Association between the Preoperative Standard Uptake Value (SUV) and Survival Outcomes after Robotic-Assisted Segmentectomy for Resectable Non-Small Cell Lung Cancer (NSCLC)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patient and Methods
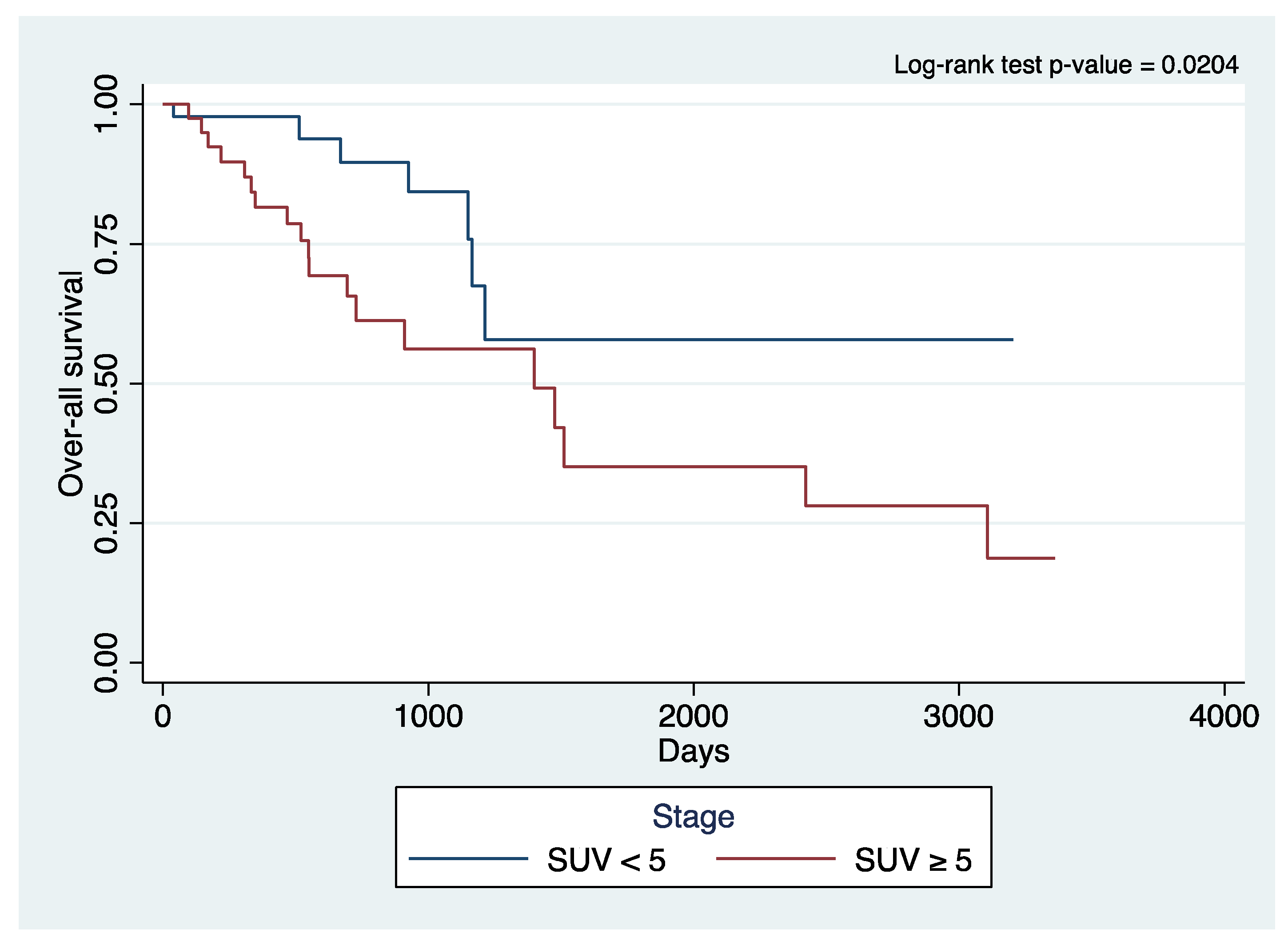
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Perroni, G.; Veronesi, G. Robotic segmentectomy: Indication and technique. J. Thorac. Dis. 2020, 12, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, S.; Zhang, R.; Jin, K.; Qian, Y.; Mao, N.; Liu, Y.; Zhang, M.; Zhang, K.; Wang, R.; et al. Identifying lung cancer patients suitable for Segmentectomy: A brief review. Front. Surg. 2021, 8, 637441. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A. Commentary: The ERS/ESTS clinical guidelines for evaluating fitness for radical treatment for lung cancer. Breathe 2009, 6, 141–145. [Google Scholar] [CrossRef]
- Charloux, A.; Quoix, E. Lung segmentectomy: Does it offer a real functional benefit over lobectomy? Eur. Respir. Rev. 2017, 26, 170079. [Google Scholar] [CrossRef] [PubMed]
- Helminen, O.; Söderström, J.; Andersen, H.; Sihvo, E. How often segmentectomy is feasible in lung cancer surgery: A population-based evaluation. Eur. J. Cardio-Thoracic Surg. 2021, 60, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Roy, P. Preoperative pulmonary evaluation for lung resection. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 296–300. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, A.; Gooding, W.E.; Santos, R.; Pettiford, B.; Ferson, P.F.; Fernando, H.C.; Urda, S.J.; Luketich, J.D.; Landreneau, R.J. Outcomes of Sublobar resection versus lobectomy for stage I non–small cell lung cancer: A 13-year analysis. Ann. Thorac. Surg. 2006, 82, 408–416. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Kamiyama, I.; Asakura, K.; Kohno, M. Thirty-day outcomes after lobectomy or segmentectomy for lung cancer surgery. Asian Cardiovasc. Thorac. Ann. 2015, 23, 828–831. [Google Scholar] [CrossRef]
- Altorki, N.K.; Wang, X.; Wigle, D.; Gu, L.; Darling, G.; Ashrafi, A.S.; Landrenau, R.; Miller, D.; Liberman, M.; Jones, D.R.; et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: Post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir. Med. 2018, 6, 915–924. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, H.; Zhao, H.; Hu, J.; Liu, L.; Liu, Y.; Li, X.; Xu, L.; Li, Y.; Lu, X.; et al. Sublobar resection is associated with better perioperative outcomes in elderly patients with clinical stage I non-small cell lung cancer: A multicenter retrospective cohort study. J. Thorac. Dis. 2019, 11, 1838–1848. [Google Scholar] [CrossRef]
- Nakamura, K.; Saji, H.; Nakajima, R.; Okada, M.; Asamura, H.; Shibata, T.; Nakamura, S.; Tada, H.; Tsuboi, M. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Ultrasound Med. Biol. 2009, 40, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Kagimoto, A.; Tsutani, Y.; Izaki, Y.; Handa, Y.; Mimae, T.; Miyata, Y.; Okada, M. Initial experience of robotic anatomical segmentectomy for non-small cell lung cancer. Ultrasound Med. Biol. 2020, 50, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, X.; Zhao, S.; Wang, J.; Zhang, W.; Sun, G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or Segmentectomy in patients with non-small cell lung cancer: A meta-analysis. BMC Cancer 2021, 21, 498. [Google Scholar] [CrossRef]
- Li, C.; Han, Y.; Han, D.; Chen, X.; Chen, K.; Cerfolio, R.J.; Li, H. Robotic approach to combined anatomic pulmonary Subsegmentectomy: Technical aspects and early results. Ann. Thorac. Surg. 2018, 107, 1480–1486. [Google Scholar] [CrossRef]
- Eguchi, T.; Miura, K.; Hamanaka, K.; Shimizu, K. Adoption of robotic core technology in minimally invasive lung Segmentectomy: Review. J. Pers. Med. 2022, 12, 1417. [Google Scholar] [CrossRef]
- Eguchi, T.; Sato, T.; Shimizu, K. Technical advances in segmentectomy for lung cancer: A minimally invasive strategy for deep, small, and impalpable tumors. Cancers 2021, 13, 3137. [Google Scholar] [CrossRef]
- Cistaro, A.; Quartuccio, N.; Mojtahedi, A.; Fania, P.; Filosso, P.L.; Campenni, A.; Ficola, U.; Baldari, S. Prediction of 2 years-survival in patients with stage I and II non-small cell lung cancer utilizing 18F-FDG PET/CT SUV quantifica. Radiol. Oncol. 2013, 47, 219–223. [Google Scholar] [CrossRef]
- Paesmans, M.; Berghmans, T.; Dusart, M.; Garcia, C.; Hossein-Foucher, C.; Lafitte, J.-J.; Mascaux, C.; Meert, A.-P.; Roelandts, M.; Scherpereel, A.; et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: Update of a systematic review and meta-analysis by the European lung cancer working party for the international association for the study of lung cancer staging project. J. Thorac. Oncol. 2010, 5, 612–619. [Google Scholar] [CrossRef]
- Mantziari, S.; Pomoni, A.; Prior, J.O.; Winiker, M.; Allemann, P.; Demartines, N.; Schäfer, M. 18f-Fdg pet/CT-derived parameters predict clinical stage and prognosis of esophageal cancer. BMC Med. Imaging 2020, 20, 7. [Google Scholar] [CrossRef]
- Hino, H.; Utsumi, T.; Maru, N.; Matsui, H.; Taniguchi, Y.; Saito, T.; Murakawa, T. Clinical impact and utility of positron emission tomography on occult lymph node metastasis and survival: Radical surgery for stage I lung cancer. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Saji, H.; Aokage, K.; Watanabe, S.-I.; Okada, M.; Mizusawa, J.; Nakajima, R.; Tsuboi, M.; Nakamura, S.; Nakamura, K.; et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J. Thorac. Cardiovasc. Surg. 2019, 158, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.J.; Wherley, E.M.; Agyabeng-Dadzie, K.; Ramsuchit, B.; Johnston, M.A.; Escalon, J.C. 500 Consecutive robotic lobectomies for non-small cell lung cancer: Perioperative and Oncologic outcomes. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2021, 16, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Chevrollier, G.S.; Nemecz, A.K.; Devin, C.; Go, K.V.; Yi, M.; Keith, S.W.; Cowan, S.W.; Evans, N.R. Early discharge does not increase readmission rates after minimally invasive anatomic lung resection. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2019, 14, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Gharagozloo, F.; Tempesta, B.; Meyer, M.; Gruessner, A. Long-term results of robotic anatomical segmentectomy for early-stage non-small-cell lung cancer. Eur. J. Cardio-Thoracic. Surg. 2018, 55, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Özyurtkan, M.O.; Kaba, E.; Ayalp, K.; Demirhan, Ö.; Uyumaz, E. Robotic anatomic lung resections: The initial experience and description of learning in 102 cases. Surg. Endosc. 2015, 30, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Terra, R.M.; Lauricella, L.L.; Haddad, R.; De-Campos, J.R.M.; Nabuco-De-Araujo, P.H.X.; Lima, C.E.T.; dos Santos, F.C.B.; Pego-Fernandes, P.M. Segmentectomia pulmonar anatômica robótica: Aspectos técnicos e desfechos. Rev. Do Colégio Bras. De Cir. 2019, 46. [Google Scholar] [CrossRef]
- Winton, T.; Livingston, R.; Johnson, D.; Rigas, J.; Johnston, M.; Butts, C.; Cormier, Y.; Goss, G.; Inculet, R.; Vallieres, E.; et al. Vinorelbine plus cisplatin vs. observation in resected non–small-cell lung cancer. N. Engl. J. Med. 2005, 352, 2589–2597. [Google Scholar] [CrossRef]
- Berghmans, T.; Dusart, M.; Paesmans, M.; Hossein-Foucher, C.; Buvat, I.; Castaigne, C.; Scherpereel, A.; Mascaux, C.; Moreau, M.; Roelandts, M.; et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): A systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J. Thorac. Oncol. 2008, 3, 6–12. [Google Scholar]
- Stiles, B.M.; Nasar, A.; Mirza, F.; Paul, S.; Lee, P.C.; Port, J.L.; McGraw, T.E.; Altorki, N.K. Ratio of Positron Emission Tomography Uptake to Tumor Size in Surgically Resected Non–Small Cell Lung Cancer. Ann. Thorac. Surg. 2013, 95, 397–404. [Google Scholar] [CrossRef]
- Blumenthaler, A.N.; Hofstetter, W.L.; Mehran, R.J.; Rajaram, R.; Rice, D.C.; Roth, J.A.; Sepesi, B.; Swisher, S.G.; Vaporciyan, A.A.; Walsh, G.L.; et al. Preoperative Maximum Standardized Uptake Value Associated with Recurrence Risk in Early Lung Cancer. Ann. Thorac. Surg. 2021, 113, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Motono, N.; Mizoguchi, T.; Ishikawa, M.; Iwai, S.; Iijima, Y.; Uramoto, H. Adaptation criterion for segmentectomy in small-sized early stage non-small cell lung cancer. Thorac. Cancer 2022, 13, 2985–2991. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, O.; Demir, F. An Investigation of the Relationship Between 18F-FDG PET/CT Parameters of Primary Tumors and Lymph Node Metastasis in Resectable Non-small Cell Lung Cancer. Curr. Radiopharm. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kamigaichi, A.; Tsutani, Y.; Mimae, T.; Miyata, Y.; Shimada, Y.; Ito, H.; Nakayama, H.; Ikeda, N.; Okada, M. Prediction of unexpected N2 disease associated with clinical T1-2N0-1M0 non–small-cell lung cancer. Clin. Lung Cancer 2020, 22, 120–126.e3. [Google Scholar] [CrossRef]
- Sun, G.; Sun, Y.; Zou, Z.; Xu, S. Analysis of segmental lymph node metastasis and clinical features in cT1N0M0 lung adenocarcinoma. BioMed Res. Int. 2020, 2020, 2842604. [Google Scholar] [CrossRef] [PubMed]
- Shiono, S.; Abiko, M.; Sato, T. Positron emission tomography/computed tomography and Lymphovascular invasion predict recurrence in stage I lung cancers. J. Thorac. Oncol. 2011, 6, 43–47. [Google Scholar] [CrossRef] [PubMed]

| Clinical Profile | Summary Statistics | |
|---|---|---|
| x ̃/n | IQR/% | |
| Age | 72 | 12 |
| Sex | ||
| Male | 41 | 40.6% |
| Female | 60 | 59.4% |
| BMI | 27.1 | 7.1 |
| Race | ||
| White | 93 | 92.1% |
| Hispanic | 2 | 2.0% |
| Black | 5 | 4.9% |
| Others | 1 | 1.0% |
| Pre-operative FEV1 | 2 | 0.745 |
| Pre-operative FEV1% | 80.5 | 31.5 |
| Co-morbidities | ||
| Hypertension | 63 | 62.4% |
| Diabetes mellitus | 16 | 15.8% |
| COPD | 42 | 41.6% |
| Congestive heart failure | 4 | 4.0% |
| ESRD | 0 | - |
| Ever-smoker | 58 | 57.4% |
| Chronic steroid use | 1 | 1.0% |
| Laterality | ||
| Left | 66 | 65.3% |
| Right | 35 | 34.7% |
| Tumor size, clinical imaging | 1.75 | 1.2 |
| Standard uptake value | ||
| SUV < 5 | 55 | 54.5% |
| SUV ≥ 5 | 46 | 45.5% |
| Pathologic Profile | Summary Statistics | |
|---|---|---|
| x ̃/n | IQR/% | |
| Tumor histology | ||
| Adenocarcinoma | 65 | 64.4% |
| Squamous | 24 | 23.8% |
| Carcinoid/Neuroendocrine | 11 | 10.9% |
| Other Lung Cancers | 1 | 0.9% |
| Tumor grade * | ||
| Well differentiated | 25 | 25.0% |
| Moderately differentiated | 50 | 50.0% |
| Poorly differentiated | 25 | 25.0% |
| Tumor size, pathologic examination | 1.8 | 1.1 |
| Lymph-vascular space invasion ** | 12 | 11.9% |
| Viscero-pleural invasion ** | 23 | 22.8% |
| Positive margins of resection | 1 | 1.0% |
| Total number of lymph nodes assessed | 10 | 7 |
| Number of mediastinal lymph nodes assessed | 5 | 4 |
| Positive lymph nodes | 10 | 9.90% |
| Pathologic stage | ||
| IA1 | 26 | 25.7% |
| IA2 | 30 | 29.7% |
| IA3 | 5 | 5.0% |
| IB | 21 | 20.8% |
| IIA | 4 | 4.0% |
| IIB | 9 | 8.9% |
| IIIA | 6 | 5.9% |
| IIIB | 0 | - |
| IIIC | 0 | - |
| IV | 0 | - |
| Surgical Profile | Summary Statistics | |
|---|---|---|
| x ̃/n | IQR/% | |
| Neoadjuvant therapy | ||
| None | 97 | 96.0% |
| Chemo/immunotherapy only | 4 | 4.0% |
| Radiotherapy only | 0 | - |
| Combination chemoradiotherapy | 0 | - |
| Operative time | 168.5 | 59 |
| Estimated blood loss | 50 | 125 |
| Intraoperative complication | 3 | 3.0% |
| Conversion to thoracotomy | 4 | 4.0% |
| Outcomes | Summary Statistics | |
|---|---|---|
| x ̃/n | IQR/% | |
| Postoperative complications * | 33 | 32.7% |
| Atrial fibrillation | 9 | 9.0% |
| Prolonged air leak | 8 | 8.0% |
| Pneumonia | 5 | 5.0% |
| Pneumothorax | 5 | 5.0% |
| Hypoxia | 5 | 5.0% |
| Empyema | 4 | 4.0% |
| Shock | 3 | 3.0% |
| Aspiration | 3 | 3.0% |
| Mucus plug | 2 | 2.0% |
| Respiratory failure | 2 | 2.0% |
| Other arrhythmia | 2 | 2.0% |
| Chyle leak | 2 | 2.0% |
| Myocardial infarction | 1 | 1.0% |
| Cardiopulmonary arrest | 1 | 1.0% |
| Hemothorax | 0 | - |
| Cerebrovascular accident | 0 | - |
| Pulmonary embolism | 0 | - |
| Length of hospital stay | 3 | 3 |
| Days with chest tube | 3 | 2 |
| Sent home with chest tube | 5 | 4.90% |
| Days at home with chest tube | 24 | 20 |
| 30-day mortality | 0 | - |
| Mortality | 25 | 24.8% |
| Time-to-mortality | 505.5 | 761 |
| Recurrence | 28 | 28.43% |
| Time-to-recurrence | 353 | 504 |
| Site of recurrence | ||
| Nodal | 4 | 13.79% ^ |
| Pleural | 2 | 6.90% |
| Local | 10 | 34.48% |
| Distant | 13 | 44.83% |
| Factor | SUV < 5 | SUV ≥ 5 | p-Value |
|---|---|---|---|
| n = 55 | n = 46 | ||
| n (%) | n (%) | ||
| Tumor histology | 0.016 | ||
| Adenocarcinoma | 42 (64.6%) | 23 (35.4%) | |
| Squamous cell | 9 (37.5%) | 15 (62.5%) | |
| Neuroendocrine | 4 (36.3%) | 8 (66.7%) | |
| Tumor grade | 0.002 | ||
| Well differentiated | 19 (76.00%) | 6 (24.00%) | |
| Moderately differentiated | 29 (58.00%) | 21 (42.00%) | |
| Poorly differentiated | 7 (28.00%) | 18 (72.00%) | |
| Pathologic stage | 0.244 | ||
| Early (stages I–II) | 53 (55.79%) | 42 (44.21%) | |
| Late (stages III–IV) | 2 (33.3%) | 4 (66.7%) | |
| Tumor size | 0.001 | ||
| T1 | 45 (67.16%) | 22 (32.84%) | |
| T2 | 9 (33.33%) | 18 (66.67%) | |
| T3 | 1 (14.29%) | 6 (85.71%0 | |
| Lymph node metastasis | 0.506 | ||
| N0 | 51 (56.04%) | 40 (43.96%) | |
| N1 + N2 | 4 (40.00%) | 6 (60.00) | |
| Lymph-vascular space invasion | 0.029 | ||
| With | 3 (25.00%) | 9 (75.00%) | |
| Without | 52 (58.43%) | 37 (41.57%) | |
| Viscero-pleural invasion | 0.008 | ||
| With | 7 (30.43%) | 16 (69.57%) | |
| Without | 48 (61.54%) | 30 (38.46%) | |
| Recurrence | <0.001 | ||
| With | 7 (25.0%) | 21 (75.0%) | |
| Without | 48 (65.8%) | 25 (34.2%) | |
| Site of recurrence | 0.047 | ||
| Nodal | 0 | 4 (100.0%) | |
| Pleural | 1 (50.0%) | 1 (50.0%) | |
| Local | 5 (55.6%) | 4 (44.4%) | |
| Distant | 1 (7.7%) | 12 (92.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboukheir Aboukheir, A.; Villanueva, E.Q., III; Garrett, J.R.; Moodie, C.C.; Tew, J.R.; Toloza, E.M.; Fontaine, J.P.; Baldonado, J.J.A.R. Association between the Preoperative Standard Uptake Value (SUV) and Survival Outcomes after Robotic-Assisted Segmentectomy for Resectable Non-Small Cell Lung Cancer (NSCLC). Cancers 2023, 15, 5379. https://doi.org/10.3390/cancers15225379
Aboukheir Aboukheir A, Villanueva EQ III, Garrett JR, Moodie CC, Tew JR, Toloza EM, Fontaine JP, Baldonado JJAR. Association between the Preoperative Standard Uptake Value (SUV) and Survival Outcomes after Robotic-Assisted Segmentectomy for Resectable Non-Small Cell Lung Cancer (NSCLC). Cancers. 2023; 15(22):5379. https://doi.org/10.3390/cancers15225379
Chicago/Turabian StyleAboukheir Aboukheir, Aihab, Emilio Q. Villanueva, III, Joseph R. Garrett, Carla C. Moodie, Jenna R. Tew, Eric M. Toloza, Jacques P. Fontaine, and Jobelle J. A. R. Baldonado. 2023. "Association between the Preoperative Standard Uptake Value (SUV) and Survival Outcomes after Robotic-Assisted Segmentectomy for Resectable Non-Small Cell Lung Cancer (NSCLC)" Cancers 15, no. 22: 5379. https://doi.org/10.3390/cancers15225379
APA StyleAboukheir Aboukheir, A., Villanueva, E. Q., III, Garrett, J. R., Moodie, C. C., Tew, J. R., Toloza, E. M., Fontaine, J. P., & Baldonado, J. J. A. R. (2023). Association between the Preoperative Standard Uptake Value (SUV) and Survival Outcomes after Robotic-Assisted Segmentectomy for Resectable Non-Small Cell Lung Cancer (NSCLC). Cancers, 15(22), 5379. https://doi.org/10.3390/cancers15225379






