Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Cell Lines
2.2. Viruses
2.3. Virus Treatment, Replication, and Quantification In Vitro
2.4. Sulforhodamine B (SRB) Cell Viability Assay
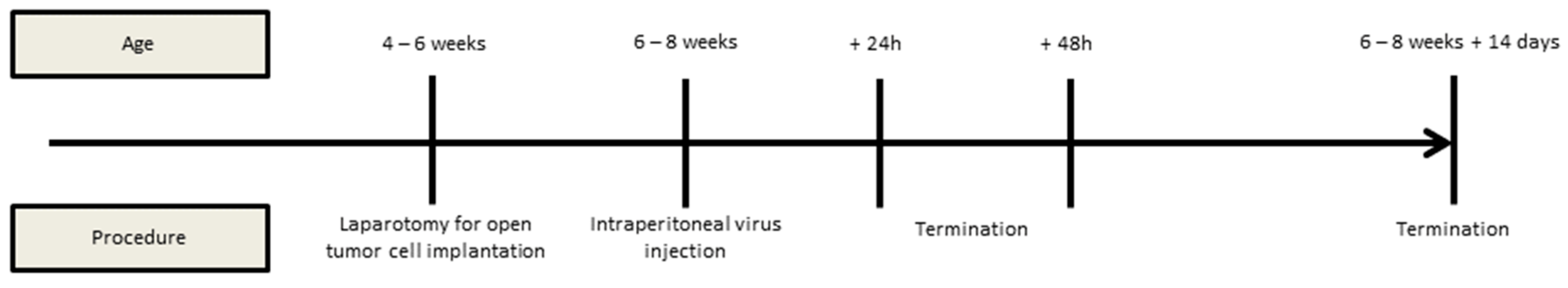
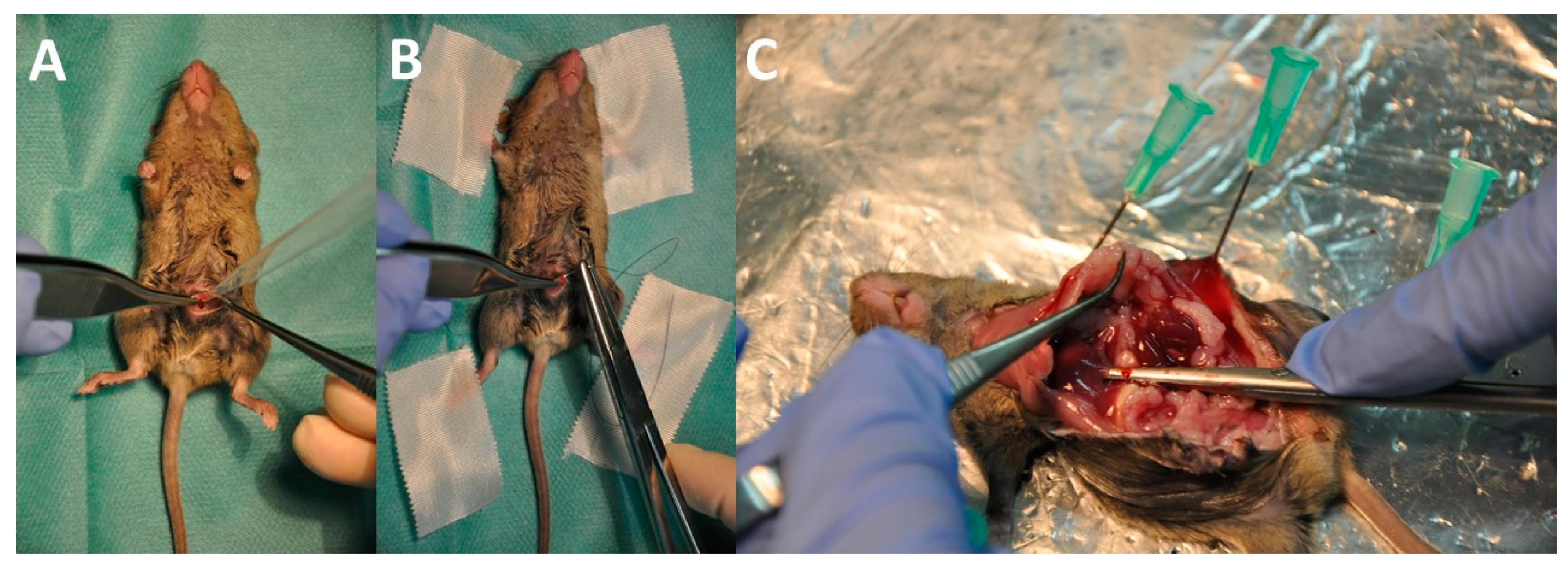
2.5. Animal Model, Interventions, and Assessment of the Peritoneal Cancer Index (PCI)
2.6. Statistical Analysis
3. Results
3.1. Oncolytic Effect of Vaccinia Virus Strains in Murine Peritoneal Mesothelioma Cell Lines
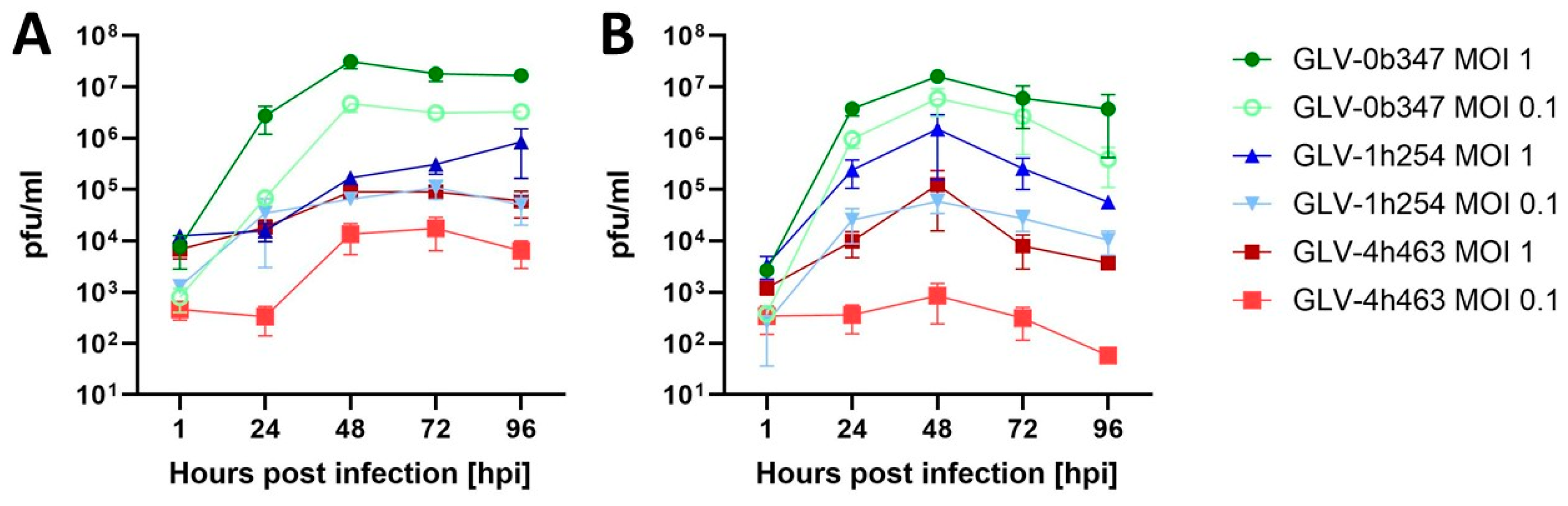
3.2. Viral Replication of Vaccinia Virus Strains in Murine Peritoneal Mesothelioma Cell Lines
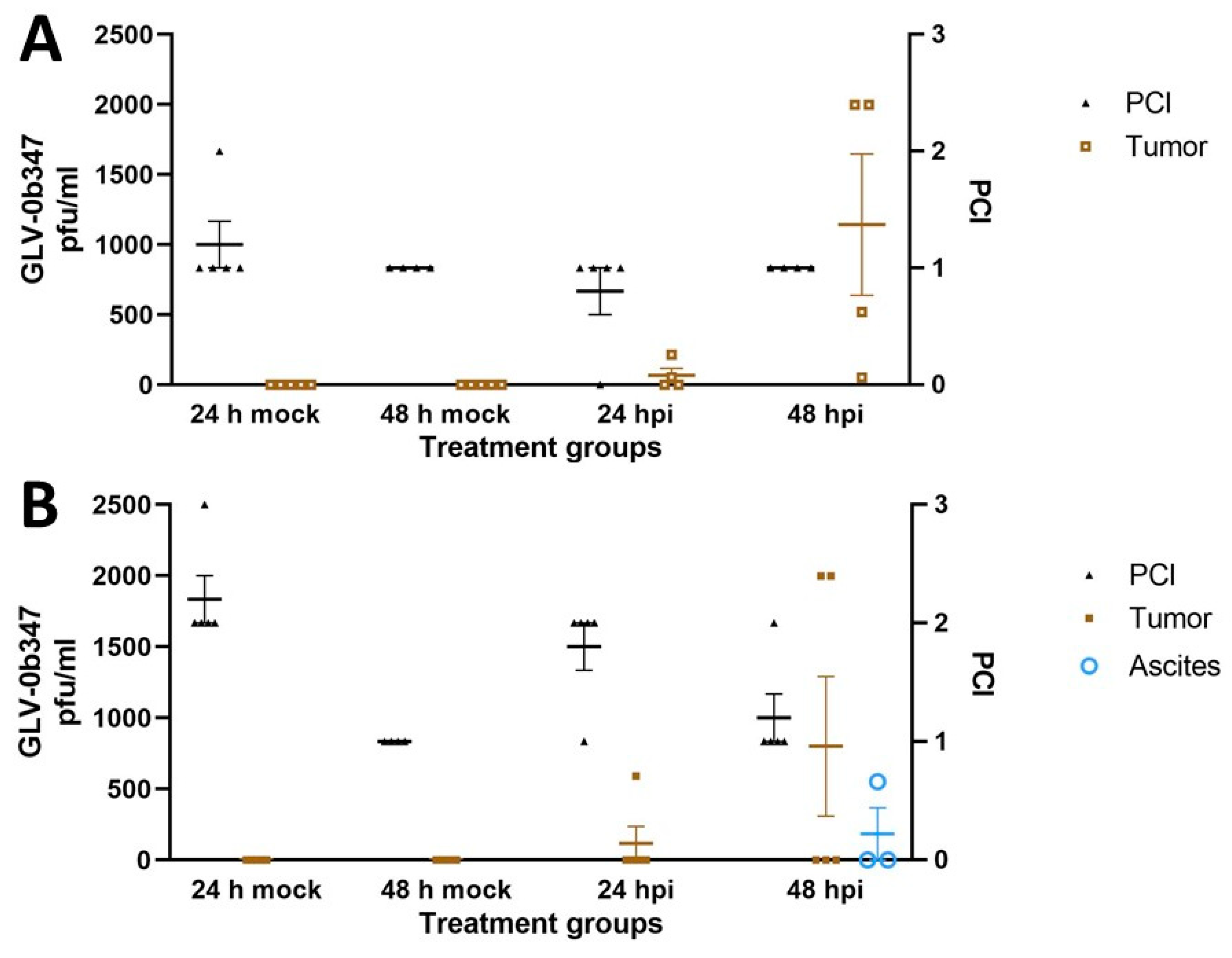
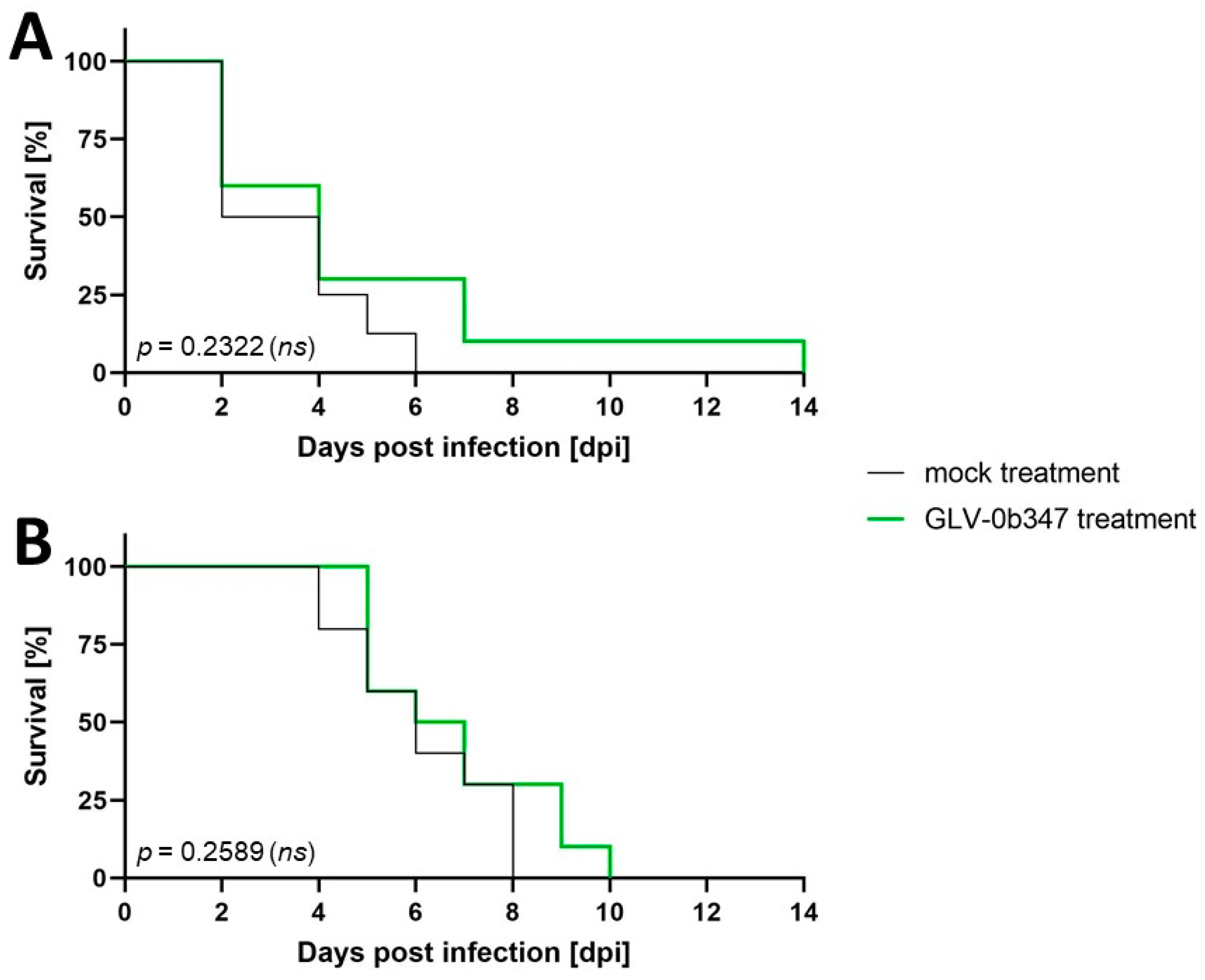
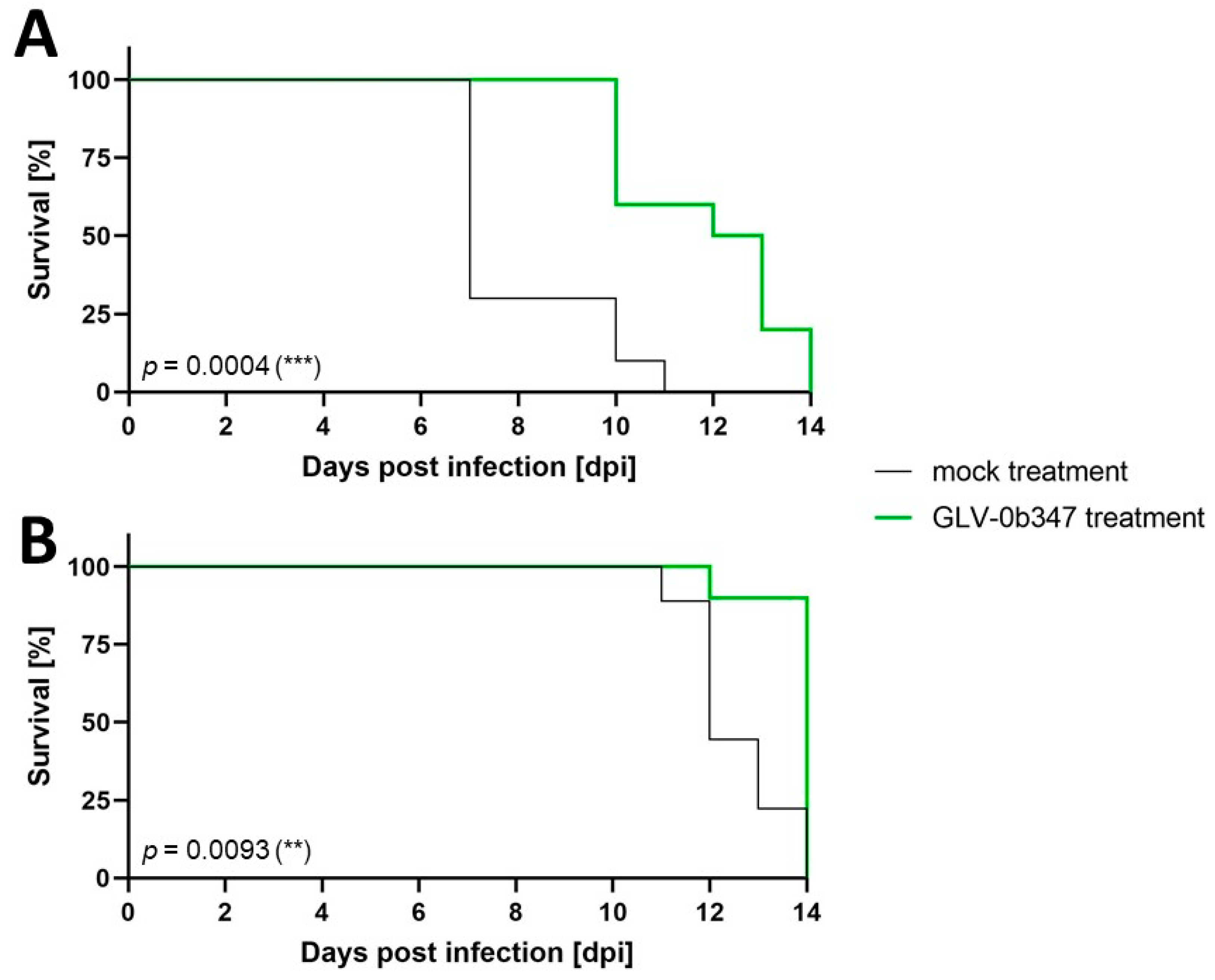
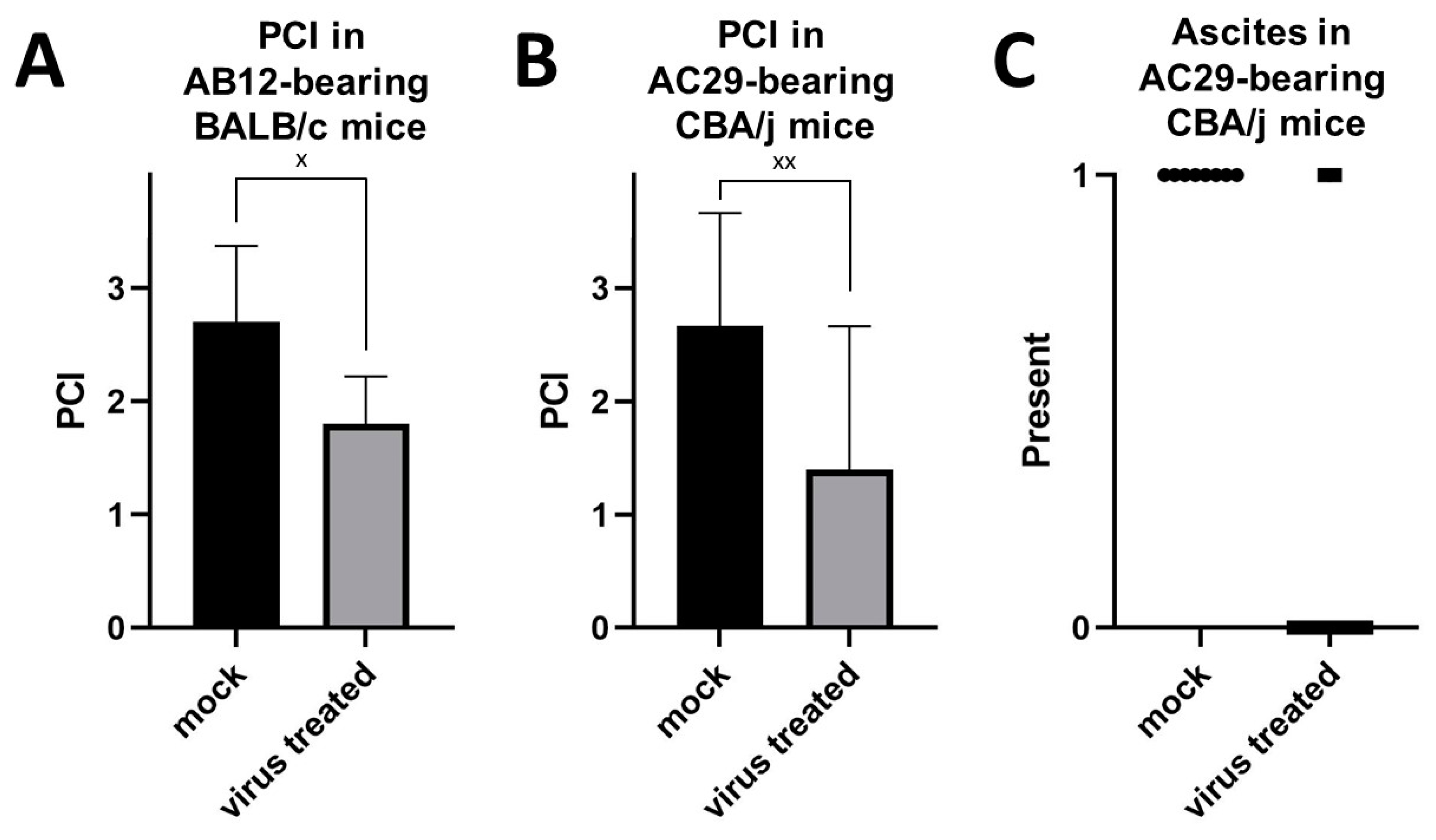
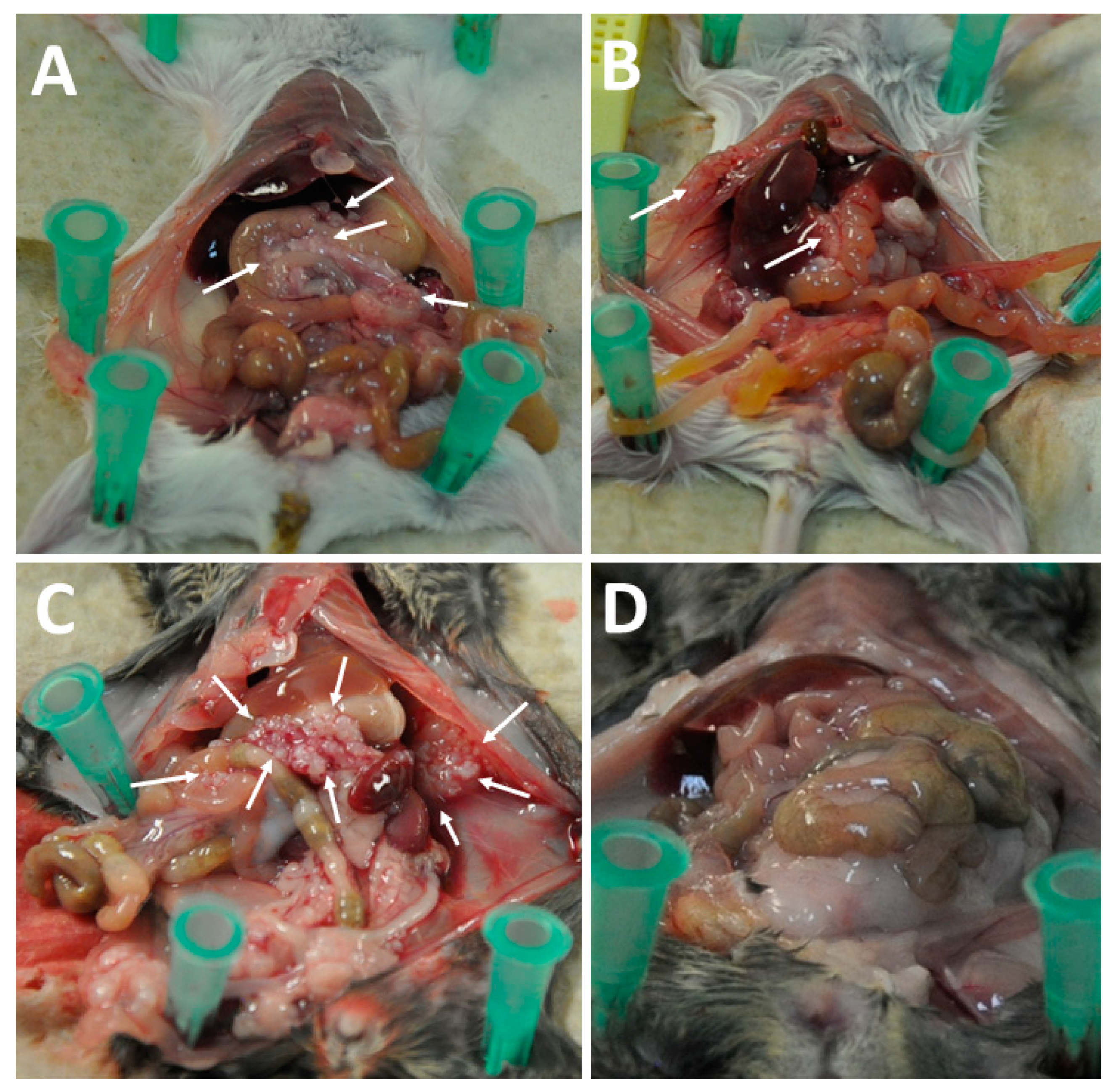
3.3. Intraperitoneal Virus Treatment of Peritoneal Mesothelioma in a Syngeneic Murine Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yano, H.; Moran, B.J.; Cecil, T.D.; Murphy, E.M. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal mesothelioma. Eur. J. Surg. Oncol. 2009, 35, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Yan, T.D.; Morris, D.L. Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: The Australian experience. J. Surg. Oncol. 2009, 99, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Deraco, M.; Baratti, D.; Kusamura, S.; Elias, D.; Glehen, O.; Gilly, F.N.; Levine, E.A.; Shen, P.; Mohamed, F.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J. Clin. Oncol. 2009, 27, 6237–6242. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.N.S.; Mehta, A.; Garcia-Fadrique, A.; Rowaiye, B.; Jenei, V.; Dayal, S.; Chandrakumaran, K.; Carr, N.; Mohamed, F.; Cecil, T.; et al. Outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma and predictors of survival. Int. J. Hyperthermia 2018, 34, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Miura, J.T.; Johnston, F.M.; Gamblin, T.C.; Turaga, K.K. Current trends in the management of malignant peritoneal mesothelioma. Ann. Surg. Oncol. 2014, 21, 3947–3953. [Google Scholar] [CrossRef]
- Kelly, E.; Russell, S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Liu, T.C.; Galanis, E.; Kirn, D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007, 4, 101–117. [Google Scholar] [CrossRef]
- Stojdl, D.F.; Lichty, B.; Knowles, S.; Marius, R.; Atkins, H.; Sonenberg, N.; Bell, J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000, 6, 821–825. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119.e1110. [Google Scholar] [CrossRef]
- Lauer, U.M.; Schell, M.; Beil, J.; Berchtold, S.; Koppenhöfer, U.; Glatzle, J.; Königsrainer, A.; Möhle, R.; Nann, D.; Fend, F.; et al. Phase I Study of Oncolytic Vaccinia Virus GL-ONC1 in Patients with Peritoneal Carcinomatosis. Clin. Cancer Res. 2018, 24, 4388–4398. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Goradel, N.H.; Alizadeh, A.; Hosseinzadeh, S.; Taghipour, M.; Ghesmati, Z.; Arashkia, A.; Negahdari, B. Oncolytic virotherapy as promising immunotherapy against cancer: Mechanisms of resistance to oncolytic viruses. Future Oncol. 2022, 18, 245–259. [Google Scholar] [CrossRef]
- Filley, A.C.; Dey, M. Immune System, Friend or Foe of Oncolytic Virotherapy? Front. Oncol. 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Acuna, S.A.; Ottolino-Perry, K.; Cako, B.; Tang, N.; Angarita, F.A.; McCart, J.A. Oncolytic vaccinia virus as an adjuvant treatment to cytoreductive surgery for malignant peritoneal mesothelioma. Ann. Surg. Oncol. 2014, 21, 2259–2266. [Google Scholar] [CrossRef]
- Van der Speeten, K.; Stuart, O.A.; Sugarbaker, P.H. Using pharmacologic data to plan clinical treatments for patients with peritoneal surface malignancy. Curr. Drug Discov. Technol. 2009, 6, 72–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurosaki, H.; Nakatake, M.; Sakamoto, T.; Kuwano, N.; Yamane, M.; Ishii, K.; Fujiwara, Y.; Nakamura, T. Anti-Tumor Effects of MAPK-Dependent Tumor-Selective Oncolytic Vaccinia Virus Armed with CD/UPRT against Pancreatic Ductal Adenocarcinoma in Mice. Cells 2021, 10, 985. [Google Scholar] [CrossRef]
- Hammad, M.; Cornejo, Y.; Flores, L.; Hyde, C.; Ngai, H.W.; Li, M.; Dellinger, T.H.; Lu, J.; Chen, N.G.; Mooney, R.; et al. Novel Chimeric Poxvirus CF17 Improves Survival in a Murine Model of Intraperitoneal Ovarian Cancer Metastasis. Mol. Ther. Oncolytics 2020, 19, 278–282. [Google Scholar] [CrossRef]
- Eveno, C.; Mojica, K.; Ady, J.W.; Thorek, D.L.; Longo, V.; Belin, L.J.; Gholami, S.; Johnsen, C.; Zanzonico, P.; Chen, N.; et al. Gene therapy using therapeutic and diagnostic recombinant oncolytic vaccinia virus GLV-1h153 for management of colorectal peritoneal carcinomatosis. Surgery 2015, 157, 331–337. [Google Scholar] [CrossRef]
- Ishikawa, W.; Kikuchi, S.; Ogawa, T.; Tabuchi, M.; Tazawa, H.; Kuroda, S.; Noma, K.; Nishizaki, M.; Kagawa, S.; Urata, Y.; et al. Boosting Replication and Penetration of Oncolytic Adenovirus by Paclitaxel Eradicate Peritoneal Metastasis of Gastric Cancer. Mol. Ther. Oncolytics 2020, 18, 262–271. [Google Scholar] [CrossRef]
- Day, G.L.; Bryan, M.L.; Northrup, S.A.; Lyles, D.S.; Westcott, M.M.; Stewart, J.H.t. Immune Effects of M51R Vesicular Stomatitis Virus Treatment of Carcinomatosis From Colon Cancer. J. Surg. Res. 2020, 245, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R.; Manning, L.S.; Whitaker, D.; Garlepp, M.J.; Robinson, B.W. Establishment of a murine model of malignant mesothelioma. Int. J. Cancer 1992, 52, 881–886. [Google Scholar] [CrossRef]
- Wang, H.; Chen, N.G.; Minev, B.R.; Zimmermann, M.; Aguilar, R.J.; Zhang, Q.; Sturm, J.B.; Fend, F.; Yu, Y.A.; Cappello, J.; et al. Optical detection and virotherapy of live metastatic tumor cells in body fluids with vaccinia strains. PLoS ONE 2013, 8, e71105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carter, M.E.; Hartkopf, A.D.; Wagner, A.; Volmer, L.L.; Brucker, S.Y.; Berchtold, S.; Lauer, U.M.; Koch, A. A Three-Dimensional Organoid Model of Primary Breast Cancer to Investigate the Effects of Oncolytic Virotherapy. Front. Mol. Biosci. 2022, 9, 826302. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Beil, J.; Berchtold, S.; Mönch, D.; Maaß, A.; Smirnow, I.; Schenk, A.; Carter, M.E.; Kloker, L.D.; Leibold, T.; et al. Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model. Viruses 2023, 15, 363. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Y.A.; Wang, E.; Chen, N.; Danner, R.L.; Munson, P.J.; Marincola, F.M.; Szalay, A.A. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007, 67, 10038–10046. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Ottow, R.T.; Steller, E.P.; Sugarbaker, P.H.; Wesley, R.A.; Rosenberg, S.A. Immunotherapy of intraperitoneal cancer with interleukin 2 and lymphokine-activated killer cells reduces tumor load and prolongs survival in murine models. Cell. Immunol. 1987, 104, 366–376. [Google Scholar] [CrossRef]
- Ali, I.H.; Jabir, M.S.; Al-Shmgani, H.S.; Sulaiman, G.M.; Sadoon, A.H. Pathological And Immunological Study On Infection With Escherichia Coli In ale BALB/c mice. J. Phys. 2018, 1003, 012009. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common pitfalls in statistical analysis: Measures of agreement. Perspect. Clin. Res. 2017, 8, 187–191. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dube, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Goere, D.; Souadka, A.; Faron, M.; Cloutier, A.S.; Viana, B.; Honore, C.; Dumont, F.; Elias, D. Extent of colorectal peritoneal carcinomatosis: Attempt to define a threshold above which HIPEC does not offer survival benefit: A comparative study. Ann. Surg. Oncol. 2015, 22, 2958–2964. [Google Scholar] [CrossRef]
- Benizri, E.I.; Bernard, J.L.; Rahili, A.; Benchimol, D.; Bereder, J.M. Small bowel involvement is a prognostic factor in colorectal carcinomatosis treated with complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2012, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Canbay, E.; Ishibashi, H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. Sci. World J. 2013, 2013, 978394. [Google Scholar] [CrossRef]
- Cohn, D.E.; Sill, M.W.; Walker, J.L.; O′Malley, D.; Nagel, C.I.; Rutledge, T.L.; Bradley, W.; Richardson, D.L.; Moxley, K.M.; Aghajanian, C. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin®) in recurrent ovarian, tubal, or peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 146, 477–483. [Google Scholar] [CrossRef]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef]
- Cavaliere, F.; De Simone, M.; Virzi, S.; Deraco, M.; Rossi, C.R.; Garofalo, A.; Di Filippo, F.; Giannarelli, D.; Vaira, M.; Valle, M.; et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur. J. Surg. Oncol. 2011, 37, 148–154. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef]
- Verma, V.; Sleightholm, R.L.; Rusthoven, C.G.; Koshy, M.; Sher, D.J.; Grover, S.; Simone, C.B., 2nd. Malignant Peritoneal Mesothelioma: National Practice Patterns, Outcomes, and Predictors of Survival. Ann. Surg. Oncol. 2018, 25, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Goere, D.; Glehen, O.; Quenet, F.; Ducreux, M.; Guilloit, J.-M.; Texier, M.; Benhamou, E.; Elias, D. Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP- NTC01226394). J. Clin. Oncol. 2018, 36, 3531. [Google Scholar] [CrossRef]
- Kowalsky, S.J.; Liu, Z.; Feist, M.; Berkey, S.E.; Ma, C.; Ravindranathan, R.; Dai, E.; Roy, E.J.; Guo, Z.S.; Bartlett, D.L. Superagonist IL-15-Armed Oncolytic Virus Elicits Potent Antitumor Immunity and Therapy That Are Enhanced with PD-1 Blockade. Mol. Ther. 2018, 26, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Moesta, A.K.; Cooke, K.; Piasecki, J.; Mitchell, P.; Rottman, J.B.; Fitzgerald, K.; Zhan, J.; Yang, B.; Le, T.; Belmontes, B.; et al. Local Delivery of OncoVEX(mGM-CSF) Generates Systemic Antitumor Immune Responses Enhanced by Cytotoxic T-Lymphocyte-Associated Protein Blockade. Clin. Cancer Res. 2017, 23, 6190–6202. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.A.; Puzanov, I.; Collichio, F.A.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J. Immunother Cancer 2023, 11, e006270. [Google Scholar] [CrossRef]
- Delaunay, T.; Nader, J.; Grard, M.; Farine, I.; Hedwig, V.; Foloppe, J.; Blondy, T.; Violland, M.; Pouliquen, D.; Grégoire, M.; et al. High Oncolytic Activity of a Double-Deleted Vaccinia Virus Copenhagen Strain against Malignant Pleural Mesothelioma. Mol. Ther. Oncolytics 2020, 18, 573–578. [Google Scholar] [CrossRef]
- Chen, N.G.; Yu, Y.A.; Zhang, Q.; Szalay, A.A. Replication efficiency of oncolytic vaccinia virus in cell cultures prognosticates the virulence and antitumor efficacy in mice. J. Transl. Med. 2011, 9, 164. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurttas, C.; Beil, J.; Berchtold, S.; Smirnow, I.; Kloker, L.D.; Sipos, B.; Löffler, M.W.; Königsrainer, A.; Mihaljevic, A.L.; Lauer, U.M.; et al. Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma. Cancers 2024, 16, 368. https://doi.org/10.3390/cancers16020368
Yurttas C, Beil J, Berchtold S, Smirnow I, Kloker LD, Sipos B, Löffler MW, Königsrainer A, Mihaljevic AL, Lauer UM, et al. Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma. Cancers. 2024; 16(2):368. https://doi.org/10.3390/cancers16020368
Chicago/Turabian StyleYurttas, Can, Julia Beil, Susanne Berchtold, Irina Smirnow, Linus D. Kloker, Bence Sipos, Markus W. Löffler, Alfred Königsrainer, André L. Mihaljevic, Ulrich M. Lauer, and et al. 2024. "Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma" Cancers 16, no. 2: 368. https://doi.org/10.3390/cancers16020368
APA StyleYurttas, C., Beil, J., Berchtold, S., Smirnow, I., Kloker, L. D., Sipos, B., Löffler, M. W., Königsrainer, A., Mihaljevic, A. L., Lauer, U. M., & Thiel, K. (2024). Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma. Cancers, 16(2), 368. https://doi.org/10.3390/cancers16020368





