Myeloproliferative Neoplasms: Diseases Mediated by Chronic Activation of Signal Transducer and Activator of Transcription (STAT) Proteins
Abstract
Simple Summary
Abstract
1. Introduction
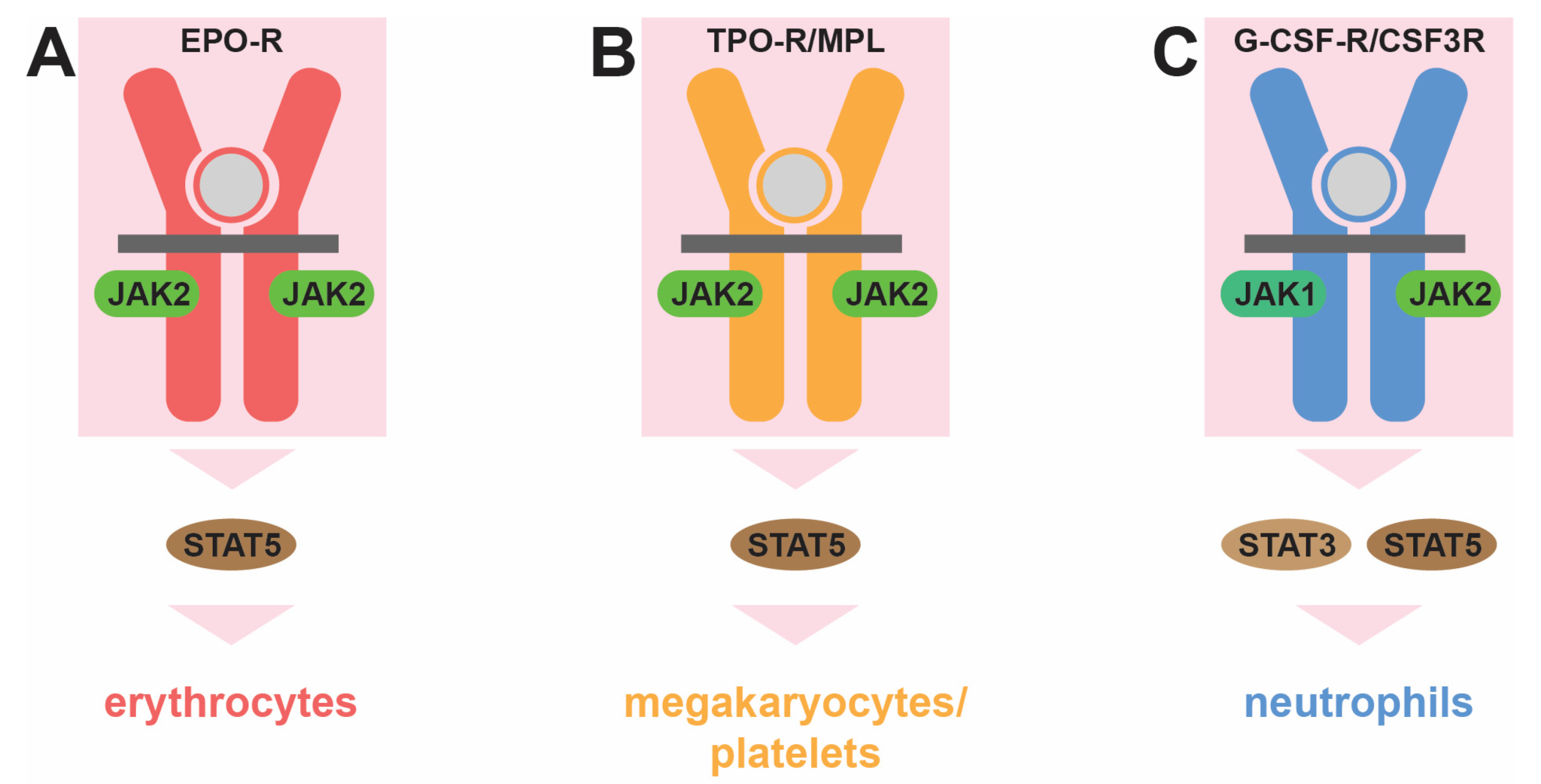
2. Normal Hematopoiesis and Its Control
3. Underlying Genetic Causes of MPNs
3.1. BCR::ABL1
3.2. JAK2
3.3. MPL
3.4. CALR
3.5. CSF3R
3.6. Other Mutations/Variants
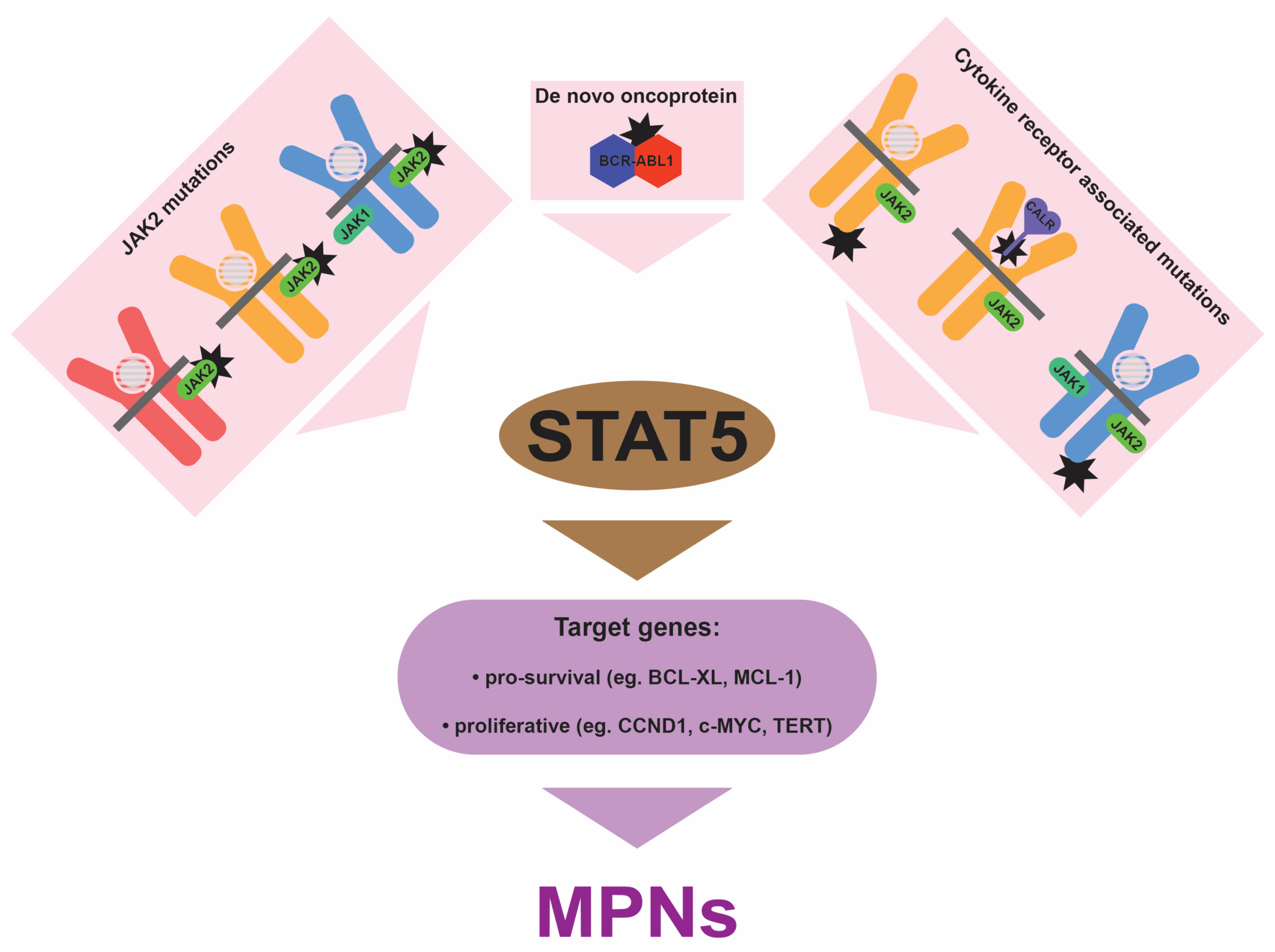
4. Central Role for STAT5
5. Implications for the Clinic
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Mughal, T.I.; Barbui, T.; Abdel-Wahab, O.; Kralovics, R.; Jamieson, C.; Kvasnicka, H.M.; Mullaly, A.; Rampal, R.; Mesa, R.; Kiladjian, J.J.; et al. Novel insights into the biology and treatment of chronic myeloproliferative neoplasms. Leuk. Lymphoma 2015, 56, 1938–1948. [Google Scholar] [CrossRef]
- Osman, A.E.G.; Deininger, M.W. Chronic myeloid leukemia: Modern therapies, current challenges and future directions. Blood Rev. 2021, 49, 100825. [Google Scholar] [CrossRef]
- Lin, Q.; Mao, L.; Shao, L.; Zhu, L.; Han, Q.; Zhu, H.; Jin, J.; You, L. Global, regional, and national burden of chronic myeloid leukemia, 1990-2017: A systematic analysis for the Global Burden of Disease study 2017. Front. Oncol. 2020, 10, 580759. [Google Scholar] [CrossRef]
- McMullin, M.F.; Anderson, L.A. Aetiology of myeloproliferative meoplasms. Cancers 2020, 12, 1810. [Google Scholar] [CrossRef]
- Baumeister, J.; Chatain, N.; Sofias, A.M.; Lammers, T.; Koschmieder, S. Progression of myeloproliferative neoplasms (MPN): Diagnostic and therapeutic perspectives. Cells 2021, 10, 3551. [Google Scholar] [CrossRef]
- Marchetti, M.; Ghirardi, A.; Masciulli, A.; Carobbio, A.; Palandri, F.; Vianelli, N.; Rossi, E.; Betti, S.; Di Veroli, A.; Iurlo, A.; et al. Second cancers in MPN: Survival analysis from an international study. Am. J. Hematol. 2020, 95, 295–301. [Google Scholar] [CrossRef]
- Edginton-White, B.; Bonifer, C. The transcriptional regulation of normal and malignant blood cell development. FEBS J. 2022, 289, 1240–1255. [Google Scholar] [CrossRef]
- Barbarani, G.; Fugazza, C.; Strouboulis, J.; Ronchi, A.E. The pleiotropic effects of GATA1 and KLF1 in physiological erythropoiesis and in dyserythropoietic disorders. Front. Physiol. 2019, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.D. C/EBPalpha in normal and malignant myelopoiesis. Int. J. Hematol. 2015, 101, 330–341. [Google Scholar] [CrossRef]
- Li, G.; Hao, W.; Hu, W. Transcription factor PU.1 and immune cell differentiation. Int. J. Mol. Med. 2020, 46, 1943–1950. [Google Scholar] [CrossRef]
- Stockley, J.; Morgan, N.V.; Bem, D.; Lowe, G.C.; Lordkipanidze, M.; Dawood, B.; Simpson, M.A.; Macfarlane, K.; Horner, K.; Leo, V.C.; et al. Enrichment of FLI1 and RUNX1 mutations in families with excessive bleeding and platelet dense granule secretion defects. Blood 2013, 122, 4090–4093. [Google Scholar] [CrossRef]
- Marin-Quilez, A.; Garcia-Tunon, I.; Fernandez-Infante, C.; Hernandez-Cano, L.; Palma-Barqueros, V.; Vuelta, E.; Sanchez-Martin, M.; Gonzalez-Porras, J.R.; Guerrero, C.; Benito, R.; et al. Characterization of the platelet phenotype caused by a germline RUNX1 variant in a CRISPR/Cas9-generated murine model. Thromb. Haemost. 2021, 121, 1193–1205. [Google Scholar] [CrossRef]
- Palii, C.G.; Cheng, Q.; Gillespie, M.A.; Shannon, P.; Mazurczyk, M.; Napolitani, G.; Price, N.D.; Ranish, J.A.; Morrissey, E.; Higgs, D.R.; et al. Single-cell proteomics reveal that quantitative changes in co-expressed lineage-specific transcription factors determine cell fate. Cell Stem Cell 2019, 24, 812–820.e815. [Google Scholar] [CrossRef]
- Caulier, A.L.; Sankaran, V.G. Molecular and cellular mechanisms that regulate human erythropoiesis. Blood 2022, 139, 2450–2459. [Google Scholar] [CrossRef]
- Hitchcock, I.S.; Hafer, M.; Sangkhae, V.; Tucker, J.A. The thrombopoietin receptor: Revisiting the master regulator of platelet production. Platelets 2021, 32, 770–778. [Google Scholar] [CrossRef]
- Liongue, C.; Wright, C.; Russell, A.P.; Ward, A.C. Granulocyte colony-stimulating factor receptor: Stimulating granulopoiesis and much more. Int. J. Biochem. Cell. Biol. 2009, 41, 2372–2375. [Google Scholar] [CrossRef]
- Awasthi, N.; Liongue, C.; Ward, A.C. STAT proteins: A kaleidoscope of canonical and non-canonical functions in immunity and cancer. J. Hematol. Oncol. 2021, 14, 198. [Google Scholar] [CrossRef]
- Kautz, L.; Nemeth, E. Molecular liaisons between erythropoiesis and iron metabolism. Blood 2014, 124, 479–482. [Google Scholar] [CrossRef]
- Shih, H.M.; Wu, C.J.; Lin, S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Grozovsky, R.; Begonja, A.J.; Liu, K.; Visner, G.; Hartwig, J.H.; Falet, H.; Hoffmeister, K.M. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat. Med. 2015, 21, 47–54. [Google Scholar] [CrossRef]
- Pedersen, C.C.; Borup, R.; Fischer-Nielsen, A.; Mora-Jensen, H.; Fossum, A.; Cowland, J.B.; Borregaard, N. Changes in gene expression during G-CSF-induced emergency granulopoiesis in humans. J. Immunol. 2016, 197, 1989–1999. [Google Scholar] [CrossRef]
- Sobah, M.L.; Liongue, C.; Ward, A.C. SOCS proteins in immunity, inflammatory diseases and immune-related cancer. Front. Med. 2021, 8, 727987. [Google Scholar] [CrossRef]
- Cross, N.C.; Daley, G.Q.; Green, A.R.; Hughes, T.P.; Jamieson, C.; Manley, P.; Mughal, T.; Perrotti, D.; Radich, J.; Skoda, R.; et al. BCR-ABL1-positive CML and BCR-ABL1-negative chronic myeloproliferative disorders: Some common and contrasting features. Leukemia 2008, 22, 1975–1989. [Google Scholar] [CrossRef][Green Version]
- Maxson, J.E.; Tyner, J.W. Genomics of chronic neutrophilic leukemia. Blood 2017, 129, 715–722. [Google Scholar] [CrossRef]
- Kjaer, L. Clonal hematopoiesis and mutations of myeloproliferative neoplasms. Cancers 2020, 12, 2100. [Google Scholar] [CrossRef]
- Bellanne-Chantelot, C.; Rabadan Moraes, G.; Schmaltz-Panneau, B.; Marty, C.; Vainchenker, W.; Plo, I. Germline genetic factors in the pathogenesis of myeloproliferative neoplasms. Blood Rev. 2020, 42, 100710. [Google Scholar] [CrossRef]
- Luque Paz, D.; Kralovics, R.; Skoda, R.C. Genetic basis and molecular profiling in myeloproliferative neoplasms. Blood 2023, 141, 1909–1921. [Google Scholar] [CrossRef]
- Nangalia, J.; Green, A.R. Myeloproliferative neoplasms: From origins to outcomes. Blood 2017, 130, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, G.; McMullin, M.F.; Mills, K. Molecular pathogenesis of the myeloproliferative neoplasms. J. Hematol. Oncol. 2021, 14, 103. [Google Scholar] [CrossRef]
- Quintas-Cardama, A.; Cortes, J. Molecular biology of BCR-ABL1-positive chronic myeloid leukemia. Blood 2009, 113, 1619–1630. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2016, 91, 252–265. [Google Scholar] [CrossRef]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.P.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef]
- Scott, L.M.; Tong, W.; Levine, R.L.; Scott, M.A.; Beer, P.A.; Stratton, M.R.; Futreal, P.A.; Erber, W.N.; McMullin, M.F.; Harrison, C.N.; et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N. Engl. J. Med. 2007, 356, 459–468. [Google Scholar] [CrossRef]
- Passamonti, F.; Elena, C.; Schnittger, S.; Skoda, R.C.; Green, A.R.; Girodon, F.; Kiladjian, J.J.; McMullin, M.F.; Ruggeri, M.; Besses, C.; et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011, 117, 2813–2816. [Google Scholar] [CrossRef]
- Wilmes, S.; Hafer, M.; Vuorio, J.; Tucker, J.A.; Winkelmann, H.; Lochte, S.; Stanly, T.A.; Pulgar Prieto, K.D.; Poojari, C.; Sharma, V.; et al. Mechanism of homodimeric cytokine receptor activation and dysregulation by oncogenic mutations. Science 2020, 367, 643–652. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. Updates in the management of polycythemia vera and essential thrombocythemia. Ther. Adv. Hematol. 2019, 10, 2040620719870052. [Google Scholar] [CrossRef]
- Ding, J.; Komatsu, H.; Wakita, A.; Kato-Uranishi, M.; Ito, M.; Satoh, A.; Tsuboi, K.; Nitta, M.; Miyazaki, H.; Iida, S.; et al. Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood 2004, 103, 4198–4200. [Google Scholar] [CrossRef]
- Plo, I.; Bellanne-Chantelot, C.; Mosca, M.; Mazzi, S.; Marty, C.; Vainchenker, W. Genetic alterations of the thrombopoietin/MPL/JAK2 axis impacting megakaryopoiesis. Front. Endocrinol. 2017, 8, 234. [Google Scholar] [CrossRef]
- Pecquet, C.; Chachoua, I.; Roy, A.; Balligand, T.; Vertenoeil, G.; Leroy, E.; Albu, R.I.; Defour, J.P.; Nivarthi, H.; Hug, E.; et al. Calreticulin mutants as oncogenic rogue chaperones for TpoR and traffic-defective pathogenic TpoR mutants. Blood 2019, 133, 2669–2681. [Google Scholar] [CrossRef]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, C.; Ma, X.; Guan, M. Clinical relevance between CALR mutation and myeloproliferative neoplasms. Stem Cell Investig. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Liongue, C.; Ward, A.C. Granulocyte colony-stimulating factor receptor mutations in myeloid malignancy. Front. Oncol. 2014, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Maxson, J.E.; Luty, S.B.; MacManiman, J.D.; Abel, M.L.; Druker, B.J.; Tyner, J.W. Ligand independence of the T618I mutation in the colony-stimulating factor 3 receptor (CSF3R) protein results from loss of O-linked glycosylation and increased receptor dimerization. J. Biol. Chem. 2014, 289, 5820–5827. [Google Scholar] [CrossRef]
- Yin, B.; Chen, X.; Gao, F.; Li, J.; Wang, H.W. Analysis of gene mutation characteristics in patients with chronic neutrophilic leukaemia. Hematology 2019, 24, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Plo, I.; Zhang, Y.; Le Couedic, J.P.; Nakatake, M.; Boulet, J.M.; Itaya, M.; Smith, S.O.; Debili, N.; Constantinescu, S.N.; Vainchenker, W.; et al. An activating mutation in the CSF3R gene induces a hereditary chronic neutrophilia. J. Exp. Med. 2009, 206, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Druhan, L.J.; McMahon, D.P.; Steuerwald, N.; Price, A.E.; Lance, A.; Gerber, J.M.; Avalos, B.R. Chronic neutrophilic leukemia in a child with a CSF3R T618I germ line mutation. Blood 2016, 128, 2097–2099. [Google Scholar] [CrossRef]
- Lee, J.; Godfrey, A.L.; Nangalia, J. Genomic heterogeneity in myeloproliferative neoplasms and applications to clinical practice. Blood Rev. 2020, 42, 100708. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Kappe, S.H.; Ploss, A.; Mikolajczak, S.A. Development of humanized mouse models to study human malaria parasite infection. Future Microbiol. 2012, 7, 657–665. [Google Scholar] [CrossRef]
- Pasquier, F.; Marty, C.; Balligand, T.; Verdier, F.; Grosjean, S.; Gryshkova, V.; Raslova, H.; Constantinescu, S.N.; Casadevall, N.; Vainchenker, W.; et al. New pathogenic mechanisms induced by germline erythropoietin receptor mutations in primary erythrocytosis. Haematologica 2018, 103, 575–586. [Google Scholar] [CrossRef]
- Zmajkovic, J.; Lundberg, P.; Nienhold, R.; Torgersen, M.L.; Sundan, A.; Waage, A.; Skoda, R.C. A gain-of-function mutation in EPO in familial erythrocytosis. N. Engl. J. Med. 2018, 378, 924–930. [Google Scholar] [CrossRef]
- Maurer, B.; Kollmann, S.; Pickem, J.; Hoelbl-Kovacic, A.; Sexl, V. STAT5A and STAT5B-twins with different personalities in hematopoiesis and leukemia. Cancers 2019, 11, 1726. [Google Scholar] [CrossRef]
- Cross, N.C.P.; Hoade, Y.; Tapper, W.J.; Carreno-Tarragona, G.; Fanelli, T.; Jawhar, M.; Naumann, N.; Pieniak, I.; Lubke, J.; Ali, S.; et al. Recurrent activating STAT5B N642H mutation in myeloid neoplasms with eosinophilia. Leukemia 2019, 33, 415–425. [Google Scholar] [CrossRef]
- Luo, Q.; Shen, J.; Yang, Y.; Tang, H.; Shi, M.; Liu, J.; Liu, Z.; Shi, X.; Yi, Y. CSF3R T618I, ASXL1 G942 fs and STAT5B N642H trimutation co-contribute to a rare chronic neutrophilic leukaemia manifested by rapidly progressive leucocytosis, severe infections, persistent fever and deep venous thrombosis. Br. J. Haematol. 2018, 180, 892–894. [Google Scholar] [CrossRef]
- Pardanani, A.; Fridley, B.L.; Lasho, T.L.; Gilliland, D.G.; Tefferi, A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood 2008, 111, 2785–2789. [Google Scholar] [CrossRef]
- Jones, A.V.; Campbell, P.J.; Beer, P.A.; Schnittger, S.; Vannucchi, A.M.; Zoi, K.; Percy, M.J.; McMullin, M.F.; Scott, L.M.; Tapper, W.; et al. The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood 2010, 115, 4517–4523. [Google Scholar] [CrossRef]
- Lesteven, E.; Picque, M.; Conejero Tonetti, C.; Giraudier, S.; Varin-Blank, N.; Velazquez, L.; Kiladjian, J.J.; Cassinat, B.; Baran-Marszak, F. Association of a single-nucleotide polymorphism in the SH2B3 gene with JAK2V617F-positive myeloproliferative neoplasms. Blood 2014, 123, 794–796. [Google Scholar] [CrossRef][Green Version]
- Rabadan Moraes, G.; Pasquier, F.; Marzac, C.; Deconinck, E.; Damanti, C.C.; Leroy, G.; El-Khoury, M.; El Nemer, W.; Kiladjian, J.J.; Raslova, H.; et al. An inherited gain-of-function risk allele in EPOR predisposes to familial JAK2(V617F) myeloproliferative neoplasms. Br. J. Haematol. 2022, 198, 131–136. [Google Scholar] [CrossRef]
- Turakhia, S.K.; Murugesan, G.; Cotta, C.V.; Theil, K.S. Thrombocytosis and STAT5 activation in chronic myelogenous leukaemia are not associated with JAK2 V617F or calreticulin mutations. J. Clin. Pathol. 2016, 69, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska-Chudy, E.; Szylberg, L.; Dworacki, G.; Mizera-Nyczak, E.; Marszalek, A. pSTAT5 and ERK exhibit different expression in myeloproliferative neoplasms. Oncol. Rep. 2017, 37, 2295–2307. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.A.; Campbell, P.J.; Scott, L.M.; Bench, A.J.; Erber, W.N.; Bareford, D.; Wilkins, B.S.; Reilly, J.T.; Hasselbalch, H.C.; Bowman, R.; et al. MPL mutations in myeloproliferative disorders: Analysis of the PT-1 cohort. Blood 2008, 112, 141–149. [Google Scholar] [CrossRef]
- Zhang, H.; Coblentz, C.; Watanabe-Smith, K.; Means, S.; Means, J.; Maxson, J.E.; Tyner, J.W. Gain-of-function mutations in granulocyte colony-stimulating factor receptor (CSF3R) reveal distinct mechanisms of CSF3R activation. J. Biol. Chem. 2018, 293, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- Sakr, H.; Clark Schneider, K.; Murugesan, G.; Bodo, J.; Hsi, E.D.; Cook, J.R. pSTAT3/pSTAT5 signaling patterns in molecularly defined subsets of myeloproliferative neoplasms. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 147–152. [Google Scholar] [CrossRef]
- Weber, A.; Borghouts, C.; Brendel, C.; Moriggl, R.; Delis, N.; Brill, B.; Vafaizadeh, V.; Groner, B. Stat5 exerts distinct, vital functions in the cytoplasm and nucleus of Bcr-Abl+ K562 and Jak2(V617F)+ HEL leukemia Cells. Cancers 2015, 7, 503–537. [Google Scholar] [CrossRef] [PubMed]
- Walz, C.; Ahmed, W.; Lazarides, K.; Betancur, M.; Patel, N.; Hennighausen, L.; Zaleskas, V.M.; Van Etten, R.A. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and JAK2(V617F) in mice. Blood 2012, 119, 3550–3560. [Google Scholar] [CrossRef]
- Yan, D.; Hutchison, R.E.; Mohi, G. Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood 2012, 119, 3539–3549. [Google Scholar] [CrossRef]
- Grisouard, J.; Shimizu, T.; Duek, A.; Kubovcakova, L.; Hao-Shen, H.; Dirnhofer, S.; Skoda, R.C. Deletion of Stat3 in hematopoietic cells enhances thrombocytosis and shortens survival in a JAK2-V617F mouse model of MPN. Blood 2015, 125, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Duek, A.; Lundberg, P.; Shimizu, T.; Grisouard, J.; Karow, A.; Kubovcakova, L.; Hao-Shen, H.; Dirnhofer, S.; Skoda, R.C. Loss of Stat1 decreases megakaryopoiesis and favors erythropoiesis in a JAK2-V617F-driven mouse model of MPNs. Blood 2014, 123, 3943–3950. [Google Scholar] [CrossRef]
- Kollmann, S.; Grundschober, E.; Maurer, B.; Warsch, W.; Grausenburger, R.; Edlinger, L.; Huuhtanen, J.; Lagger, S.; Hennighausen, L.; Valent, P.; et al. Twins with different personalities: STAT5B-but not STAT5A-has a key role in BCR/ABL-induced leukemia. Leukemia 2019, 33, 1583–1597. [Google Scholar] [CrossRef]
- Belizaire, R.; Koochaki, S.H.J.; Udeshi, N.D.; Vedder, A.; Sun, L.; Svinkina, T.; Hartigan, C.; McConkey, M.; Kovalcik, V.; Bizuayehu, A.; et al. CBL mutations drive PI3K/AKT signaling via increased interaction with LYN and PIK3R1. Blood 2021, 137, 2209–2220. [Google Scholar] [CrossRef]
- Grand, F.H.; Hidalgo-Curtis, C.E.; Ernst, T.; Zoi, K.; Zoi, C.; McGuire, C.; Kreil, S.; Jones, A.; Score, J.; Metzgeroth, G.; et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood 2009, 113, 6182–6192. [Google Scholar] [CrossRef]
- Maslah, N.; Cassinat, B.; Verger, E.; Kiladjian, J.J.; Velazquez, L. The role of LNK/SH2B3 genetic alterations in myeloproliferative neoplasms and other hematological disorders. Leukemia 2017, 31, 1661–1670. [Google Scholar] [CrossRef]
- Mandal, M.; Powers, S.E.; Maienschein-Cline, M.; Bartom, E.T.; Hamel, K.M.; Kee, B.L.; Dinner, A.R.; Clark, M.R. Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nat. Immunol. 2011, 12, 1212–1220. [Google Scholar] [CrossRef]
- Yoo, K.H.; Oh, S.; Kang, K.; Hensel, T.; Robinson, G.W.; Hennighausen, L. Loss of EZH2 results in precocious mammary gland development and activation of STAT5-dependent genes. Nucleic Acids Res. 2015, 43, 8774–8789. [Google Scholar] [CrossRef]
- Goyal, H.; Chachoua, I.; Pecquet, C.; Vainchenker, W.; Constantinescu, S.N. A p53-JAK-STAT connection involved in myeloproliferative neoplasm pathogenesis and progression to secondary acute myeloid leukemia. Blood Rev. 2020, 42, 100712. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.C.; Fan, A.; Ward, A.C.; Liongue, C.; Lewis, R.S.; Cheng, S.H.; Chan, P.K.; Yip, S.F.; Liang, R.; Leung, A.Y. A novel zebrafish jak2a(V581F) model shared features of human JAK2(V617F) polycythemia vera. Exp. Hematol. 2009, 37, 1379–1386. [Google Scholar] [CrossRef]
- Lewis, R.S.; Stephenson, S.E.M.; Ward, A.C. Constitutive activation of zebrafish Stat5 expands hematopoietic cell populations in vivo. Exp. Hematol. 2006, 34, 179–187. [Google Scholar] [CrossRef]
- Harrison, D.A.; Binari, R.; Nahreini, T.S.; Gilman, M.; Perrimon, N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995, 14, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.S.; Melnick, M.B.; Perrimon, N. Marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell 1996, 84, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.O.L.; Cramer, S.D.; Winer, H.Y.; Hixon, J.A.; Li, W.; Yunes, J.A.; Durum, S.K. Mutations that collaborate with IL-7Ra signaling pathways to drive ALL. Adv. Biol. Regul. 2021, 80, 100788. [Google Scholar] [CrossRef]
- Teramo, A.; Barila, G.; Calabretto, G.; Vicenzetto, C.; Gasparini, V.R.; Semenzato, G.; Zambello, R. Insights into genetic landscape of large granular lymphocyte leukemia. Front. Oncol. 2020, 10, 152. [Google Scholar] [CrossRef]
- Andersson, E.I.; Bruck, O.; Braun, T.; Mannisto, S.; Saikko, L.; Lagstrom, S.; Ellonen, P.; Leppa, S.; Herling, M.; Kovanen, P.E.; et al. STAT3 mutation is associated with STAT3 activation in CD30+ ALK− ALCL. Cancers 2020, 12, 702. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Vannucchi, A.M. Current management strategies for polycythemia vera and essential thrombocythemia. Blood Rev. 2020, 42, 100714. [Google Scholar] [CrossRef]
- Ge, H.; Wang, C.; Tian, C.; Diao, Y.; Wang, W.; Ma, X.; Zhang, J.; Li, H.; Zhao, Z.; Zhu, L. Efficacy of WWQ-131, a highly selective JAK2 inhibitor, in mouse models of myeloproliferative neoplasms. Biomed. Pharmacother. 2022, 156, 113884. [Google Scholar] [CrossRef]
- Wang, X.; Haylock, D.; Hu, C.S.; Kowalczyk, W.; Jiang, T.; Qiu, J.; Mosoyan, G.; He, W.; Marshall, N.; Mascarenhas, J.; et al. A thrombopoietin receptor antagonist is capable of depleting myelofibrosis hematopoietic stem and progenitor cells. Blood 2016, 127, 3398–3409. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Wingelhofer, B.; Maurer, B.; Heyes, E.C.; Cumaraswamy, A.A.; Berger-Becvar, A.; de Araujo, E.D.; Orlova, A.; Freund, P.; Ruge, F.; Park, J.; et al. Pharmacologic inhibition of STAT5 in acute myeloid leukemia. Leukemia 2018, 32, 1135–1146. [Google Scholar] [CrossRef]
- Hadzijusufovic, E.; Keller, A.; Berger, D.; Greiner, G.; Wingelhofer, B.; Witzeneder, N.; Ivanov, D.; Pecnard, E.; Nivarthi, H.; Schur, F.K.M.; et al. STAT5 is expressed in CD34+/CD38− stem cells and serves as a potential molecular target in Ph-negative myeloproliferative neoplasms. Cancers 2020, 12, 1021. [Google Scholar] [CrossRef]
- Juen, L.; Brachet-Botineau, M.; Parmenon, C.; Bourgeais, J.; Herault, O.; Gouilleux, F.; Viaud-Massuard, M.C.; Prie, G. New inhibitor targeting signal transducer and activator of transcription 5 (STAT5) signaling in myeloid leukemias. J. Med. Chem. 2017, 60, 6119–6136. [Google Scholar] [CrossRef]
- Liao, Z.; Gu, L.; Vergalli, J.; Mariani, S.A.; De Dominici, M.; Lokareddy, R.K.; Dagvadorj, A.; Purushottamachar, P.; McCue, P.A.; Trabulsi, E.; et al. Structure-based screen identifies a potent small molecule inhibitor of Stat5a/b with therapeutic potential for prostate cancer and chronic myeloid leukemia. Mol. Cancer Ther. 2015, 14, 1777–1793. [Google Scholar] [CrossRef]
- Elumalai, N.; Berg, A.; Rubner, S.; Blechschmidt, L.; Song, C.; Natarajan, K.; Matysik, J.; Berg, T. Rational development of Stafib-2: A selective, nanomolar inhibitor of the transcription factor STAT5b. Sci. Rep. 2017, 7, 819. [Google Scholar] [CrossRef] [PubMed]
- Mencalha, A.L.; Du Rocher, B.; Salles, D.; Binato, R.; Abdelhay, E. LLL-3, a STAT3 inhibitor, represses BCR-ABL-positive cell proliferation, activates apoptosis and improves the effects of Imatinib mesylate. Cancer Chemother. Pharmacol. 2010, 65, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, J.; Shi, M.; Zhao, S.; Bai, W.; Cao, W.; Tu, Z.; Huang, Z.; Feng, W. Targeted blockage of signal transducer and activator of transcription 5 signaling pathway with decoy oligodeoxynucleotides suppresses leukemic K562 cell growth. DNA Cell Biol. 2011, 30, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Turkson, J.; Zhang, S.; Mora, L.B.; Burns, A.; Sebti, S.; Jove, R. A novel platinum compound inhibits constitutive Stat3 signaling and induces cell cycle arrest and apoptosis of malignant cells. J. Biol. Chem. 2005, 280, 32979–32988. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, K.; Zhang, S.; Guida, W.C.; Blaskovich, M.A.; Greedy, B.; Lawrence, H.R.; Yip, M.L.; Jove, R.; McLaughlin, M.M.; Lawrence, N.J.; et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc. Natl. Acad. Sci. USA 2007, 104, 7391–7396. [Google Scholar] [CrossRef] [PubMed]
- Bartalucci, N.; Calabresi, L.; Balliu, M.; Martinelli, S.; Rossi, M.C.; Villeval, J.L.; Annunziato, F.; Guglielmelli, P.; Vannucchi, A.M. Inhibitors of the PI3K/mTOR pathway prevent STAT5 phosphorylation in JAK2V617F mutated cells through PP2A/CIP2A axis. Oncotarget 2017, 8, 96710–96724. [Google Scholar] [CrossRef]
- Fenerich, B.A.; Fernandes, J.C.; Rodrigues Alves, A.P.N.; Coelho-Silva, J.L.; Scopim-Ribeiro, R.; Scheucher, P.S.; Eide, C.A.; Tognon, C.E.; Druker, B.J.; Rego, E.M.; et al. NT157 has antineoplastic effects and inhibits IRS1/2 and STAT3/5 in JAK2(V617F)-positive myeloproliferative neoplasm cells. Signal Transduct. Target. Ther. 2020, 5, 5. [Google Scholar] [CrossRef]
- Austin, R.J.; Straube, J.; Bruedigam, C.; Pali, G.; Jacquelin, S.; Vu, T.; Green, J.; Grasel, J.; Lansink, L.; Cooper, L.; et al. Distinct effects of ruxolitinib and interferon-alpha on murine JAK2V617F myeloproliferative neoplasm hematopoietic stem cell populations. Leukemia 2020, 34, 1075–1089. [Google Scholar] [CrossRef]

| MPN Category | Genetic Change | Frequency |
|---|---|---|
| CML | BCR::ABL1 | >95% |
| PV | JAK2 GOF | ~98% |
| ET | JAK2 GOF | 55% |
| MPL GOF | 5–7% | |
| CALR GOF | 25–30% | |
| PMF | JAK2 GOF | 60% |
| MPL GOF | 7–10% | |
| CALR GOF | 20–30% | |
| Hereditary thrombocytosis | THPO GOF | nd |
| JAK2 GOF | nd | |
| MPL GOF | nd | |
| Hereditary erythrocytosis | EPOR GOF | nd |
| EPO GOF | nd | |
| VHL LOF | nd | |
| CNL | CSF3R GOF | >80% |
| Hypereosinophilic syndrome | STAT5B GOF | nd |
| Disease | Treatment | Effect | Application |
|---|---|---|---|
| CML | imatinib, dasatinib, nilotinib, bosutinib, ponatinib | ABL1-selective inhibitors | 1st line therapy |
| PV | hydroxyurea, phlebotomy | cytoreductive | 1st line therapy |
| aspirin (low dose) | antithrombotic | 1st line therapy | |
| ruxolitinib | JAK2-selective inhibitors | 2nd line therapy | |
| MF | ruxolitinib, fedratinib, pacritinib | JAK2-selective inhibitors | 1st line therapy |
| Various MPNs | interferon α | antiproliferative, pro-apoptotic | alternative therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liongue, C.; Ward, A.C. Myeloproliferative Neoplasms: Diseases Mediated by Chronic Activation of Signal Transducer and Activator of Transcription (STAT) Proteins. Cancers 2024, 16, 313. https://doi.org/10.3390/cancers16020313
Liongue C, Ward AC. Myeloproliferative Neoplasms: Diseases Mediated by Chronic Activation of Signal Transducer and Activator of Transcription (STAT) Proteins. Cancers. 2024; 16(2):313. https://doi.org/10.3390/cancers16020313
Chicago/Turabian StyleLiongue, Clifford, and Alister C. Ward. 2024. "Myeloproliferative Neoplasms: Diseases Mediated by Chronic Activation of Signal Transducer and Activator of Transcription (STAT) Proteins" Cancers 16, no. 2: 313. https://doi.org/10.3390/cancers16020313
APA StyleLiongue, C., & Ward, A. C. (2024). Myeloproliferative Neoplasms: Diseases Mediated by Chronic Activation of Signal Transducer and Activator of Transcription (STAT) Proteins. Cancers, 16(2), 313. https://doi.org/10.3390/cancers16020313







