Simple Summary
Immune-related adverse events (irAEs) are becoming an increasingly prevalent and well-studied phenomenon. Little is known about how clinical practice has evolved to incorporate our growing knowledge on the subject. We identified temporal trends in irAE management as well as factors associated with improved outcomes among these patients such as timely specialty consultation. These findings can improve the quality of management algorithms for immune-related adverse events at our institution and may inform policies in other institutions.
Abstract
Understanding of immune-related adverse events (irAEs) has evolved rapidly, and management guidelines are continually updated. We explored temporal changes in checkpoint inhibitor-induced irAE management at a tertiary cancer care center to identify areas for improvement. We conducted a single-center retrospective study of patients who developed a gastrointestinal, pulmonary, renal, or cardiac irAE between July and 1 October in 2019 or 2021. We collected patient demographic and clinical information up to 1 year after toxicity. Endoscopic evaluation and specialty follow-up after discharge for patients with gastrointestinal irAEs declined between the 2019 and 2021 periods. Symptom duration and steroid taper attempts also declined. For pulmonary irAEs, rates of specialty consultation, hospital admission and readmission, and mortality improved in 2021 compared with 2019. Follow-up rates after hospital discharge were consistently low (<50%) in both periods. For cardiac irAEs, consultation with a cardiologist was frequent and prompt in both periods. Outpatient treatment and earlier specialty consultation improved outcomes with gastrointestinal irAEs. Our study exploring irAE practice changes over time identified areas to improve management; specifically, timely specialty consultation was associated with better outcomes for gastrointestinal irAEs. These findings can help improve the quality of management algorithms at our institution and may inform policies in other institutions.
1. Introduction
Immune checkpoint inhibitors are an increasingly popular treatment option for a growing number of cancers. Immune checkpoint inhibitors such as anti-programmed death protein-1/ligand-1 (PD-1/PD-L1) and anti-cytotoxic T-lymphocyte associated protein-4 (CTLA-4) work to induce an immune antitumor response by stimulating T-cell activity and proliferation. This nonspecific activation of T cells can give rise to inflammatory toxicities in any organ system in the body, referred to collectively as immune-related adverse events (irAEs). Such irAEs are estimated to occur in 34–76% of patients, with around 5–20% developing severe grades of toxicity (≥3, as determined by the Common Terminology Criteria for Adverse Events 5.0 [CTCAEv5]) depending on the class of immune checkpoint inhibitor used [1,2]. Gastrointestinal and pulmonary irAEs are among the most common manifestations of immunotherapy toxicity, and cardiac and renal irAEs are rarer but serious presentations [3]. Mortality rates have been estimated at 0.3–1.3% with colitis, pneumonitis, carditis, hepatitis, and neurotoxicity among the most fatal toxicities [4]. Early recognition and treatment of irAEs is crucial given the associated morbidity and mortality, as well as the impact on the feasibility of future immune checkpoint inhibition [5].
Guidelines regarding the management and treatment of irAEs are rapidly changing and evolving. Management of irAEs is usually supportive and strongly relies on early recognition and prompt intervention with strategies tailored to the organ system affected [6]. The first step in managing irAEs begins with a thorough evaluation to exclude other possible diagnoses and establish a clinical symptom grade. CTCAEv5 grading is the current standard to stratify patients and guide their treatment plan. Patients with grade 1 irAEs typically receive supportive treatment, whereas those with grade 2 irAEs may additionally receive corticosteroids. The mainstay of treating grade ≥ 2 irAEs is immunosuppression with corticosteroids and frequently immunomodulatory agents such as selective immunosuppressive therapy (SIT; e.g., infliximab, vedolizumab) or intravenous immunoglobulins [6].
Our understanding of irAEs has changed rapidly. The growing body of literature on irAEs possesses immense translational value to inform clinical practice, especially considering that many centers and physicians have limited exposure to these cases. It is therefore essential to investigate how well clinical practice has kept up with evolving guidelines to identify and address any ostensible shortcomings. Three studies so far have shown that the implementation of a dedicated irAE team to improve various management parameters led to significantly better patient outcomes [7,8,9]. With this in mind, the aim of the current study was to evaluate the changes in irAE management in a tertiary care cancer center over a 2-year period and to identify aspects of management that may be associated with better patient outcomes.
2. Materials and Methods
2.1. Study Design and Patient Selection
The current study was a single-center retrospective chart review of all patients who developed an irAE between 1 July and 1 October in 2019 or 2021. Any patient with a new, clinically confirmed gastrointestinal, pulmonary, renal, or cardiac irAE in these time windows was included. These specific toxicities were chosen because they are either very common (gastrointestinal) or have high mortality (pulmonary, cardiac) or hospitalization (gastrointestinal, renal) rates. Patients were considered to have a confirmed irAE if the diagnosis was made by a specialty team, the symptoms were managed as an irAE, or irAE was suspected and no other diagnosis was likely. Patients with established alternate diagnoses or unlikely diagnoses were not included in the analysis. Patients were identified using a natural language processing model. Other systemic toxicities, while important, were not included because they can for the most part be managed on an outpatient basis with a lower risk for mortality or future complications.
2.2. Natural Language Processing Model
Systems analysts at the study hospital worked closely with clinical staff to develop a dictionary and rules-based annotator for immunotoxicity and irAEs. These dictionaries and negations were used to create a custom annotator using IBM Content Analytics (New York, NY, USA), which reviewed clinical notes daily to select potential cases of irAEs. A set of filters and business rules for post-processing were developed with the help of the clinical staff. These filters and rules allowed for cross note validation of the natural language processing model findings and improved the precision of the output. Filters and rules included filtering for nonspecific text patterns, validating treatment using immunotherapeutic agents, separating concurrent and sequential agents, and identifying the first visit for an irAE.
2.3. Data Collection
Patients were followed for up to 1 year after diagnosis or until 1 July of the subsequent year, whichever came first. We collected data from patient charts, including patient demographics, oncologic history, irAE management (treatment, specialty consultation, procedural evaluation), and irAE outcome (hospitalization, symptom improvement, symptom recurrence, clinical remission, and all-cause mortality). We also collected information regarding peak CTCAEv5 grade and CTCAEv5 grade at the time of specialty consultation for gastrointestinal and pulmonary irAEs.
2.4. IrAE Management
Treatment options varied by organ system involved and were chosen based on current irAE management guidelines [6]. Treatments included loperamide, mesalamine, corticosteroids, and selective immunosuppressant treatment (SIT (e.g., infliximab, vedolizumab) for gastrointestinal toxicities; bronchodilators, corticosteroids, and SIT for pulmonary toxicities; hydration, dialysis, corticosteroids, and SIT for renal toxicities; and heart failure medications (e.g., ACE inhibitors, angiotensin receptor blockers, angiotensin receptor/neprilysin inhibitors, mineralocorticoid receptor antagonists), diuretics, corticosteroids, SIT, immunoglobulins, and plasmapheresis for cardiac toxicities.
Specialty consultation refers to outpatient consultation with a specialist prior to hospitalization, inpatient consultation with a specialist, or follow-up with a specialist after hospital discharge. Patients who presented to an emergency department or were admitted to a hospital inpatient unit on or within 1 day of presentation were excluded from any analysis involving outpatient consultation or treatment because these patients would not have had the opportunity to receive either.
Procedures included endoscopy for colitis, bronchoscopy for pneumonitis, and biopsy for nephritis and carditis.
2.5. IrAE Outcomes
Symptom improvement was defined as the patient having a sustained decrease in symptom severity for more than 30 days as described by the oncologist’s or specialist’s progress notes. Clinical remission was defined as complete resolution of the patient’s symptoms by the end of their follow-up period.
2.6. Statistical Analysis
Data were analyzed using SPSS 26.0 (Chicago, IL, USA). Comparisons were made between the 2019 and 2021 cohorts. The distribution of continuous variables was summarized by medians and interquartile ranges. The distribution of categorical variables was summarized in terms of frequencies and percentages. Chi-square and Fisher exact tests were used to evaluate associations between two categorical variables. Mann–Whitney U tests were used to compare continuous variables between two groups. Univariate logistic regression was used to identify relationships between different management parameters and outcomes among patients with gastrointestinal toxicities. For all analyses, p < 0.05 was considered statistically significant.
3. Results
3.1. Overall Number of irAE Cases by Organ System as Baseline
Using a natural language processing model, we were able to identify suspected cases of irAEs in the 2019 and 2021 periods at our institution across the entire year. We found that gastrointestinal irAEs were the most common (571 cases in 2019 and 718 cases in 2021), followed by endocrine irAEs (556 and 638, respectively) and cutaneous irAEs (465 and 506, respectively). Cardiac (116 in 2019 and 155 in 2021) and renal (117 in 2019 and 212 in 2021) irAEs were the third and fifth least frequent, respectively. More details can be found in Supplementary Figure S1.
3.2. Demographics
A total of 181 patients were found to have irAEs in the designated three-month period and included in our analysis as shown in the patient selection flowchart in Figure 1. Most of our patients were white (87.8%) and male (68.5%), with a median age of 67 years (interquartile range 58–73 years). The most common malignancies were melanoma (21.5%), lung cancer (24.3%), and genitourinary (26.0%) cancer, and most patients had stage IV disease (69.6%). PD-1/PD-L1 blockade was the most common form of immunotherapy (56.4% of patients), followed by combination therapy (36.5%) and single-agent CTLA-4 blockade (7.2%). Around two-thirds of patients (62.4%) were alive at the end of the follow-up period. This information is summarized in Table 1, with a detailed summary by organ system available in Supplementary Table S1.
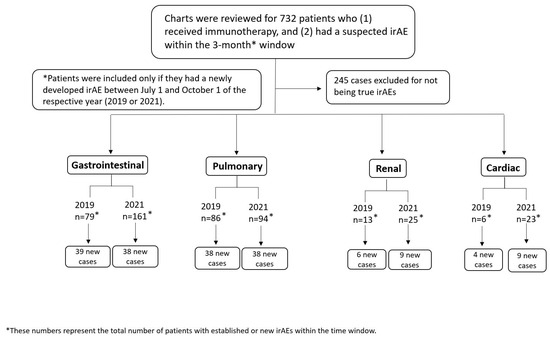
Figure 1.
Patient selection flowchart for patients with immune-related adverse events (irAEs).

Table 1.
Cohort demographic information, n = 181.
3.3. Gastrointestinal irAEs
3.3.1. Comparison of Management in 2019 and 2021
A total of 77 new gastrointestinal irAEs were diagnosed during the periods studied, 39 in 2019 and 38 in 2021, with no significant differences in the severity of diarrhea (p = 1.000) or colitis (p = 0.492) between years (Supplementary Table S2). Our findings suggested lower rates of outpatient consultation with a gastroenterologist in 2021 compared to 2019. We observed a slight decrease in rates of treatment prior to hospitalization and inpatient consultation with a gastroenterologist in 2021. We also saw a higher rate of hospitalization and hospital readmission rates in 2021 compared to 2019. None of these differences achieved statistical significance however (p > 0.05). More importantly, there was a significant decline between the 2019 and 2021 periods in rates of follow-up with a gastroenterologist after discharge (92% in 2019 compared with 48% in 2021, p = 0.007), endoscopic evaluation (95% compared with 61%, p < 0.001), and SIT use (74% compared with 42%, p = 0.004). In terms of outcomes, clinical remission and recurrence rates were similar, with significant improvements in symptom duration (median 43.5 days in 2019 compared with 24.5 days in 2021, p = 0.036) and the need for more than two steroid tapering courses (15% compared with 0%, p = 0.014). More comparisons between the two periods can be found in Table 2, with a breakdown by diarrhea and colitis severity found in Supplementary Tables S3 and S4. A graphical representation of the case load and hospitalization and procedure rates, median durations of symptoms and hospitalization, and times to procedure and follow-up can be found in Supplemental Figures S2, S3 and S4, respectively.

Table 2.
Gastrointestinal immune-related adverse-event clinical management and disease outcomes in 2019 and 2021.
3.3.2. Differences in Management between Patients Who Did and Did Not Receive an Outpatient Consultation with a Gastroenterologist
Of the 50 patients whose gastrointestinal irAE was diagnosed prior to hospitalization, 39 were seen by a gastroenterologist at our hospital, and 11 were not (Supplementary Table S5). Patients who were seen by a gastroenterologist had similar rates of outpatient treatment to that of those who were not (87% compared with 64%), as well as similar rates of hospitalization (49% compared with 64%), symptom recurrence (38% compared with 45%), and clinical remission (92% compared with 82%). None of these differences were significant (p > 0.05). Patients who were seen by a gastroenterologist were also more likely to receive follow-up after hospital discharge (84% compared with 29%, p = 0.007) and more likely to undergo endoscopic evaluation (82% compared with 45%, p = 0.023). There was no significant difference in diarrhea or colitis severity between the two groups.
3.3.3. Univariate Analysis for Relationships between Management Parameters
A summary of the univariate analysis conducted to evaluate associations between different parameters can be found in Supplementary Table S6. Outpatient treatment was associated with less recurrence (odds ratio [OR] 0.2, p = 0.005), as were SIT use (OR 0.3, p = 0.012) and initial symptom improvement within 30 days of diagnosis (OR 0.04, p < 0.001). A longer duration of both symptoms (OR 1.02, p = 0.018) and initial steroid use (OR 1.02, p = 0.025) predicted a need for more than one steroid tapering attempt. Finally, early consultation with a gastroenterologist (up to 2 weeks after initiation of steroid therapy) was associated with a lower likelihood of colitis recurrence (OR 0.4, p = 0.047), hospital readmission (OR 0.2, p = 0.012), and need for more than one steroid tapering attempt (OR 0.3, p = 0.024).
3.4. Pulmonary irAE Management in 2019 and 2021
A total of 76 new pulmonary irAEs were diagnosed in the periods studied, with 38 cases per period. Pneumonitis severity significantly differed between the two periods (78.9% of patients had grade 3 and above pneumonitis in 2019 compared with 57.9% in 2021, p = 0.048), as shown in Supplementary Table S2. Management of pulmonary irAEs had improved in several ways by 2021. Specifically, rates of outpatient consultation with a pulmonologist prior to hospitalization improved from 67% in 2019 to 93% in 2021 (p = 0.055), as did rates of hospitalization (95% compared with 68%, p = 0.011), hospital readmission (47% compared with 31%, p = 0.179), the need for more than one steroid tapering attempt (42% compared with 24%, p = 0.140), and all-cause mortality (68% compared with 32%, p = 0.003). Rates of follow-up with a pulmonologist after hospital discharge and bronchoscopic evaluation remained low across both periods, hovering just under 50%. Further details can be found in Table 3, with a breakdown by pneumonitis severity found in Supplementary Table S7. No subgroup analyses could be carried out owing to small sample sizes, and univariate analysis revealed no significant correlations except for an association between early consultation with a pulmonologist and lower likelihood of hospitalization (OR 0.13, p = 0.003). A graphical representation of case load and hospitalization and procedure rates, median durations of symptoms and hospitalization, and times to procedure and follow-up can be found in Supplemental Figures S2, S3 and S4, respectively.

Table 3.
Pneumonitis clinical management and disease outcomes in 2019 and 2021.
3.5. Renal irAE Management in 2019 and 2021
A total of 15 patients developed renal irAEs within the periods studied, 6 in 2019 and 9 in 2021. The main differences between management between the two periods were a shorter median time to consultation with a nephrologist (median 67.5 days in 2019 compared with 3 days in 2021, p = 0.059), a higher rate of inpatient consultation with a nephrologist (33% compared with 83%, p = 0.226), and a higher rate of SIT use (0% compared with 44%, p = 0.103). All other parameters were consistent across the two periods except for hospital readmission rates, which increased in 2021 (0% compared with 50%, p = 0.228). A summary of these findings can be found in Table 4. A graphical representation of case load and hospitalization and procedure rates, median durations of symptoms and hospitalization, and times to procedure and follow-up can be found in Supplemental Figures S2–S4, respectively.

Table 4.
Nephritis and carditis clinical management and disease outcomes in 2019 and 2021.
3.6. Cardiac irAE Management in 2019 and 2021
A total of 13 patients developed cardiac irAEs within the periods studied, 4 in 2019 and 9 in 2021. All patients who developed cardiac irAEs were hospitalized, with only one patient diagnosed as an outpatient and seen by a cardiologist prior to hospitalization. All patients received inpatient consultation with a cardiologist as well as cardiac imaging. All patients received prompt consultation with a cardiologist, at a median of 1 day after diagnosis in both periods. Management did not differ between the two periods, but there appeared to be a trend towards higher rates of symptom improvement in 2021 compared with 2019 (89% compared with 50%, p = 0.203) and lower readmission rates (11% compared with 25%, p = 0.202). A summary of these findings can be found in Table 4. A graphical representation of case load and hospitalization and procedure rates, median durations of symptoms and hospitalization, and times to procedure and follow-up can be found in Supplemental Figures S2–S4, respectively.
4. Discussion
Our study is one of the few quality improvement projects exploring the management of irAEs. We studied changes in the management of irAEs between 2019 and 2021 and identified key aspects associated with better outcomes. We found that overall, patient outcomes improved from 2019 to 2021 despite a decline in performance for a few parameters, namely rates of procedural evaluation and post-discharge follow-up. We also noted that timely specialty consultation may be associated with a lower frequency of outcomes such as hospital admission and readmission, disease recurrence, and the need for multiple steroid tapering attempts. Our findings highlight the potential overburdening of specialty services and the importance of implementing treatment algorithms that ensure adequate and prompt patient follow-up.
Immune-mediated diarrhea and colitis is among the most common irAEs and the most frequently severe [3]. Considering the breadth of the literature on the subject, it was surprising to see that rates of endoscopic evaluation and SIT use dropped considerably between 2019 and 2021 when studies have shown that these interventions are associated with positive outcomes [10,11]. Adding to the complexity of this issue is the fact that despite a decline in these key parameters, several patient outcomes such as remission and recurrence rates, symptom duration, and the need for multiple steroid tapering attempts actually improved by 2021. Together, these findings seem to suggest that our management of immune-mediated diarrhea and colitis has improved over the years, but there is potential for further improvement.
We observed no differences in disease severity between 2019 and 2021. This suggests that any worsening in parameters is related to a difference in practice rather than in clinical presentation. There are many potential explanations for these changes in practice, as described in Figure 2. The impact of the COVID-19 pandemic cannot be overstated. Multiple studies have shown that consultation [12,13] and procedure [14,15] rates dramatically decreased following the start of the pandemic. Other studies stress the impact of the pandemic-related economic recession on health insurance coverage [16]. At a more basic level, the ever-growing number of patients presenting to the hospital with irAEs may lead to overwhelming the specialty consultation system. Regarding SIT use in particular, better management of immune-mediated diarrhea and colitis could have allowed symptoms to resolve before SIT was needed. This is supported by the decreased need for multiple steroid tapering attempts. Another layer to this could be the increasing comfort of primary oncology teams in handling irAEs without specialty consultation. The establishment of a dedicated irAE committee at the study hospital has helped facilitate this by making great efforts to educate primary providers via lectures, developed algorithms, and educational phone applications.
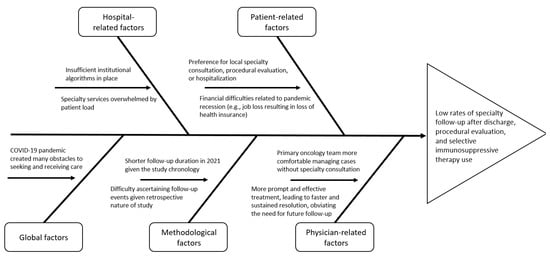
Figure 2.
Fishbone diagram exploring reasons for changes in clinical practice.
The increasing comfort of primary oncology teams in handling certain irAEs is a positive development, but it should not detract from the significance of specialty consultation. Our findings show that specialty consultation—especially early in the disease course—may be linked to better patient outcomes either directly or indirectly through an association with other aspects of management that are correlated with outcomes, such as outpatient treatment. This supports findings from a previous quality improvement project showing that implementation of a specialty service dedicated to gastrointestinal irAEs was associated with shorter symptom duration, higher follow-up rates for gastrointestinal irAEs after hospital discharge, fewer hospital readmissions, and less disease recurrence [8]. This previous study additionally found that patients with immune-mediated diarrhea and colitis who followed up with a gastroenterologist had longer overall survival. However, the utility of specialty services to mitigate poor outcomes after irAEs would ideally be studied in a randomized controlled trial comparing usual care to systematic, early specialty service consultation.
Pulmonary irAEs, although not as common as gastrointestinal irAEs, are very serious and potentially lethal [17,18]. For this reason, prompt and effective management is critical to ensure patient survival. The current study showed that there have been marked improvements in consultation with a pulmonologist both on an outpatient basis prior to hospitalization and on an inpatient basis. This is likely due to an increased institutional effort to host educational events to raise awareness regarding these toxicities. However, our study showed a few shortcomings as well. SIT use decreased from 18% in 2019 to 8% in 2021, which may be due to the lower frequency of high-grade pulmonary irAEs in 2021 compared with 2019. This change in severity likely had broad downstream impacts on other metrics and may have also affected parameters such as hospitalization, readmission, and mortality rates, and therefore, it is not possible to conclude that consultation with a pulmonologist had a causal role in mitigating poor outcomes after pneumonitis. Furthermore, rates of follow-up with a pulmonologist after discharge and bronchoscopy rates were below 50% in both periods studied. Although bronchoscopy is contingent on several factors, including patient safety, ideally follow-up after hospital discharge should be universal. The low rate of follow-up is likely multifactorial, but considering the information in Figure 2, we would have expected a drop in these parameters. The fact that the rates stayed consistent across the two periods studied is reassuring, but further improvements may be beneficial for patients with pneumonitis.
Although we cannot say for sure whether the improvement in mortality was due to an improvement in pneumonitis management or a difference in pneumonitis severity, this is still a notable improvement for this particular irAE. Immune-mediated pneumonitis, unlike some other irAEs, is associated with mortality; a study of patients presenting to the emergency department with irAEs showed that the presence of pneumonitis was associated with worse overall survival [19,20,21,22]. This is a difficult irAE to manage in the acute care setting given the complexity of the affected population, including a range of underlying cancers, comorbidities, and alternative diagnoses. Given the challenge in diagnosing pneumonitis, increasing the rate of consultation with pulmonologists and up-front diagnostic bronchoscopy is likely to be beneficial, but definitive proof would require comparison to usual care in a randomized controlled trial.
In a similar vein, immune-mediated myocarditis is a relatively rare irAE with a high reported mortality [23]. Our findings reflect this; all patients who presented with carditis were hospitalized for this condition and received rapid and comprehensive multidisciplinary management efforts: 100% of patients received a consultation with a cardiologist and 90% of patients underwent a biopsy, most within days of presentation. Patients with pulmonary and cardiac irAEs in our analysis had the highest and second-highest mortality rates, respectively.
As a quality improvement project, our study identified key areas that need to be worked on to enhance patient outcomes. The implementation of automatic order sets in particular may help to ensure a timely diagnosis and workup and improve consultation, treatment, and procedure rates. These types of interventions have already shown benefits in the early diagnosis and treatment of various other pathologies, including sepsis, at multiple institutions [24]. The creation of a dedicated irAE group available on demand may also facilitate improved management of irAEs, as demonstrated by the implementation of such a service at multiple cancer centers [7,8,9]. Nonetheless, our study is not without limitations. Our study is retrospective, which may have impacted the accuracy of data collected from electronic health records. Additionally, we may have missed information regarding management that was carried out at local institutions. The power of our statistical analysis may have been limited by our sample size and time reviewed. Reviewing more patients over a longer follow-up period may help delineate clearer trends in patient management and outcomes. It is also difficult to assess the extent of the impact of the COVID-19 pandemic on health care. Finally, our study explored irAEs in only four organ systems. There would be merit in expanding the sample size and including irAEs from other organ systems such as cutaneous or neurological irAEs.
5. Conclusions
The current study is one of the few qualitative studies exploring current practice in managing irAEs. The changes in practice between 2019 and 2021 that we focused on likely reflect a variety of factors, including an evolving knowledge of this disease, as well as the impact of the COVID-19 pandemic. Although the overall irAE outcomes among the toxicities that we described have improved in recent years, there is room for more improvement. Ensuring adequate and timely consultation, procedural evaluation, and the use of SIT could help reduce hospital readmission and disease recurrence, though prospective studies are needed to confirm these findings. Together, these findings can help improve the quality of irAE management algorithms at our institution and potentially others.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16020369/s1, Table S1: Demographic information for patients who developed immune-related adverse events in 2019 and 2021 by type of event, n = 181; Table S2: Immune-related adverse event Common Terminology Criteria for Adverse Events 5.0 grades in 2019 and 2021; Table S3: Gastrointestinal immune-related adverse event clinical management and outcomes by diarrhea grade in 2019 and 2021; Table S4: Gastrointestinal immune-related adverse event clinical management and outcomes by colitis grade in 2019 and 2021; Table S5: Management outcomes in patients who received an outpatient consultation with a gastroenterologist and those who did not across both years; Table S6: Univariate analysis of factors associated with various outcomes in gastrointestinal immune-related adverse events; Table S7: Pneumonitis clinical management and disease outcomes in 2019 and 2021 for peak pneumonitis grade 3+; Figure S1: Total number of immunotoxicity cases among different systems in 2019 and 2021; Figure S2: Summary of immune-related adverse event incidence and management; Figure S3: (a): Median duration of symptoms; (b): Median duration of hospitalization for immune-related adverse events; Figure S4: (a): Median time to procedure; (b): Median time to IM follow-up after hospital discharge.
Author Contributions
Y.W. was the senior author of the study; she developed the concept, designed the study, interpreted the results, ensured that data accuracy and integrity were preserved at all stages, agreed to be accountable for all aspects of the study, was in charge of the overall direction and planning of the study, and contributed to the writing of the manuscript, with input from all authors. M.S. (Malek Shatila), F.E., A.R.T., A.G.K., B.Z., S.N., M.S. (Mianen Sun), S.F. and J.J. collected the data for the study. M.S. (Malek Shatila) conducted and interpreted the analysis. M.S. (Malek Shatila) and J.S.S. wrote the manuscript. A.A., A.S., N.L.P., M.C.F.-V., M.S.G., A.S.T. and H.C.Z. critically revised the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of The University of Texas MD Anderson Cancer Center (2021-0100).
Informed Consent Statement
Patient consent was waived for this study given its retrospective nature.
Data Availability Statement
The datasets used and analyzed in this study are available from the corresponding author on reasonable request.
Acknowledgments
Medical editing of this paper was provided by Editing Services, Research Medical Library, at The University of Texas MD Anderson Cancer Center.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PD-1/PD-L1 | programmed death 1/ligand 1 |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| irAE | immune-related adverse event |
| CTCAEv5 | Common Terminology Criteria for Adverse Events version 5 |
| SIT | selective immunosuppressive therapy |
References
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Colen, R.R.; Bilen, M.A.; Hess, K.R.; Hajjar, J.; Suarez-Almazor, M.E.; Alshawa, A.; Hong, D.S.; Tsimberidou, A.; Janku, F.; et al. Incidence of immune-related adverse events and its association with treatment outcomes: The MD Anderson Cancer Center experience. Investig. New Drugs 2017, 36, 638–646. [Google Scholar] [CrossRef]
- Michot, J.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Naidoo, J.; Zhang, J.; Lipson, E.J.; Forde, P.M.; Suresh, K.; Moseley, K.F.; Mehta, S.; Kwatra, S.G.; Parian, A.M.; Kim, A.K.; et al. A Multidisciplinary Toxicity Team for Cancer Immunotherapy–Related Adverse Events. J. Natl. Compr. Cancer Netw. 2019, 17, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Saji, A.; Chopra, M.; Jacob, J.; Altan, M.; Alhalabi, O.; Shah, A.Y.; Qiao, W.; Wang, Y.; Thomas, A. Implementing an immunotherapy toxicity (IOTOX) GI service improves outcomes in patients with immune-mediated diarrhea and colitis. J. Cancer Res. Clin. Oncol. 2022, 149, 5841–5852. [Google Scholar] [CrossRef]
- Zubiri, L.; Molina, G.E.; Mooradian, M.J.; Cohen, J.; Durbin, S.M.; Petrillo, L.; Boland, G.M.; Juric, D.; Dougan, M.; Thomas, M.F.; et al. Effect of a multidisciplinary Severe Immunotherapy Complications Service on outcomes for patients receiving immune checkpoint inhibitor therapy for cancer. J. Immunother. Cancer 2021, 9, e002886. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Luo, W.; Qiao, W.; Raju, G.S.; Wang, Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 2018, 6, 95. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Wang, X.; Mallepally, N.; Chen, E.; Altan, M.; Bresalier, R.S.; Charabaty, A.; Dadu, R.; Jazaeri, A.; et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor–induced colitis. J. Immunother. Cancer 2019, 7, 93. [Google Scholar] [CrossRef]
- Baugh, J.J.; White, B.A.; McEvoy, D.; Yun, B.J.; Brown, D.F.; Raja, A.S.; Dutta, S. The cases not seen: Patterns of emergency department visits and procedures in the era of COVID-19. Am. J. Emerg. Med. 2020, 46, 476–481. [Google Scholar] [CrossRef]
- Schäfer, I.; Hansen, H.; Menzel, A.; Eisele, M.; Tajdar, D.; Lühmann, D.; Scherer, M. The effect of COVID-19 pandemic and lockdown on consultation numbers, consultation reasons and performed services in primary care: Results of a longitudinal observational study. BMC Fam. Pract. 2021, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.; Sayuk, G.; Elwing, J.; Wilgus, N.; Dieckgraefe, B.; Presti, M. Colonoscopy Following COVID-19 Delays in Procedures: Risk Stratification for Procedures Is Critical. Gastro Hep Adv. 2022, 1, 546–548. [Google Scholar] [CrossRef]
- Cadoni, S.; Ishaq, S.; Hassan, C.; Bhandari, P.; Neumann, H.; Kuwai, T.; Uedo, N.; Parra-Blanco, A.; Mulder, C.J.; Binmoeller, K.F.; et al. COVID-19 pandemic impact on colonoscopy service and suggestions for managing recovery. Endosc. Int. Open 2020, 8, E985–E989. [Google Scholar] [CrossRef]
- Woolhandler, S.; Himmelstein, D.U. Intersecting U.S. Epidemics: COVID-19 and Lack of Health Insurance. Ann. Intern. Med. 2020, 173, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Tone, M.; Izumo, T.; Awano, N.; Kuse, N.; Inomata, M.; Jo, T.; Yoshimura, H.; Minami, J.; Takada, K.; Miyamoto, S.; et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac. Cancer 2019, 10, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Psoter, K.J.; Voong, K.R.; Shankar, B.; Forde, P.M.; Ettinger, D.S.; Marrone, K.A.; Kelly, R.J.; Hann, C.L.; Levy, B.; et al. Impact of Checkpoint Inhibitor Pneumonitis on Survival in NSCLC Patients Receiving Immune Checkpoint Immunotherapy. J. Thorac. Oncol. 2018, 14, 494–502. [Google Scholar] [CrossRef]
- El Majzoub, I.; Qdaisat, A.; Thein, K.Z.; Win, M.A.; Han, M.M.; Jacobson, K.; Chaftari, P.S.; Prejean, M.; Reyes-Gibby, C.; Yeung, S.-C.J. Adverse Effects of Immune Checkpoint Therapy in Cancer Patients Visiting the Emergency Department of a Comprehensive Cancer Center. Ann. Emerg. Med. 2018, 73, 79–87. [Google Scholar] [CrossRef]
- Sheshadri, A.; Goizueta, A.A.; Shannon, V.R.; London, D.; Garcia-Manero, G.; Kantarjian, H.M.; Ravandi-Kashani, F.; Kadia, T.M.; Konopleva, M.Y.; DiNardo, C.D.; et al. Pneumonitis after immune checkpoint inhibitor therapies in patients with acute myeloid leukemia: A retrospective cohort study. Cancer 2022, 128, 2736–2745. [Google Scholar] [CrossRef]
- Altan, M.; Soto, F.; Xu, T.; Wilson, N.; Franco-Vega, M.; Clavijo, C.S.; Shannon, V.; Faiz, S.; Gandhi, S.; Lin, S.; et al. Pneumonitis After Concurrent Chemoradiation and Immune Checkpoint Inhibition in Patients with Locally Advanced Non-small Cell Lung Cancer. Clin. Oncol. 2023, 35, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Altan, M.; Soto, F.; Zhong, L.L.; Akhmedzhanov, F.O.; Wilson, N.R.; Zarifa, A.; Albittar, A.A.; Yang, V.; Lewis, J.; Rinsurongkawong, W.; et al. Incidence and Risk Factors for Pneumonitis Associated With Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer: A Single Center Experience. Oncologist 2023, 28, e1065–e1074. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.L.; Segura, A.; Lelenwa, L.; Siddiqui, B.A.; Subudhi, S.K.; Lopez-Mattei, J.; Durand, J.B.; Deswal, A.; Zhao, B.; Buja, L.M.; et al. Immune checkpoint inhibitor myocarditis: Elucidating the spectrum of disease through endomyocardial biopsy. Eur. J. Heart Fail. 2021, 23, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Gatewood, M.O.; Wemple, M.; Greco, S.; Kritek, P.A.; Durvasula, R. A quality improvement project to improve early sepsis care in the emergency department. BMJ Qual. Saf. 2015, 24, 787–795. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).