Influencing Factors on the Quality of Lymph Node Dissection for Stage IA Non-Small Cell Lung Cancer: A Retrospective Nationwide Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diagnosis, Surgical Treatment, and Reporting of Treatment Results for Lung Cancer in Poland
2.2. Study Design
- Right upper paratracheal (2R), right lower paratracheal (4R), and subcarinal (7) nodes for right upper and right middle lobectomy, and for right upper bilobectomy.
- Right lower paratracheal (4R), subcarinal (7), and paraesophageal (8) or pulmonary ligament (9) nodes for right lower lobectomy.
- Aorto-pulmonary window (5), paraaortic (6), and subcarinal (7) nodes for left upper lobectomy.
- Subcarinal (7), paraesophageal (8), and pulmonary ligament (9) nodes for left lower lobectomy.
- All right-sided stations (2R, 4R, 7, and 8 or 9) for right lower bilobectomy.
2.3. Statistical Analyses
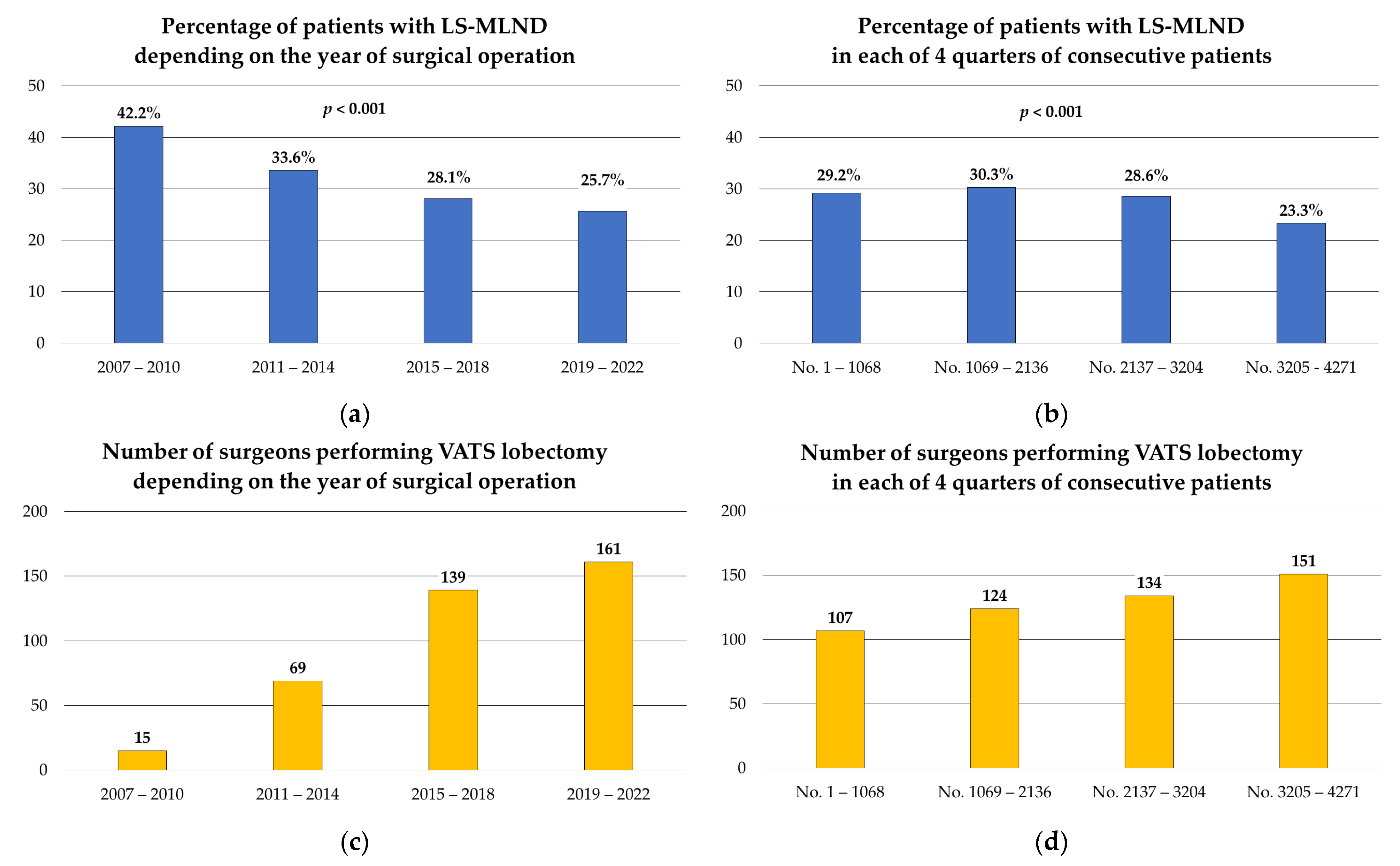
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Howlader, N. SEER Cancer Statistics Review, 1975–2011. Available online: https://seer.cancer.gov/archive/csr/1975_2014/ (accessed on 20 October 2023).
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.W.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Oudkerk, M.; Devaraj, A.; Vliegenthart, R.; Henzler, T.; Prosch, H.; Heussel, C.P.; Bastarrika, G.; Sverzellati, N.; Mascalchi, M.; Delorme, S.; et al. European position statement on lung cancer screening. Lancet Oncol. 2017, 18, e754–e766. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, R.; Marjanski, T.; Binczyk, F.; Polanska, J.; Sawicka, W.; Rzyman, W. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: A propensity score-matched analysis. Eur. J. Cardiothorac. Surg. 2018, 54, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Piwkowski, C.; Gabryel, P.; Campisi, A.; Orłowski, T.M.; Zieliński, M.; Rzyman, W.; Kowalewski, J.; Czyżewski, D.; Grochowski, Z.; Wójcik, J.; et al. Ninety-day mortality of thoracoscopic vs open lobectomy: A large multicenter cohort study. Ann. Thorac. Surg. 2023, 115, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Coco, D.; Leanza, S. Current perspective on uniportal and multiportal video-assisted thoracic surgery during lobectomy for lung cancer. Kardiochir. Torakochir. Pol. 2022, 19, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, X.; Zhao, S.; Wang, J.; Zhang, W.; Sun, G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: A meta-analysis. BMC Cancer 2021, 21, 498. [Google Scholar] [CrossRef]
- Servais, E.L.; Miller, D.L.; Thibault, D.; Hartwig, M.G.; Kosinski, A.S.; Stock, C.T.; Price, T.; Quadri, S.M.; D’Agostino, R.S.; Burfeind, W.R. Conversion to thoracotomy during thoracoscopic vs robotic lobectomy: Predictors and outcomes. Ann. Thorac. Surg. 2022, 114, 409–417. [Google Scholar] [CrossRef]
- Novellis, P.; Bottoni, E.; Voulaz, E.; Cariboni, U.; Testori, A.; Bertolaccini, L.; Giordano, L.; Dieci, E.; Granato, L.; Vanni, E.; et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: Comparison of costs and outcomes at a single institute. J. Thorac. Dis. 2018, 10, 790–798. [Google Scholar] [CrossRef]
- Ginsberg, R.J.; Rubinstein, L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann. Thorac. Surg. 1995, 60, 615–622. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Seg-mentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or sublobar resection for peripheral stage IA non–small-cell lung cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.; Petersen, R.H.; Ricciardi, S.; Patel, A.; Lodhia, J.V.; Gooseman, M.R.; Brunelli, A.; Dunning, J.; Fang, W.; Gossot, D.; et al. European guidelines for the surgical management of pure ground-glass opacities and part-solid nodules: Task Force of the European Association of Cardio-Thoracic Surgery and the European Society of Thoracic Surgeons. Eur. J. Cardiothorac. Surg. 2023, 64, ezad222. [Google Scholar] [CrossRef] [PubMed]
- Galanis, M.; Leivaditis, V.; Gioutsos, K.; Panagiotopoulos, I.; Kyratzopoulos, A.; Mulita, F.; Papaporfyriou, A.; Verras, G.I.; Tasios, K.; Antzoulas, A.; et al. Segmentectomy versus lobectomy. Which factors are decisive for an optimal oncological outcome? Kardiochir. Torakochir. Pol. 2023, 20, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R. The evolving concept of complete resection in lung cancer surgery. Cancers 2021, 13, 2583. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, B.; Zirafa, C.C.; Filosso, P.L.; Stefani, A.; Romano, G.; Davini, F.; Melfi, F. The Role of Lymphadenectomy in Early-Stage NSCLC. Cancers 2023, 15, 3735. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.-B.; Yang, J.; Zeng, T.-S.; Long, H.; Fu, J.-H.; Zhang, L.-J.; Lin, P.; Wang, X.; Rong, T.-H.; Hou, X.; et al. Incidence and distribution of lobe-specific mediastinal lymph node metastasis in non-small cell lung cancer: Data from 4511 resected cases. Ann. Surg. Oncol. 2018, 25, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.Y.; Zhou, J.; Wang, R.L.; Jiang, R.; Zhu, D.X.; Tang, X.J.; Zhou, Q. Lobe-specific lymph node dissection for clinical early-stage (cIa) peripheral non-small cell lung cancer patients: What and how? Ann. Surg. Oncol. 2020, 27, 472–480. [Google Scholar] [CrossRef]
- Shapiro, M.; Kadakia, S.; Lim, J.; Breglio, A.; Wisnivesky, J.P.; Kaufman, A.; Lee, D.S.; Flores, R.M. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest 2013, 144, 1615–1621. [Google Scholar] [CrossRef]
- Woo, W.; Shin, J.I.; Kipkorir, V.; Yang, Y.H.; Lee, S.; Lee, C.Y. Clinical benefits of lobe-specific lymph node dissection in surgery for non-small cell lung cancer: A systematic review and meta-analysis. JTO Clin. Res. Rep. 2023, 4, 100516. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, Z.; Cheng, D.; Hao, X.; Pu, Q.; Liu, L. Lobe-specific node dissection can be a suitable alternative to systematic lymph node dissection in highly selective early-stage non-small-cell lung cancer patients: A meta-analysis. Ann. Thorac. Cardiovasc. Surg. 2021, 27, 143–150. [Google Scholar] [CrossRef]
- Kakuturu, J.; Abbas, G.; Toker, A. Evaluating the quality of lymphadenectomy in lung cancer resections: A narrative review. AME Surg. J. 2022, 2, 1–8. [Google Scholar] [CrossRef]
- Ray, M.A.; Smeltzer, M.P.; Faris, N.R.; Osarogiagbon, R.U. Survival after mediastinal node dissection, systematic sampling, or neither for early stage NSCLC. J. Thorac. Oncol. 2020, 15, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Gabryel, P.; Skrzypczak, P.; Campisi, A.; Kasprzyk, M.; Roszak, M.; Piwkowski, C. Predictors of long-term survival of thoracoscopic lobectomy for stage ia non-small cell lung cancer: A large retrospective cohort study. Cancers 2023, 15, 3877. [Google Scholar] [CrossRef] [PubMed]
- Osarogiagbon, R.U.; Yu, X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013, 96, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Butnor, K.J.; Asamura, H.; Travis, W.D. Node doubt: Rigorous surgical nodal procurement combined with thorough pathologic evaluation improves non-small cell lung carcinoma staging accuracy. Ann. Thorac. Surg. 2016, 102, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Gabryel, P.; Roszak, M.; Skrzypczak, P.; Gabryel, A.; Zielińska, D.; Sielewicz, M.; Campisi, A.; Kasprzyk, M.; Piwkowski, C. Identification of factors related to the quality of lymphadenectomy for lung cancer: Secondary analysis of prospective randomized trial data. J. Clin. Med. 2023, 12, 3780. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, U.; Barańska, K.; Michałek, I.; Olasek, P.; Miklewska, M.; Didkowska, J.A. Cancer in Poland in 2020. Narodowy Instytut Onkologii. 2020. Available online: https://onkologia.org.pl/en/publications (accessed on 23 July 2023).
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. In Non-Small Cell Lung Cancer. Version 3.2022; 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/35545176/ (accessed on 4 November 2023).
- Rami-Porta, R.; Call, S.; Dooms, C.; Obiols, C.; Sánchez, M.; Travis, W.D.; Vollmer, I. Lung cancer staging: A concise update. Eur. Resp. J. 2018, 51, 1800190. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Hirmas, N.; Fanti, S.; Paez, D.; Abuhijla, F.; Al-Rimawi, D.; Al-Rasheed, U.; Abdeljalil, R.; Hawari, F.; Alrabi, K.; et al. Impact of (18)F-FDG PET/CT, CT and EBUS/TBNA on preoperative mediastinal nodal staging of NSCLC. BMC Med. Imaging. 2021, 21, 49. [Google Scholar] [CrossRef]
- Edwards, T.; Balata, H.; Elshafi, M.; Foden, P.; Bishop, P.; Fontaine, E.; Jones, M.; Krysiak, P.; Rammohan, K.; Shah, R.; et al. Adequacy of intraoperative nodal staging during surgical resection of NSCLC: Influencing factors and its relationship to survival. J. Thorac. Oncol. 2017, 12, 1845–1850. [Google Scholar] [CrossRef][Green Version]
- Pawelczyk, K.; Blasiak, P.; Szromek, M.; Nowinska, K.; Marciniak, M. Assessment of adequacy of intraoperative nodal staging and factors influencing the lack of its compliance with recommendations in the surgical treatment of non-small cell lung cancer (NSCLC). J. Thorac. Dis. 2018, 10, 4902–4911. [Google Scholar] [CrossRef]
- Bott, M.J.; Patel, A.P.; Crabtree, T.D.; Colditz, G.A.; Kreisel, D.; Krupnick, A.S.; Patterson, G.A.; Broderick, S.; Meyers, B.F.; Puri, V. Pathologic upstaging in patients undergoing resection for stage I non-small cell lung cancer: Are there modifiable predictors? Ann. Thorac. Surg. 2015, 100, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.M.; Liu, C.Y.; Lin, M.W.; Hsu, H.H.; Chen, J.S. Factors associated with nodal upstaging in clinical T1a-bN0M0 non-small cell lung cancers. Cancers 2022, 14, 1277. [Google Scholar] [CrossRef]
- Chmielewska, I.; Stencel, K.; Kalinka, E.; Ramlau, R.; Krawczyk, P. Neoadjuvant and adjuvant immunotherapy in non-small cell lung cancer—Clinical trials experience. Cancers 2021, 13, 5048. [Google Scholar] [CrossRef]
- McKenna, R.J. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac. Surg. Clin. 2008, 18, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Paladini, P.; Meniconi, F.; Ghisalberti, M.; Luzzi, L.; Ligabue, T.; de Leonibus, L.; Corzani, R. Review of the learning curve of video-assisted thoracic surgery & robotic-assisted thoracic surgery lobectomies—Similarities and differences. Curr. Chall. Thorac. Surg. 2021, 3, 16. [Google Scholar]
- Amore, D.; Curcio, C. Steps in the development of a VATS lobectomy program. J. Vis. Surg. 2017, 3, 104. [Google Scholar] [CrossRef][Green Version]
- Petersen, R.H.; Hansen, H.J. Learning curve associated with VATS lobectomy. Ann. Cardiothorac. Surg. 2012, 1, 47–50. [Google Scholar] [CrossRef]
- Gonfiotti, A.; Bongiolatti, S.; Borgianni, S.; Borrelli, R.; Jaus, M.O.; Politi, L.; Tancredi, G.; Viggiano, D.; Voltolini, L. Development of a video-assisted thoracoscopic lobectomy program in a single institution: Results before and after completion of the learning curve. J. Cardiothorac. Surg. 2016, 11, 130. [Google Scholar] [CrossRef][Green Version]
- Mazzella, A.; Olland, A.; Falcoz, P.E.; Renaud, S.; Santelmo, N.; Massard, G. Video-assisted thoracoscopic lobectomy: Which is the learning curve of an experienced consultant? J. Thorac. Dis. 2016, 8, 2444–2453. [Google Scholar] [CrossRef]
- Osarogiagbon, R.U.; Ogbata, O.; Yu, X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann. Thorac. Surg. 2014, 97, 385–393. [Google Scholar] [CrossRef]
- Krantz, S.B.; Lutfi, W.; Kuchta, K.; Wang, C.H.; Kim, K.W.; Howington, J.A. Improved Lymph Node Staging in Early-Stage Lung Cancer in the National Cancer Database. Ann. Thorac. Surg. 2017, 104, 1805–1814. [Google Scholar] [CrossRef]
- Mroczkowski, P.; Dziki, Ł.; Vosikova, T.; Otto, R.; Mercz-Sadowska, A.; Zajdel, R.; Zajdel, K.; Lippert, H.; Jannasch, O. Rectal cancer: Are 12 lymph nodes the limit? Cancers 2023, 15, 3447. [Google Scholar] [CrossRef]
- Shapiro, M.; Mhango, G.; Kates, M.; Weiser, T.S.; Chin, C.; Swanson, S.J.; Wisnivesky, J.P. Extent of lymph node resection does not increase perioperative morbidity and mortality after surgery for stage I lung cancer in the elderly. Eur. J. Surg. Oncol. 2012, 38, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Darling, G.E.; Allen, M.S.; Decker, P.A.; Ballman, K.; Malthaner, R.A.; Inculet, R.I.; Jones, D.R.; McKenna, R.J.; Landreneau, R.J.; Rusch, V.W.; et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J. Thorac. Cardiovasc. Surg. 2011, 141, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, J.; Chen, Q.; Jiang, J. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e109979. [Google Scholar] [CrossRef] [PubMed]
- Taleb, N.N. Black swans and the domains of statistics. Am. Stat. 2007, 61, 198–200. [Google Scholar] [CrossRef]
- Safdie, F.M.; Sanchez, M.V.; Sarkaria, I.S. Prevention and management of intraoperative crisis in VATS and open chest surgery: How to avoid emergency conversion. J. Vis. Surg. 2017, 3, 87. [Google Scholar] [CrossRef][Green Version]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Ray, M.A.; Fehnel, C.; Akinbobola, O.; Faris, N.R.; Taylor, M.; Pacheco, A.; Smeltzer, M.P.; Osarogiagbon, R.U. Comparative effectiveness of a lymph node collection kit versus heightened awareness on lung cancer surgery quality and outcomes. J. Thorac. Oncol. 2021, 16, 774–783. [Google Scholar] [CrossRef]
- Osarogiagbon, R.U.; Smeltzer, M.P.; Faris, N.R.; Ray, M.A.; Fehnel, C.; Ojeabulu, P.; Akinbobola, O.; Meadows-Taylor, M.; McHugh, L.M.; Halal, A.M.; et al. Outcomes after use of a lymph node collection kit for lung cancer surgery: A pragmatic, population-based, multi-institutional, staggered implementation study. J. Thorac. Oncol. 2021, 16, 630–642. [Google Scholar] [CrossRef]
- Smeltzer, M.P.; Faris, N.R.; Fehnel, C.; Akinbobola, O.; Saulsberry, A.; Meadows-Taylor, M.; Pacheco, A.; Ray, M.; Osarogiagbon, R.U. Impact of a Lymph Node Specimen Collection Kit on the Distribution and Survival Implications of the Proposed Revised Lung Cancer Residual Disease Classification: A Propensity-Matched Analysis. JTO Clin. Res. Rep. 2021, 2, 100161. [Google Scholar] [CrossRef] [PubMed]
- Gabryel, P.; Kasprzyk, M.; Roszak, M.; Campisi, A.; Smoliński, S.; Zieliński, P.; Piwkowski, C. Comparison of the LigaSure™ bipolar vessel sealer to monopolar electrocoagulation for thoracoscopic lobectomy and lymphadenectomy: A prospective randomized controlled trial. Surg. Endosc. 2023, 37, 4449–4457. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, S.; Yajima, T.; Ohtaki, Y.; Ito, T.; Kosaka, T.; Shirabe, K. Tips on lymph node dissection using energy devices: A narrative review. AME Surg. J. 2022, 2, 1–7. [Google Scholar] [CrossRef]
- ESTS Quality Certification Program. Available online: https://www.ests.org/professional_development/accreditation.aspx (accessed on 4 November 2023).
- ESTS Silverbook, Database Annual Report 2023. Available online: https://www.ests.org/ests_database/database_reports/database_reports_silver_books.aspx (accessed on 4 November 2023).
- Osarogiagbon, R.U.; Sareen, S.; Eke, R.; Yu, X.; McHugh, L.M.; Kernstine, K.H.; Putnam, J.B.; Robbins, E.T. Audit of lymphadenectomy in lung cancer resections using a specimen collection kit and checklist. Ann. Thorac. Surg. 2015, 99, 421–427. [Google Scholar] [CrossRef]
- Riquet, M.; Legras, A.; Mordant, P.; Rivera, C.; Arame, A.; Gibault, L.; Foucault, C.; Dujon, A.; Le Pimpec Barthes, F. Number of mediastinal lymph nodes in non-small cell lung cancer: A Gaussian curve, not a prognostic factor. Ann. Thorac. Surg. 2014, 98, 224–231. [Google Scholar] [CrossRef]
- Van de Graaf, F.W.; Eryigit, Ö.; Lange, J.F. Current perspectives on video and audio recording inside the surgical operating room: Results of a cross-disciplinary survey. Updates Surg. 2021, 73, 2001–2007. [Google Scholar] [CrossRef]
- Eckhoff, J.A.; Rosman, G.; Altieri, M.S.; Speidel, S.; Stoyanov, D.; Anvari, M.; Meier-Hein, L.; März, K.; Jannin, P.; Pugh, C.; et al. SAGES consensus recommendations on surgical video data use, structure, and exploration (for research in artificial intelligence, clinical quality improvement, and surgical education). Surg. Endosc. 2023, 37, 8690–8707. [Google Scholar] [CrossRef]
| Lobe-Specific Lymph Node Dissection | p-Value | ||
|---|---|---|---|
| Non L-SMLND (n = 3081) | L-SMLND (n = 1190) | ||
| Age, years, mean (SD) | 65.1 (SD: 8.1) | 65.9 (SD: 7.8) | 0.794 |
| Male, n (%) | 1408 (45.7) | 568 (47.7) | 0.233 |
| Smoking history, n (%) | 2052 (66.6) | 934 (78.5) | <0.001 * |
| BMI, kg/m2, mean (SD) | 26.9 (SD: 4.9) | 27.6 (SD: 5.1) | 0.040 * |
| ppFEV1%, mean (SD) | 89.0 (SD: 19.4) | 88.6 (SD: 19.1) | 0.631 |
| Comorbidities, n (%) | 2444 (79.3) | 932 (78.3) | 0.469 |
| Chronic obstructive pulmonary disease | 542 (17.6) | 274 (23.0) | <0.001 * |
| Coronary arterial disease | 433 (14.1) | 158 (13.3) | 0.510 |
| Cerebrovascular disease | 40 (1.3) | 23 (1.9) | 0.123 |
| Peripheral arterial disease | 103 (3.3) | 53 (4.5) | 0.083 |
| Hypertension | 1571 (51.0) | 605 (50.8) | 0.930 |
| Diabetes mellitus | 462 (15.0) | 185 (15.5) | 0.652 |
| Chronic kidney disease | 24 (0.8) | 16 (1.3) | 0.085 |
| Other neoplasm | 502 (16.3) | 173 (14.5) | 0.159 |
| ThRCRI, n (%) | 0.806 | ||
| Group A (n = 3622) | 2610 (84.7) | 1012 (85.1) | |
| Group B (n = 644) | 469 (15.2) | 175 (14.7) | |
| Group C (n = 5) | 2 (0.1) | 3 (0.2) | |
| CCI, median (IQR) | 3 (IQR, 2 to 4) | 3 (IQR, 2 to 4) | 0.587 |
| Patients with PET-CT | 972 (31.5) | 521 (43.8) | <0.001 * |
| Patients with EBUS-TBNA | 364 (11.8) | 114 (9.6) | 0.038 * |
| Patients with mediastinoscopy | 25 (0.8) | 19 (1.6) | 0.023 * |
| Lobe-Specific Lymph Node Dissection | p-Value | ||
|---|---|---|---|
| Non L-SMLND (n = 3081) | L-SMLND (n = 1190) | ||
| Side of surgery, n (%) | <0.001 * | ||
| Right (n = 2543) | 1719 (67.6) | 824 (32.4) | |
| Left (n = 1728) | 1362 (78.8) | 366 (21.2) | |
| Type of surgery, n (%) | <0.001 * | ||
| Right upper lobectomy (n = 1415) | 946 (66.9) | 469 (33.1) | |
| Right middle lobectomy (n = 361) | 266 (73.7) | 95 (26.3) | |
| Right lower lobectomy (n = 739) | 485 (65.6) | 254 (34.4) | |
| Right upper bilobectomy (n = 14) | 9 (64.3) | 5 (35.7) | |
| Right lower bilobectomy (n = 14) | 13 (92.9) | 1 (7.1) | |
| Left upper lobectomy (n = 1055) | 767 (72.7) | 288 (27.3) | |
| Left lower lobectomy (n = 673) | 595 (88.4) | 78 (11.6) | |
| Surgeons’ experience, n (%) | 0.003 * | ||
| Initial 50 VATS lobectomies (n = 2523) | 1863 (73.8) | 660 (26.2) | |
| Later lobectomies (>50) (n = 1748) | 1218 (69.7) | 530 (30.3) | |
| Histology, n (%) | 0.059 | ||
| Adenocarcinoma (n = 2363) | 1694 (71.7) | 669 (28.3) | |
| Squamous cell carcinoma (n = 1036) | 731 (70.6) | 305 (29.4) | |
| Other types (n = 872) | 656 (75.2) | 216 (24.8) | |
| pT, n (%) | <0.001 * | ||
| pT 1a (n = 442) | 336 (76.0) | 106 (24.0) | |
| pT 1b (n = 2136) | 1566 (73.3) | 570 (26.7) | |
| pT 1c (n = 1691) | 1177 (69.6) | 514 (30.4) | |
| Number of lymph nodes stations removed, median (IQR) | |||
| N1 stations | 2 (IQR, 1 to 2) | 2 (IQR, 1 to 2) | <0.001 * |
| N2 stations | 2 (IQR, 1 to 3) | 4 (IQR, 3 to 4) | <0.001 * |
| Total number of lymph nodes stations | 4 (IQR, 3 to 5) | 6 (IQR, 5 to 6) | <0.001 * |
| Number of lymph nodes removed, median (IQR) | |||
| N1 lymph nodes | 4 (IQR, 2 to 6) | 4 (IQR, 2 to 7) | <0.001 * |
| N2 lymph nodes | 4 (IQR, 2 to 6) | 8 (IQR, 5 to 12) | <0.001 * |
| Total number of lymph nodes | 8 (IQR, 5 to 12) | 12 (IQR, 8 to 18) | <0.001 * |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Smoking | 1.265 | 0.897 to 1.784 | 0.181 |
| BMI | 1.030 | 0.999 to 1.063 | 0.058 |
| COPD | 1.252 | 0.863 to 1.817 | 0.237 |
| PET-CT | 3.238 | 2.315 to 4.529 | <0.001 * |
| Mediastinoscopy | 1.934 | 0.119 to 31.561 | 0.643 |
| EBUS-TBNA | 0.889 | 0.572 to 1.380 | 0.599 |
| pT | 1.292 | 1.009 to 1.653 | 0.042 * |
| Surgeon’s experience | 1.959 | 1.432 to 2.679 | <0.001 * |
| No. of surgery (quarters) | 0.647 | 0.474 to 0.884 | 0.006 * |
| Side of surgery | 0.816 | 0.083 to 8.005 | 0.862 |
| Right upper lobectomy | 1.493 | 0.153 to 14.557 | 0.730 |
| Right middle lobectomy | 0.952 | 0.094 to 9.655 | 0.967 |
| Right lower lobectomy | 1.577 | 0.159 to 15.589 | 0.697 |
| Right upper bilobectomy | 3.570 | 0.128 to 99.477 | 0.453 |
| Left upper lobectomy | 1.306 | 0.133 to 12.856 | 0.819 |
| Left lower lobectomy | 0.422 | 0.041 to 4.333 | 0.468 |
| Lobe-Specific Lymph Node Dissection | p-Value | ||
|---|---|---|---|
| Non L-SMLND (n = 1184) | L-SMLND (n = 1184) | ||
| Complications, n (%) | 255 (21.5) | 272 (23.5) | 0.258 |
| Prolonged air leak > 5 days | 102 (8.6) | 107 (9.0) | 0.717 |
| Residual air space | 39 (3.3) | 24 (2.0) | 0.055 |
| Re-drainage | 35 (3.0) | 25 (2.1) | 0.191 |
| Atrial arrythmia | 39 (3.3) | 31 (2.6) | 0.332 |
| Transfusion | 37 (3.1) | 43 (3.6) | 0.495 |
| Pneumonia | 16 (1.4) | 22 (1.9) | 0.326 |
| Bronchoscopy for atelectasis | 7 (0.6) | 14 (1.2) | 0.125 |
| Surgery for postoperative bleeding | 9 (0.8) | 12 (1.0) | 0.511 |
| Surgery for other postoperative complications | 12 (1.0) | 17 (1.4) | 0.350 |
| Delirium | 5 (0.4) | 6 (0.5) | 0.762 |
| Prolonged intubation | 5 (0.4) | 5 (0.4) | 1.000 |
| Bronchopleural fistula | 1 (0.1) | 1 (0.1) | 1.000 |
| Chylothorax | 1 (0.1) | 5 (0.4) | 0.102 |
| Recurrent laryngeal nerve palsy | 1 (0.1) | 2 (0.2) | 0.563 |
| Pulmonary embolism | 1 (0.1) | 4 (0.3) | 0.179 |
| Myocardial infarction | 1 (0.1) | 1 (0.1) | 1.000 |
| Cerebrovascular complications | 1 (0.1) | 4 (0.3) | 0.179 |
| Kidney failure | 0 | 2 (0.2) | 0.157 |
| Other complications | 22 (1.9) | 25 (2.1) | 0.658 |
| Chest tube duration, days, median (IQR) | 4 (IQR, 3 to 5) | 3 (IQR, 2 to 5) | <0.001 * |
| Hospital stay, days, median (IQR) | 6 (IQR, 4 to 7) | 6 (IQR, 4 to 7) | 0.870 |
| In-hospital death | 3 (0.3) | 7 (0.6) | 0.205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabryel, P.; Skrzypczak, P.; Roszak, M.; Campisi, A.; Zielińska, D.; Bryl, M.; Stencel, K.; Piwkowski, C. Influencing Factors on the Quality of Lymph Node Dissection for Stage IA Non-Small Cell Lung Cancer: A Retrospective Nationwide Cohort Study. Cancers 2024, 16, 346. https://doi.org/10.3390/cancers16020346
Gabryel P, Skrzypczak P, Roszak M, Campisi A, Zielińska D, Bryl M, Stencel K, Piwkowski C. Influencing Factors on the Quality of Lymph Node Dissection for Stage IA Non-Small Cell Lung Cancer: A Retrospective Nationwide Cohort Study. Cancers. 2024; 16(2):346. https://doi.org/10.3390/cancers16020346
Chicago/Turabian StyleGabryel, Piotr, Piotr Skrzypczak, Magdalena Roszak, Alessio Campisi, Dominika Zielińska, Maciej Bryl, Katarzyna Stencel, and Cezary Piwkowski. 2024. "Influencing Factors on the Quality of Lymph Node Dissection for Stage IA Non-Small Cell Lung Cancer: A Retrospective Nationwide Cohort Study" Cancers 16, no. 2: 346. https://doi.org/10.3390/cancers16020346
APA StyleGabryel, P., Skrzypczak, P., Roszak, M., Campisi, A., Zielińska, D., Bryl, M., Stencel, K., & Piwkowski, C. (2024). Influencing Factors on the Quality of Lymph Node Dissection for Stage IA Non-Small Cell Lung Cancer: A Retrospective Nationwide Cohort Study. Cancers, 16(2), 346. https://doi.org/10.3390/cancers16020346








