–60% | 1 | 3.12 |
| 60–70% | 3 | 9.68 |
| 70–80% | 10 | 32.26 |
| 80–90% | 3 | 9.68 |
| >90% | 8 | 25.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacała, A.; Dorochowicz, M.; Korbecki, A.; Sobański, M.; Puła, M.; Patrzałek, D.; Janczak, D.; Guziński, M. Transarterial Bleomycin–Lipiodol Chemoembolization for the Treatment of Giant Hepatic Hemangiomas: An Assessment of Effectiveness. Cancers 2024, 16, 380. https://doi.org/10.3390/cancers16020380
Kacała A, Dorochowicz M, Korbecki A, Sobański M, Puła M, Patrzałek D, Janczak D, Guziński M. Transarterial Bleomycin–Lipiodol Chemoembolization for the Treatment of Giant Hepatic Hemangiomas: An Assessment of Effectiveness. Cancers. 2024; 16(2):380. https://doi.org/10.3390/cancers16020380
Chicago/Turabian StyleKacała, Arkadiusz, Mateusz Dorochowicz, Adrian Korbecki, Michał Sobański, Michał Puła, Dariusz Patrzałek, Dariusz Janczak, and Maciej Guziński. 2024. "Transarterial Bleomycin–Lipiodol Chemoembolization for the Treatment of Giant Hepatic Hemangiomas: An Assessment of Effectiveness" Cancers 16, no. 2: 380. https://doi.org/10.3390/cancers16020380
APA StyleKacała, A., Dorochowicz, M., Korbecki, A., Sobański, M., Puła, M., Patrzałek, D., Janczak, D., & Guziński, M. (2024). Transarterial Bleomycin–Lipiodol Chemoembolization for the Treatment of Giant Hepatic Hemangiomas: An Assessment of Effectiveness. Cancers, 16(2), 380. https://doi.org/10.3390/cancers16020380