Integrated Diagnostics of Thyroid Nodules
Abstract
Simple Summary
Abstract
1. Introduction
- Initial assessment of patients with thyroid nodules should be based on clinical history, clinical examination, and measurement of TSH level.
- Thyroid US and its scoring systems are important for the risk stratification of thyroid nodules.
- Thyroid scintigraphy is generally performed in patients with low to low-normal TSH value and nodules >1 cm in size.
- FNAC is performed for non-autonomous thyroid nodules, according to US scoring systems.
- [99mTc]Tc-MIBI and [18F]FDG PET/CT are recommended in cytologically indeterminate thyroid nodules to reduce “diagnostic” surgeries.
- The clinical value of AI in the evaluation of thyroid nodules needs to be determined.
2. Clinical History and Clinical Examination
2.1. Clinical History
2.2. Clinical Examination
3. Thyroid Laboratory, Imaging, and Cytopathology
3.1. Laboratory Medicine
3.2. Thyroid Ultrasound
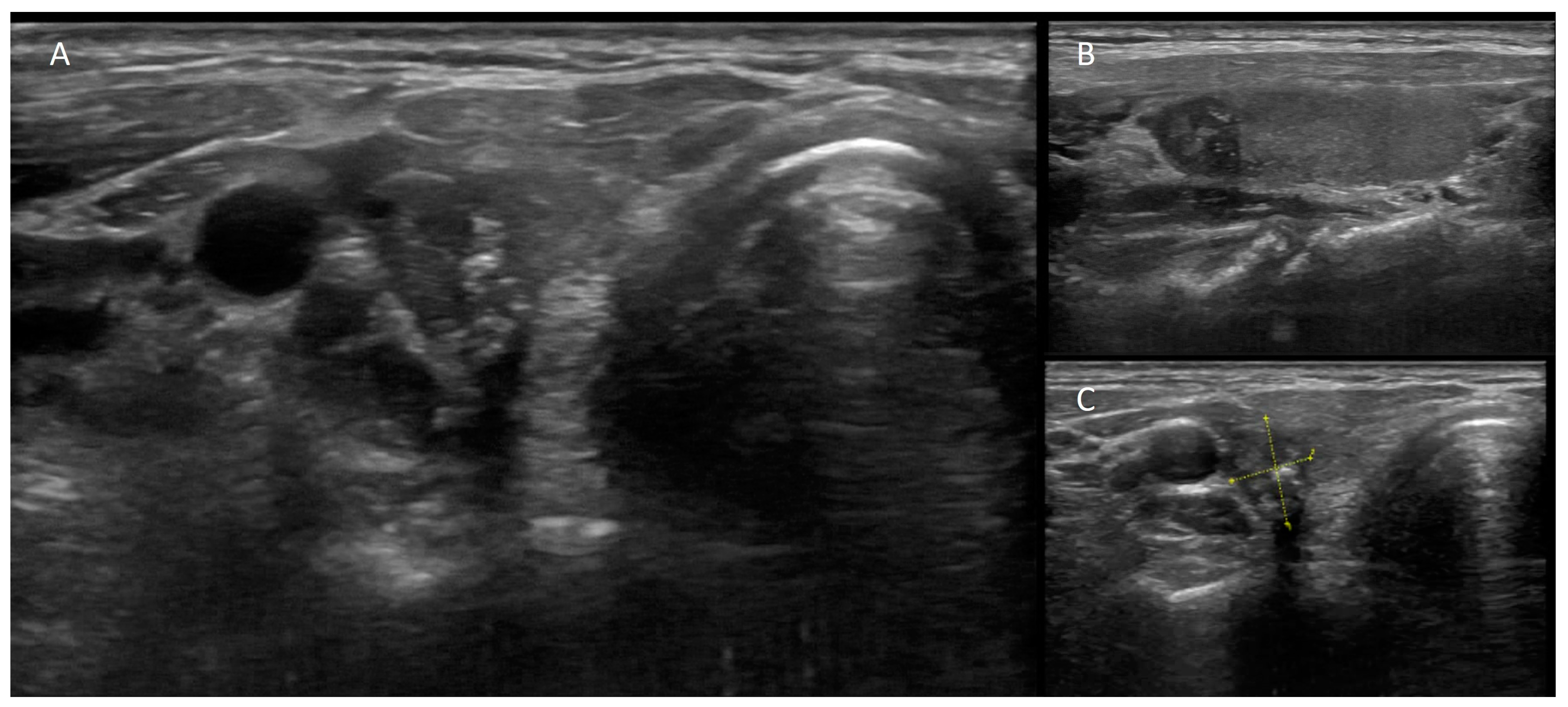
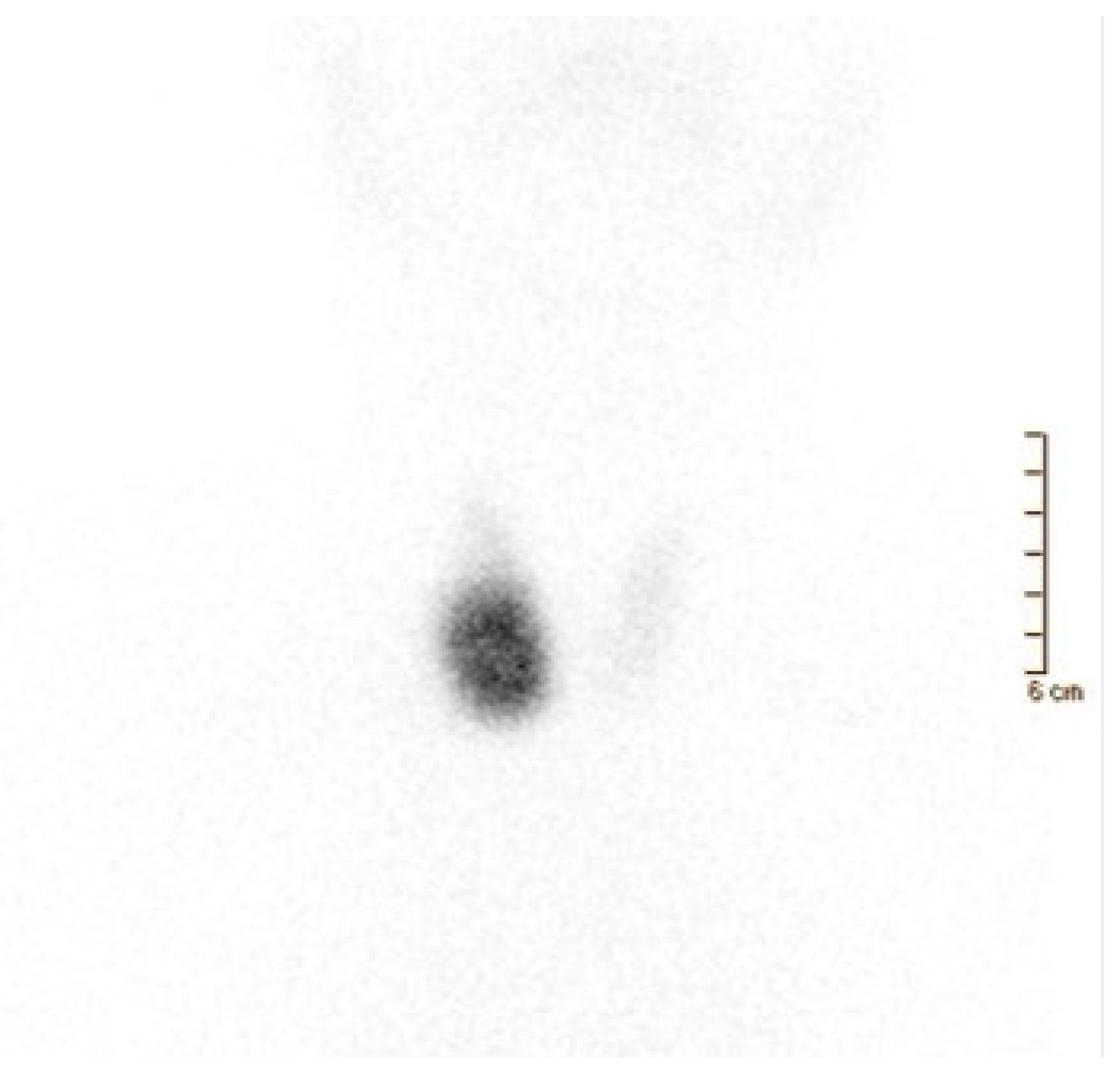
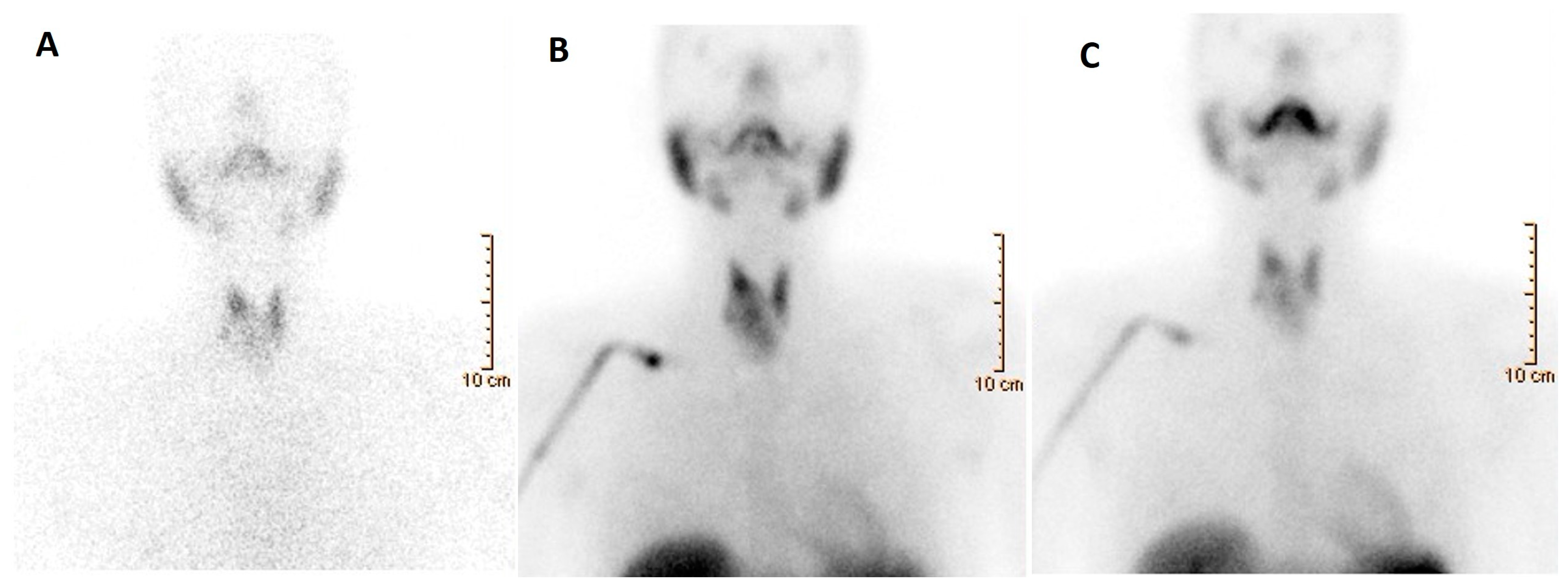
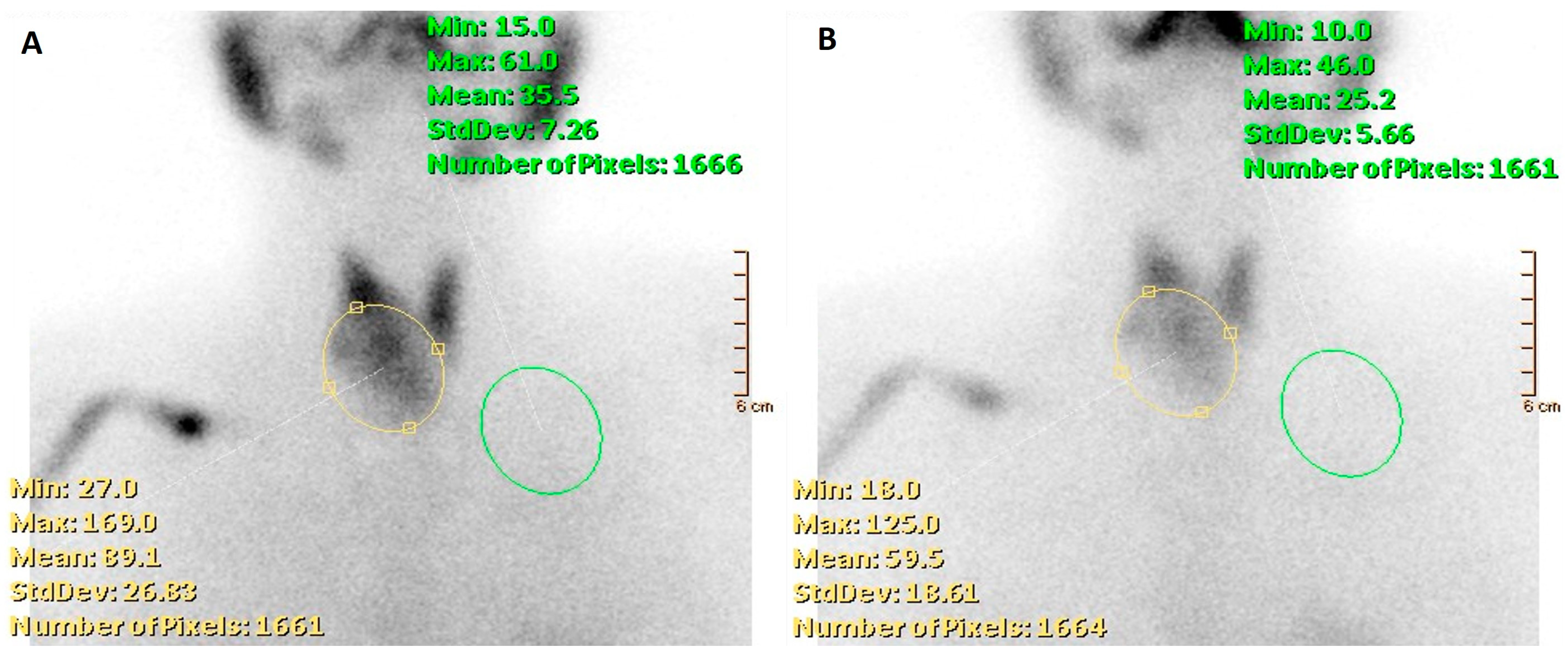
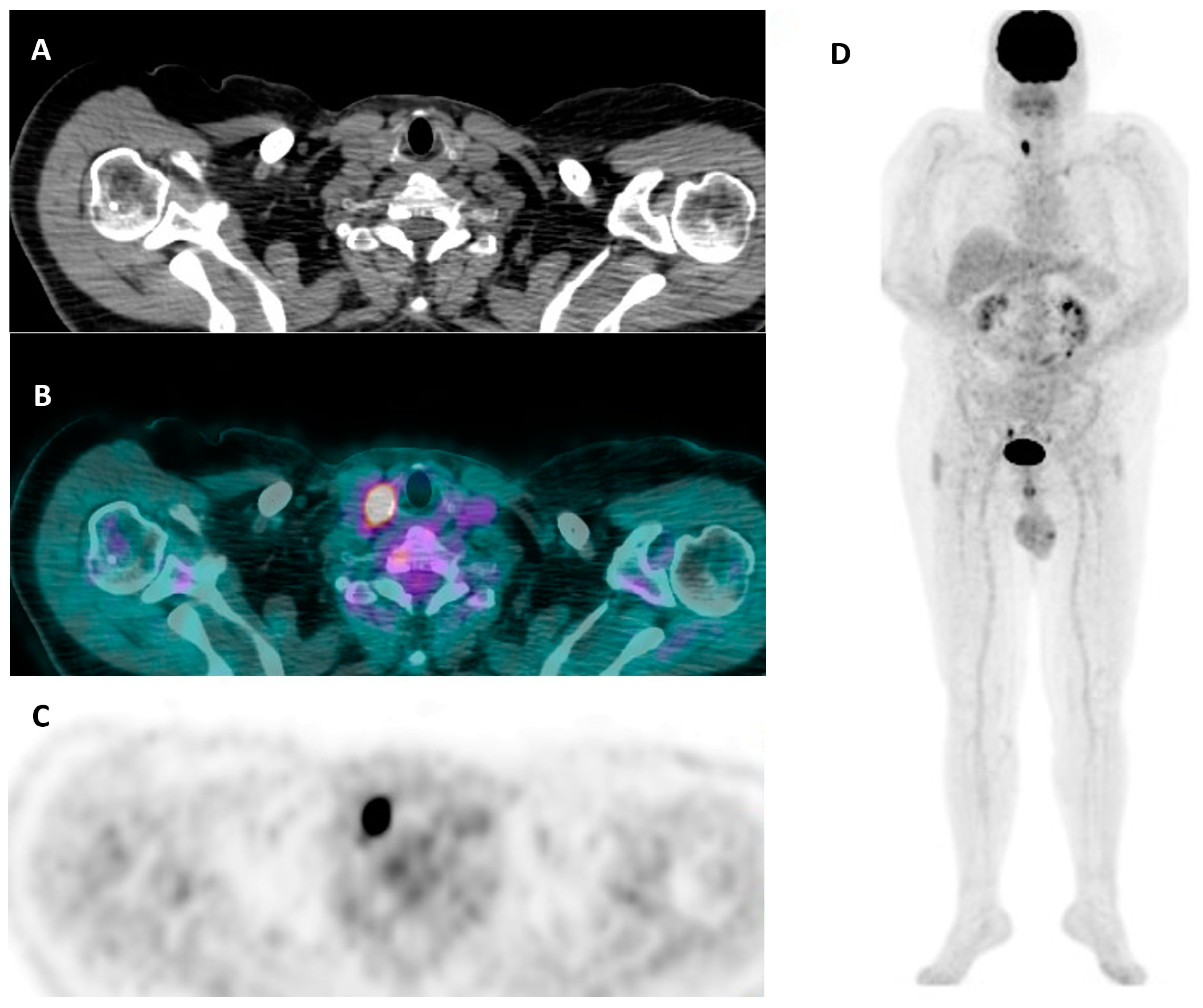
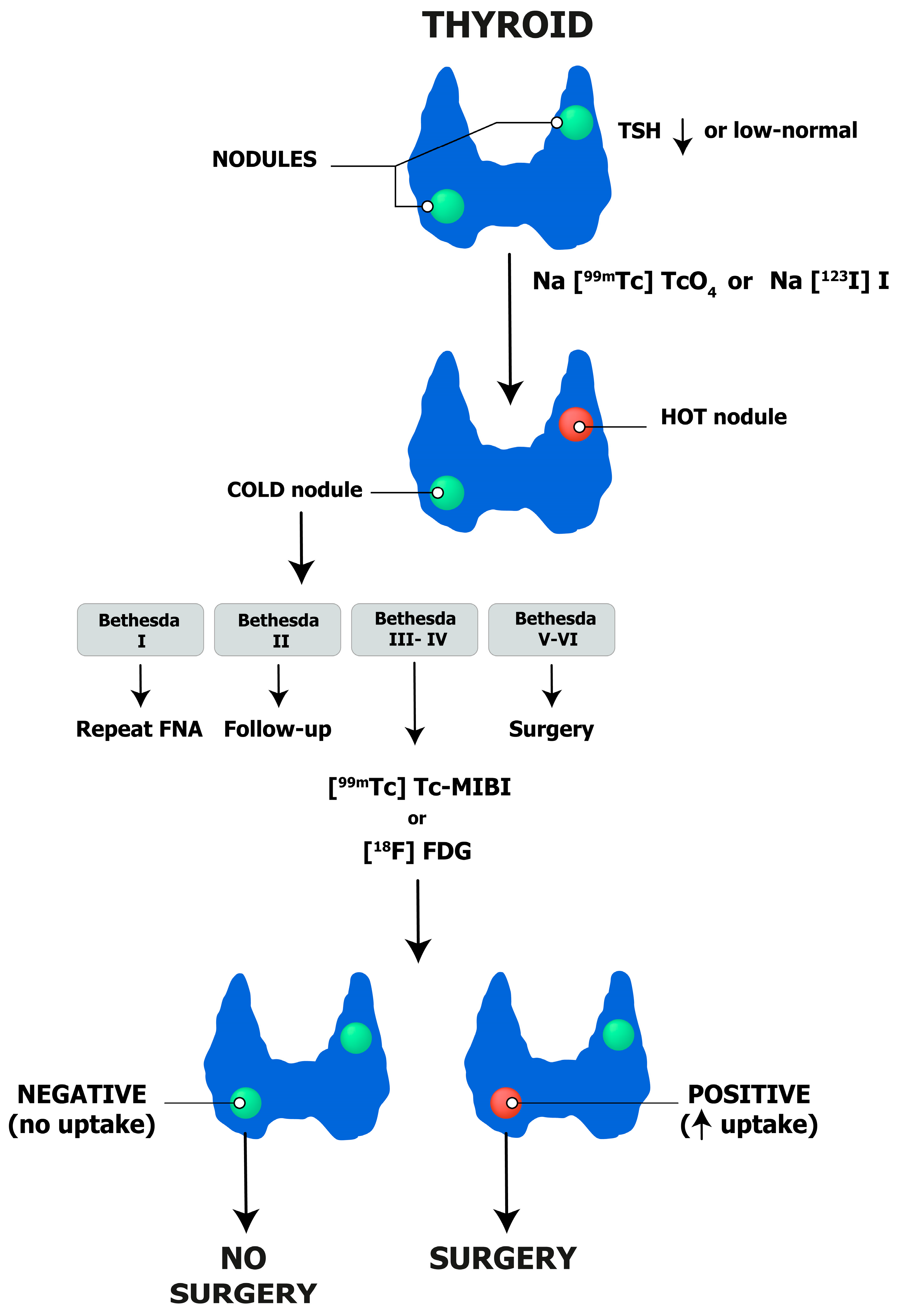
3.3. Nuclear Medicine
3.4. Fine-Needle Aspiration Cytology and Cytopathology
- Preprocedural
- Specimen Staining
- Material Adequacy and False-Negative Results
- Comparison of Aspiration and Capillary Action
- Comparison with Core-Needle Biopsy
4. Molecular Biomarkers
5. Integrated Diagnostics of Thyroid Nodules
6. Perspectives
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, A.M.; Braverman, L.E.; Pearce, E.N. History of U.S. Iodine Fortification and Supplementation. Nutrients 2012, 4, 1740–1746. [Google Scholar] [CrossRef]
- Popoveniuc, G.; Jonklaas, J. Thyroid Nodules. Med. Clin. N. Am. 2012, 96, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Jolanta, M.D.; Bogsrud, T.V. Nuclear Medicine in Evaluation and Therapy of Nodular Thyroid. In Thyroid Nodules; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Alexander, E.K.; Cibas, E.S. Diagnosis of Thyroid Nodules. Lancet Diabetes Endocrinol. 2022, 10, 533–539. [Google Scholar] [CrossRef]
- Schenke, S.A.; Kreissl, M.C.; Grunert, M.; Hach, A.; Haghghi, S.; Kandror, T.; Peppert, E.; Rosenbaum-Krumme, S.; Ruhlmann, V.; Stahl, A.; et al. Distribution of Functional Status of Thyroid Nodules and Malignancy Rates of Hyperfunctioning and Hypofunctioning Thyroid Nodules in Germany. Nuklearmedizin 2022, 61, 376–384. [Google Scholar] [CrossRef]
- Hegedüs, L. Clinical Practice. The Thyroid Nodule. N. Engl. J. Med. 2004, 351, 1764–1771. [Google Scholar] [CrossRef]
- Durante, C.; Costante, G.; Lucisano, G.; Bruno, R.; Meringolo, D.; Paciaroni, A.; Puxeddu, E.; Torlontano, M.; Tumino, S.; Attard, M.; et al. The Natural History of Benign Thyroid Nodules. JAMA 2015, 313, 926–935. [Google Scholar] [CrossRef]
- Fisher, S.B.; Perrier, N.D. The Incidental Thyroid Nodule. CA Cancer J. Clin. 2018, 68, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very High Prevalence of Thyroid Nodules Detected by High Frequency (13 MHz) Ultrasound Examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Hilmi, O.; England, J.; Tolley, N. An Update on the Management of Thyroid Nodules: Rationalising the Guidelines. J. Laryngol. Otol. 2023, 137, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Yousem, D.M.; Huang, T.; Loevner, L.A.; Langlotz, C.P. Clinical and Economic Impact of Incidental Thyroid Lesions Found with CT and MR. Am. J. Neuroradiol. 1997, 18, 1423–1428. [Google Scholar]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for Thyroid Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Schenke, S.A.; Groener, D.; Grunert, M.; Stahl, A.R. Integrated Thyroid Imaging: Ultrasound and Scintigraphy. In Integrated Diagnostics and Theranostics of Thyroid Diseases; Giovanella, L., Ed.; Springer: Cham, Switzerland, 2023; pp. 25–62. [Google Scholar] [CrossRef]
- de Koster, E.J.; Morreau, H.; Bleumink, G.S.; van Engen-van Grunsven, A.C.H.; de Geus-Oei, L.-F.; Links, T.P.; Wakelkamp, I.M.M.J.; Oyen, W.J.G.; Vriens, D. Molecular Diagnostics and [18F]FDG-PET/CT in Indeterminate Thyroid Nodules: Complementing Techniques or Waste of Valuable Resources? Thyroid, 2023; online ahead of print. [Google Scholar] [CrossRef]
- de Koster, E.J.; Vriens, D.; van Aken, M.O.; Dijkhorst-Oei, L.T.; Oyen, W.J.G.; Peeters, R.P.; Schepers, A.; de Geus-Oei, L.F.; van den Hout, W.B. FDG-PET/CT in Indeterminate Thyroid Nodules: Cost-Utility Analysis alongside a Randomised Controlled Trial. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3452–3469. [Google Scholar] [CrossRef] [PubMed]
- Wale, A.; Miles, K.A.; Young, B.; Zammit, C.; Williams, A.; Quin, J.; Dizdarevic, S. Combined (99m)Tc-Methoxyisobutylisonitrile Scintigraphy and Fine-Needle Aspiration Cytology Offers an Accurate and Potentially Cost-Effective Investigative Strategy for the Assessment of Solitary or Dominant Thyroid Nodules. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 105–115. [Google Scholar] [CrossRef]
- Heinzel, A.; Müller, D.; Behrendt, F.F.; Giovanella, L.; Mottaghy, F.M.; Verburg, F.A. Thyroid Nodules with Indeterminate Cytology: Molecular Imaging with 99mTc-Methoxyisobutylisonitrile (MIBI) Is More Cost-Effective than the Afirma Gene Expression Classifier. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Capezzone, M.; Marchisotta, S.; Cantara, S.; Busonero, G.; Brilli, L.; Pazaitou-Panayiotou, K.; Carli, A.F.; Caruso, G.; Toti, P.; Capitani, S.; et al. Familial Non-Medullary Thyroid Carcinoma Displays the Features of Clinical Anticipation Suggestive of a Distinct Biological Entity. Endocr. Relat. Cancer 2008, 15, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.C. Familial Nonmedullary Thyroid Carcinoma: A Meta-Review of Case Series. Thyroid 1997, 7, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Stoffer, S.S.; van Dyke, D.L.; Bach, J.V.; Szpunar, W.; Weiss, L. Familial Papillary Carcinoma of the Thyroid. Am. J. Med. Genet. 1986, 25, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Moses, W.; Weng, J.; Kebebew, E. Prevalence, Clinicopathologic Features, and Somatic Genetic Mutation Profile in Familial versus Sporadic Nonmedullary Thyroid Cancer. Thyroid 2011, 21, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, H.; Benavidez, J.; Poehls, J.L.; Youngwirth, L.; Chen, H.; Sippel, R.S. In Patients with Thyroid Cancer of Follicular Cell Origin, a Family History of Nonmedullary Thyroid Cancer in One First-Degree Relative Is Associated with More Aggressive Disease. Thyroid 2012, 22, 3–8. [Google Scholar] [CrossRef]
- Park, Y.J.; Ahn, H.Y.; Choi, H.S.; Kim, K.W.; Park, D.J.; Cho, B.Y. The Long-Term Outcomes of the Second Generation of Familial Nonmedullary Thyroid Carcinoma Are More Aggressive than Sporadic Cases. Thyroid 2012, 22, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Balinisteanu, I.; Panzaru, M.C.; Caba, L.; Ungureanu, M.C.; Florea, A.; Grigore, A.M.; Gorduza, E.V. Cancer Predisposition Syndromes and Thyroid Cancer: Keys for a Short Two-Way Street. Biomedicines 2023, 11, 2143. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.S.; Gharib, H. Epidemiology of Thyroid Nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Bomeli, S.R.; LeBeau, S.O.; Ferris, R.L. Evaluation of a Thyroid Nodule. Otolaryngol. Clin. N. Am. 2010, 43, 229. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.L.; Jabbour, N.; Ogilvie, J.B.; Ohori, N.P.; Carty, S.E.; Yim, J.H. The Incidence of Cancer and Rate of False-Negative Cytology in Thyroid Nodules Greater than or Equal to 4 Cm in Size. Surgery 2007, 142, 837–844.e3. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.N.; Shah, M.D.; Palme, C.E.; Hall, F.T.; Eski, S.; Freeman, J.L. Risk Factors for Well-Differentiated Thyroid Carcinoma in Patients with Thyroid Nodular Disease. Otolaryngol. Head Neck Surg. 2008, 139, 21–26. [Google Scholar] [CrossRef]
- Koulouri, O.; Moran, C.; Halsall, D.; Chatterjee, K.; Gurnell, M. Pitfalls in the Measurement and Interpretation of Thyroid Function Tests. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 745–762. [Google Scholar] [CrossRef]
- D’Aurizio, F.; Kratzsch, J.; Gruson, D.; Petranović Ovčariček, P.; Giovanella, L. Free Thyroxine Measurement in Clinical Practice: How to Optimize Indications, Analytical Procedures, and Interpretation Criteria While Waiting for Global Standardization. Crit. Rev. Clin. Lab. Sci. 2023, 60, 101–140. [Google Scholar] [CrossRef]
- Giovanella, L.; Petranović Ovčariček, P. Functional and Molecular Thyroid Imaging. Q. J. Nucl. Med. Mol. Imaging 2022, 66, 86–92. [Google Scholar] [CrossRef]
- Kronenberg, H.M.; Melmed, S.; Larsen, P.R.; Polonsky, K.S. Principles of Endocrinology. In Williams Textbook of Endocrinology; Melmed, S., Polonsky, K.S., Larsen, P.R., Kronenberg, H.M., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 3–12. [Google Scholar]
- Giovanella, L.; Avram, A.M.; Ovčariček, P.P.; Clerc, J. Thyroid Functional and Molecular Imaging. Presse Med. 2022, 51, 104116. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.M.; Spencer, C.A. Laboratory Medicine Practice Guidelines: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Clin. Endocrinol. 2003, 58, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Giovanella, L. Reflex TSH Strategy: The Good, the Bad and the Ugly. Clin. Chem. Lab. Med. 2019, 58, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, M.T. Biochemical Testing of the Thyroid: TSH Is the Best and, Oftentimes, Only Test Needed—A Review for Primary Care. Clin. Med. Res. 2016, 14, 83–92. [Google Scholar] [CrossRef]
- Giovanella, L.; D’Aurizio, F.; Campenni’, A.; Ruggeri, R.; Baldari, S.; Verburg, F.; Trimboli, P.; Ceriani, L. Searching For The Most Effective Thyrotropin (TSH) Threshold To Rule-Out Autonomously Functioning Thyroid Nodules In Iodine Deficient Regions. Endocrine 2016, 54, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Repplinger, D.; Bargren, A.; Zhang, Y.W.; Adler, J.T.; Haymart, M.; Chen, H. Is Hashimoto’s Thyroiditis a Risk Factor for Papillary Thyroid Cancer? J. Surg. Res. 2008, 150, 49–52. [Google Scholar] [CrossRef]
- Giovanella, L.; D’Aurizio, F.; Algeciras-Schimnich, A.; Görges, R.; Petranovic Ovcaricek, P.; Tuttle, R.M.; Visser, W.E.; Verburg, F.A. Thyroglobulin and Thyroglobulin Antibody: An Updated Clinical and Laboratory Expert Consensus. Eur. J. Endocrinol. 2023, 189, R11–R27. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; MacHens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Verbeek, H.H.; de Groot, J.W.B.; Sluiter, W.J.; Kobold, A.C.M.; van den Heuvel, E.R.; Plukker, J.T.; Links, T.P. Calcitonin Testing for Detection of Medullary Thyroid Cancer in People with Thyroid Nodules. Cochrane Database Syst. Rev. 2020, 3, CD010159. [Google Scholar] [CrossRef]
- Rago, T.; Vitti, P. Role of Thyroid Ultrasound in the Diagnostic Evaluation of Thyroid Nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 913–928. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P. American association of clinical endocrinologists, american college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard Ba, G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. Guidelines for the Management of Thyroid Cancer. Clin. Endocrinol. 2014, 81 (Suppl. 1), 1–122. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.K.; Asadollahi, S.; Durante, C.; Hegedüs, L.; Papini, E.; Tessler, F.N. An International Survey on Utilization of Five Thyroid Nodule Risk Stratification Systems: A Needs Assessment with Future Implications. Thyroid 2022, 32, 675–681. [Google Scholar] [CrossRef]
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European Thyroid Association Clinical Practice Guidelines for Thyroid Nodule Management. Eur. Thyroid J. 2023, 12, e230067. [Google Scholar] [CrossRef]
- Seifert, P.; Schenke, S.; Zimny, M.; Stahl, A.; Grunert, M.; Klemenz, B.; Freesmeyer, M.; Kreissl, M.C.; Herrmann, K.; Görges, R. Diagnostic Performance of Kwak, EU, ACR, and Korean TIRADS as Well as ATA Guidelines for the Ultrasound Risk Stratification of Non-Autonomously Functioning Thyroid Nodules in a Region with Long History of Iodine Deficiency: A German Multicenter Trial. Cancers 2021, 13, 4467. [Google Scholar] [CrossRef]
- Kuru, B.; Kefeli, M.; Danaci, M. Comparison of 5 Thyroid Ultrasound Stratification Systems for Differentiation of Benign and Malignant Nodules and to Avoid Biopsy Using Histology as Reference Standard. Endocr. Pract. 2021, 27, 1093–1099. [Google Scholar] [CrossRef]
- Li, X.; Peng, C.; Liu, Y.; Hu, Y.; Yang, L.; Yu, Y.; Zeng, H.; Huang, W.; Li, Q.; Tao, N.; et al. Modified American College of Radiology Thyroid Imaging Reporting and Data System and Modified Artificial Intelligence Thyroid Imaging Reporting and Data System for Thyroid Nodules: A Multicenter Retrospective Study. Thyroid, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Giovanella, L.; Avram, A.M.; Iakovou, I.; Kwak, J.; Lawson, S.A.; Lulaj, E.; Luster, M.; Piccardo, A.; Schmidt, M.; Tulchinsky, M.; et al. EANM Practice Guideline/SNMMI Procedure Standard for RAIU and Thyroid Scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2514–2525. [Google Scholar] [CrossRef]
- Giovanella, L.; Ceriani, L.; Treglia, G. Role of Isotope Scan, Including Positron Emission Tomography/Computed Tomography, in Nodular Goitre. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Hetrakul, N.; Civelek, A.C.; Stagg, C.A.; Udelsman, R. In Vitro Accumulation of Technetium-99m-Sestamibi in Human Parathyroid Mitochondria. Surgery 2001, 130, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Karamzade-Ziarati, N.; Manafi-Farid, R.; Ataeinia, B.; Langsteger, W.; Pirich, C.; Mottaghy, F.M.; Beheshti, M. Molecular Imaging of Bone Metastases Using Tumor-Targeted Tracers. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Delmaire, C.; Savatovsky, J.; Boulanger, T.; Dhermain, F.; Le Rhun, E.; Météllus, P.; Gerber, S.; Carsin-Nicole, B.; Petyt, G. Imagerie Des Métastases Cérébrales. Cancer/Radiothérapie 2015, 19, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhan, H.; Sun, D. Comparison of 99mTc-MIBI Scintigraphy, Ultrasound, and Mammography for the Diagnosis of BI-RADS 4 Category Lesions. BMC Cancer 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Hurtado-López, L.M.; Arellano-Montaño, S.; Torres-Acosta, E.M.; Zaldivar-Ramirez, F.R.; Duarte-Torres, R.M.; Alonso-De-Ruiz, P.; Martínez-Duncker, I.; Martínez-Duncker, C. Combined Use of Fine-Needle Aspiration Biopsy, MIBI Scans and Frozen Section Biopsy Offers the Best Diagnostic Accuracy in the Assessment of the Hypofunctioning Solitary Thyroid Nodule. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1273–1279. [Google Scholar] [CrossRef]
- Giovanella, L.; Campenni, A.; Treglia, G.; Verburg, F.A.; Trimboli, P.; Ceriani, L.; Bongiovanni, M. Molecular Imaging with (99m)Tc-MIBI and Molecular Testing for Mutations in Differentiating Benign from Malignant Follicular Neoplasm: A Prospective Comparison. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1018–1026. [Google Scholar] [CrossRef]
- Campennì, A.; Giovanella, L.; Siracusa, M.; Alibrandi, A.; Pignata, S.A.; Giovinazzo, S.; Trimarchi, F.; Ruggeri, R.M.; Baldari, S. (99m)Tc-Methoxy-Isobutyl-Isonitrile Scintigraphy Is a Useful Tool for Assessing the Risk of Malignancy in Thyroid Nodules with Indeterminate Fine-Needle Cytology. Thyroid 2016, 26, 1101–1109. [Google Scholar] [CrossRef]
- Campennì, A.; Siracusa, M.; Ruggeri, R.M.; Laudicella, R.; Pignata, S.A.; Baldari, S.; Giovanella, L. Differentiating Malignant from Benign Thyroid Nodules with Indeterminate Cytology by 99m Tc-MIBI Scan: A New Quantitative Method for Improving Diagnostic Accuracy. Sci. Rep. 2017, 7, 6147. [Google Scholar] [CrossRef]
- Schenke, S.A.; Campennì, A.; Tuncel, M.; Bottoni, G.; Sager, S.; Crncic, T.B.; Rozic, D.; Görges, R.; Özcan, P.P.; Groener, D.; et al. Diagnostic Performance of 99mTc-Methoxy-Isobuty-Isonitrile (MIBI) for Risk Stratification of Hypofunctioning Thyroid Nodules: A European Multicenter Study. Diagnostics 2022, 12, 1358. [Google Scholar] [CrossRef]
- Schenke, S.A.; Campenni, A.; Tuncel, M.; Piccardo, A.; Sager, S.; Bogovic Crncic, T.; Rozic, D.; Goerges, R.; Özcan Kara, P.P.; Groener, D.; et al. A Multicenter Survey of Current Practices of 99mTc-Methoxy-Isobutyl-Isonitrile (MIBI) Imaging for the Diagnosis of Thyroid Nodules: More Standardization Is Essential. Clin. Transl. Imaging 2021, 9, 413–422. [Google Scholar] [CrossRef]
- Giovanella, L.; Suriano, S.; Ricci, R.; Ceriani, L.; Verburg, F.A. Postsurgical Thyroid Remnant Estimation by (99m) Tc-Pertechnetate Scintigraphy Predicts Radioiodine Ablation Effectiveness in Patients with Differentiated Thyroid Carcinoma. Head Neck 2011, 33, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Saggiorato, E.; Angusti, T.; Rosas, R.; Martinese, M.; Finessi, M.; Arecco, F.; Trevisiol, E.; Bergero, N.; Puligheddu, B.; Volante, M.; et al. 99mTc-MIBI Imaging in the Presurgical Characterization of Thyroid Follicular Neoplasms: Relationship to Multidrug Resistance Protein Expression. J. Nucl. Med. 2009, 50, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Piga, M.; Cocco, M.C.; Serra, A.; Boi, F.; Loy, M.; Mariotti, S. The Usefulness of 99mTc-SestaMIBI Thyroid Scan in the Differential Diagnosis and Management of Amiodarone-Induced Thyrotoxicosis. Eur. J. Endocrinol. 2008, 159, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.A.; Westcott, J.; Lichtenstein, M.; Toh, H.B.; Gunawardana, D.; Better, N.; Forehan, S.; Sivaratnam, D. Quantitative Assessment of Thyroid-to-Background Ratio Improves the Interobserver Reliability of Technetium-99m Sestamibi Thyroid Scintigraphy for Investigation of Amiodarone-Induced Thyrotoxicosis. Nucl. Med. Commun. 2015, 36, 356–362. [Google Scholar] [CrossRef]
- Hervás Morón, A. PET-CT in Oncology. Clin. Transl. Oncol. 2007, 9, 473–474. [Google Scholar] [CrossRef]
- Meyer, H.J.; Wienke, A.; Surov, A. Associations between GLUT Expression and SUV Values Derived from FDG-PET in Different Tumors-A Systematic Review and Meta Analysis. PLoS ONE 2019, 14, e0217781. [Google Scholar] [CrossRef]
- de Koster, E.J.; de Geus-Oei, L.F.; Brouwers, A.H.; van Dam, E.W.C.M.; Dijkhorst-Oei, L.T.; van Engen-van Grunsven, A.C.H.; van den Hout, W.B.; Klooker, T.K.; Netea-Maier, R.T.; Snel, M.; et al. [18F]FDG-PET/CT to Prevent Futile Surgery in Indeterminate Thyroid Nodules: A Blinded, Randomised Controlled Multicentre Trial. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1970–1984. [Google Scholar] [CrossRef]
- Vriens, D.; De Wilt, J.H.W.; Van Der Wilt, G.J.; Netea-Maier, R.T.; Oyen, W.J.G.; De Geus-Oei, L.F. The Role of [18F]-2-Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography in Thyroid Nodules with Indeterminate Fine-Needle Aspiration Biopsy: Systematic Review and Meta-Analysis of the Literature. Cancer 2011, 117, 4582–4594. [Google Scholar] [CrossRef]
- Wang, N.; Zhai, H.; Lu, Y. Is Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography Useful for the Thyroid Nodules with Indeterminate Fine Needle Aspiration Biopsy? A Meta-Analysis of the Literature. J. Otolaryngol. Head Neck Surg. 2013, 42, 38. [Google Scholar] [CrossRef]
- Castellana, M.; Trimboli, P.; Piccardo, A.; Giovanella, L.; Treglia, G. Performance of 18 F-FDG PET/CT in Selecting Thyroid Nodules with Indeterminate Fine-Needle Aspiration Cytology for Surgery. A Systematic Review and a Meta-Analysis. J. Clin. Med. 2019, 8, 1333. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Milan, L.; Piccardo, A.; Bottoni, G.; Cuzzocrea, M.; Paone, G.; Ceriani, L. Radiomics Analysis Improves 18FDG PET/CT-Based Risk Stratification of Cytologically Indeterminate Thyroid Nodules. Endocrine 2022, 75, 202–210. [Google Scholar] [CrossRef] [PubMed]
- de Koster, E.J.; Noortman, W.A.; Mostert, J.M.; Booij, J.; Brouwer, C.B.; de Keizer, B.; de Klerk, J.M.H.; Oyen, W.J.G.; van Velden, F.H.P.; de Geus-Oei, L.F.; et al. Quantitative Classification and Radiomics of [18F]FDG-PET/CT in Indeterminate Thyroid Nodules. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2174–2188. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Moon, W.J.; So, L.J.; Jeong, H.L.; Dong, G.N.; Baek, J.H.; Young, H.L.; Kim, J.; Hyun, S.K.; Jun, S.B.; Dong, H.L. Benign and Malignant Thyroid Nodules: US Differentiation--Multicenter Retrospective Study. Radiology 2008, 247, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, E.K.; Park, S.I.; Kim, B.M.; Kwak, J.Y.; Kim, S.J.; Youk, J.H.; Park, S.H. US-Guided Fine-Needle Aspiration of Thyroid Nodules: Indications, Techniques, Results. Radiographics 2008, 28, 1869–1886. [Google Scholar] [CrossRef] [PubMed]
- Black, J.M. Anticoagulation in Elective Surgery. Plast. Surg. Nurs. 2004, 24, 8–11. [Google Scholar] [CrossRef]
- Oertel, Y.C. Fine-Needle Aspiration of the Thyroid: Technique and Terminology. Endocrinol. Metab. Clin. N. Am. 2007, 36, 737–751. [Google Scholar] [CrossRef]
- Santos, J.E.; Leiman, G. Nonaspiration Fine Needle Cytology. Application of a New Technique to Nodular Thyroid Disease. Acta Cytol. 1988, 32, 353–356. [Google Scholar]
- Degirmenci, B.; Haktanir, A.; Albayrak, R.; Acar, M.; Sahin, D.A.; Sahin, O.; Yucel, A.; Caliskan, G. Sonographically Guided Fine-Needle Biopsy of Thyroid Nodules: The Effects of Nodule Characteristics, Sampling Technique, and Needle Size on the Adequacy of Cytological Material. Clin. Radiol. 2007, 62, 798–803. [Google Scholar] [CrossRef]
- Titton, R.L.; Gervais, D.A.; Boland, G.W.; Maher, M.M.; Mueller, P.R. Sonography and Sonographically Guided Fine-Needle Aspiration Biopsy of the Thyroid Gland: Indications and Techniques, Pearls and Pitfalls. AJR Am. J. Roentgenol. 2003, 181, 267–271. [Google Scholar] [CrossRef]
- Quinn, S.F.; Nelson, H.A.; Demlow, T.A. Thyroid Biopsies: Fine-Needle Aspiration Biopsy versus Spring-Activated Core Biopsy Needle in 102 Patients. J. Vasc. Interv. Radiol. 1994, 5, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Taki, S.; Kakuda, K.; Kakuma, K.; Annen, Y.; Katada, S.; Yamashita, R.; Kosugi, M.; Michigishi, T.; Tonami, N. Thyroid Nodules: Evaluation with US-Guided Core Biopsy with an Automated Biopsy Gun. Radiology 1997, 202, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Pusztaszeri, M.; Rossi, E.D.; Auger, M.; Baloch, Z.; Bishop, J.; Bongiovanni, M.; Chandra, A.; Cochand-Priollet, B.; Fadda, G.; Hirokawa, M.; et al. The Bethesda System for Reporting Thyroid Cytopathology: Proposed Modifications and Updates for the Second Edition from an International Panel. Acta Cytol. 2016, 60, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian Consensus for the Classification and Reporting of Thyroid Cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Cross, P.; Chandra, A.; Giles, T.; Johnson, S.; Kocjan, G.; Poller, D. Guidance on the Reporting of Thyroid Cytology Specimens; The Royal College of Pathologists: London, UK, 2016. [Google Scholar]
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 1039–1044. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Bellevicine, C.; Troncone, G.; Sykiotis, G.P. Approach to Cytological Indeterminate Thyroid Nodules. Gland Surg. 2019, 8 (Suppl. 2), S98–S104. [Google Scholar] [CrossRef]
- Vignali, P.; Macerola, E.; Poma, A.M.; Sparavelli, R.; Basolo, F. Indeterminate Thyroid Nodules: From Cytology to Molecular Testing. Diagnostics 2023, 13, 3008. [Google Scholar] [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS Mutations in Thyroid Benign Nodules and Non-Medullary Thyroid Cancer. Cancers 2021, 13, 3785. [Google Scholar] [CrossRef]
- Bardet, S.; Goardon, N.; Lequesne, J.; Vaur, D.; Ciappuccini, R.; Leconte, A.; Monpeyssen, H.; Saguet-Rysanek, V.; Clarisse, B.; Lasne-Cardon, A.; et al. Diagnostic and Prognostic Value of a 7-Panel Mutation Testing in Thyroid Nodules with Indeterminate Cytology: The SWEETMAC Study. Endocrine 2021, 71, 407–417. [Google Scholar] [CrossRef]
- Alzumaili, B.; Sadow, P.M. Update on Molecular Diagnostics in Thyroid Pathology: A Review. Genes 2023, 14, 1314. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.K.; Lee, S.; Kim, S.J.; Jee, H.G.; Kim, B.A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.K.; Shin, J.Y.; et al. Comprehensive Analysis of the Transcriptional and Mutational Landscape of Follicular and Papillary Thyroid Cancers. PLoS Genet. 2016, 12, e1006239. [Google Scholar] [CrossRef]
- Labourier, E.; Fahey, T.J. Preoperative Molecular Testing in Thyroid Nodules with Bethesda VI Cytology: Clinical Experience and Review of the Literature. Diagn. Cytopathol. 2021, 49, E175–E180. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, R.; Shen, X.; Zhu, G.; Li, B.; Xing, M. The Genetic Duet of BRAF V600E and TERT Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J. Nucl. Med. 2020, 61, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Eloy, C.; Russ, G.; Suciu, V.; Johnson, S.J.; Rossi, E.D.; Pantanowitz, L.; Vielh, P. Preoperative Diagnosis of Thyroid Nodules: An Integrated Multidisciplinary Approach. Cancer Cytopathol. 2022, 130, 320–325. [Google Scholar] [CrossRef]
- Iglesias, P.; Acosta, M.; Sánchez, R.; Fernández-Reyes, M.J.; Mon, C.; Díez, J.J. Ambulatory Blood Pressure Monitoring in Patients with Hyperthyroidism before and after Control of Thyroid Function. Clin. Endocrinol. 2005, 63, 66–72. [Google Scholar] [CrossRef]
- Jansen, T.; Stikkelbroeck, N.; van de Ven, A.; van Engen-van Grunsven, I.; Janssen, M.; Bonenkamp, H.; Gotthardt, M.; Netea-Maier, R.T. Clinical Characteristics, Diagnostic Approach and Outcome of Thyroid Incidental Findings vs. Clinically Overt Thyroid Nodules: An Observational Single-Centre Study. Cancers 2023, 15, 2350. [Google Scholar] [CrossRef]
- Vuong, H.G.; Ngo, H.T.T.; Bychkov, A.; Jung, C.K.; Vu, T.H.; Lu, K.B.; Kakudo, K.; Kondo, T. Differences in Surgical Resection Rate and Risk of Malignancy in Thyroid Cytopathology Practice between Western and Asian Countries: A Systematic Review and Meta-Analysis. Cancer Cytopathol. 2020, 128, 238–249. [Google Scholar] [CrossRef]
- Sakai, T.; Sugitani, I.; Ebina, A.; Fukuoka, O.; Toda, K.; Mitani, H.; Yamada, K. Active Surveillance for T1bN0M0 Papillary Thyroid Carcinoma. Thyroid 2019, 29, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, A.; Kudo, T.; Ito, Y.; Oda, H.; Sasai, H.; Higashiyama, T.; Fukushima, M.; Masuoka, H.; Kihara, M.; Miya, A. Estimation of the Lifetime Probability of Disease Progression of Papillary Microcarcinoma of the Thyroid during Active Surveillance. Surgery 2018, 163, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Lippi, G. Integrated Diagnostics: The Future of Diagnostic Medicine? In Integrated Diagnostics and Theranostics of Thyroid Diseases; Giovanella, L., Ed.; Springer: Cham, Switzerland, 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Lai, M.; Feng, B.; Yao, J.; Wang, Y.; Pan, Q.; Chen, Y.; Chen, C.; Feng, N.; Shi, F.; Tian, Y.; et al. Value of Artificial Intelligence in Improving the Accuracy of Diagnosing TI-RADS Category 4 Nodules. Ultrasound Med. Biol. 2023, 49, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Wetterstrand, M.S. The Cost of Sequencing a Human Genome. Available online: https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost (accessed on 21 December 2023).
- Dlamini, Z.; Francies, F.Z.; Hull, R.; Marima, R. Artificial Intelligence (AI) and Big Data in Cancer and Precision Oncology. Comput. Struct. Biotechnol. J. 2020, 18, 2300–2311. [Google Scholar] [CrossRef]
- Seyala, N.; Abdullah, S.N. Cluster Analysis on Longitudinal Data of Patients with Kidney Dialysis Using a Smoothing Cubic B-Spline Model. Int. J. Math. Stat. Comput. Sci. 2024, 2, 85–95. [Google Scholar] [CrossRef]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial Intelligence in Digital Pathology—New Tools for Diagnosis and Precision Oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Milan, L. Artificial Intelligence and Machine Learning in Integrated Diagnostic. In Integrated Diagnostics and Theranostics of Thyroid Diseases; Giovanella, L., Ed.; Springer: Cham, Switzerland, 2023; pp. 5–11. [Google Scholar] [CrossRef]
- Ali, A.M.; Mohammed, M.A. A Comprehensive Review of Artificial Intelligence Approaches in Omics Data Processing: Evaluating Progress and Challenges. Int. J. Math. Stat. Comput. Sci. 2024, 2, 114–167. [Google Scholar] [CrossRef]


| Indications |
|---|
|
|
|
|
|
| Category | US Features | Malignancy Risk, % | Recommendations |
|---|---|---|---|
| EU-TIRADS 1: Normal | No nodules | None | None |
| EU-TIRADS 2: benign | Pure cyst, Entirely spongiform | 0 | No FNA required (unless for therapeutic purposes/to relieve compression) |
| EU-TIRADS 3: low risk | Ovoid, smooth isoechoic/hyperechoic No features of high suspicion | 2–4 | >20 mm FNA |
| EU-TIRADS 4: intermediate risk | Ovoid, smooth, mildly hypoechoic No features of high suspicion | 6–17 | >15 mm FNA |
| EU-TIRADS 5: high risk | At least 1 of the following features of high suspicion:
| 26–87 | >10 mm FNA, <10 mm: consider FNA or active surveillance |
| Sonographic Pattern | US Features | Estimated Risk of Malignancy, % | FNA Size Cutoff (Largest Dimension) |
|---|---|---|---|
| High suspicion | Solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, micro-lobulated), microcalcifications, taller-than-wide shape, rim calcifications with small extrusive soft tissue component, evidence of extrathyroidal extension | >70–90 | Recommend FNA at ≥1 cm |
| Intermediate suspicion | Hypoechoic solid nodule with smooth margins without microcalcifications, ETE, or taller-than-wide shape | 10–20 | Recommend FNA at ≥1 cm |
| Low suspicion | Isoechoic or hyperechoic solid nodule, or partially cystic nodule with eccentric solid areas, without microcalcification, irregular margin or ETE, or taller-than-wide shape | 5–10 | Recommend FNA at ≥1.5 cm |
| Very low suspicion | Spongiform or partially cystic nodules without any of the sonographic features described in low-, intermediate-, or high-suspicion patterns | <3 | Consider FNA at ≥2 cm Observation without FNA is also a reasonable option |
| Benign | Purely cystic nodules (no solid component) | <1 | No biopsy |
| (a) | |||
| Category | Malignancy Risk, % | Recommendations | |
| EU-TIRADS 1: Normal | None | None | |
| EU-TIRADS 2 | benign, malignancy risk 0%; | No FNA required (unless for therapeutic purposes/to relieve compression) | |
| EU-TIRADS 3 | low risk, malignancy risk 2–4% | >20 mm FNA | |
| EU-TIRADS 4 | intermediate risk, malignancy risk 6–17% | >15 mm FNA | |
| EU-TIRADS 5 | high risk, malignancy risk 26–87% | >10 mm FNA, <10 mm: consider FNA or active surveillance | |
| (b) | |||
| Category | Points | Malignancy Risk, % | Recommendations |
| TR1: | 0 points | benign (aggregate risk level 0.3%); | No FNA required |
| TR2: | 2 points | not suspicious (aggregate risk level 1.5%); | No FNA required |
| TR3: | 3 points | mildly suspicious (aggregate risk level 4.8%); | ≥25 mm FNA |
| TR4: | 4–6 points | moderately suspicious (aggregate risk level 5.9–12.8%); | ≥15 mm FNA |
| TR5: | 7 points or more | highly suspicious (aggregate risk level 20.8–68.4% for 10 points). | ≥10 mm FNA, |
| (c) | |||
| Category | Malignancy Risk, % | Recommendations | |
| Benign | Risk level < 1% | No FNA required | |
| Very low suspicion | Risk level < 3% | Consider FNA at ≥2 cm Observation without FNA is also a reasonable option | |
| Low suspicion | Risk level 5–10% | Recommend FNA at ≥1.5 cm | |
| Intermediate suspicion | Risk level 10–20% | Recommend FNA at ≥1 cm | |
| High suspicion | Risk level > 70–90% | Recommend FNA at ≥1 cm | |
| Diagnostic Category | Risk of Malignancy if NIFTP ≠ CA (%) | Risk of Malignancy if NIFTP = CA (%) | Usual Management |
|---|---|---|---|
| I. Non-diagnostic or unsatisfactory | 5–10 | 5–10 | Repeat FNA with ultrasound guidance |
| II. Benign | 0–3 | 0–3 | Clinical and sonographic follow-up |
| III. Atypia of undetermined significance or follicular lesion of undetermined significance | 6–18 | ∼10–30 | Repeat FNA, molecular testing, or lobectomy |
| IV. Follicular neoplasm or suspicious for a follicular neoplasm | 10–40 | 25–40 | Molecular testing, lobectomy |
| V. Suspicious for malignancy | 45–60 | 50–75 | Near-total thyroidectomy or lobectomy |
| VI. Malignant | 94–96 | 97–99 | Near-total thyroidectomy or lobectomy |
| RCPath | Bethesda | Italian SIAPEC-AIT |
|---|---|---|
| Thy1 Non-diagnostic for cytological diagnosis Thy1c Non-diagnostic for cytological diagnosis—cystic lesion |
| TIR1 Non-diagnostic TIR1c Non-diagnostic cystic |
| Thy2 Non-neoplastic Thy2c Non-neoplastic, cystic lesion | Consistent with a benign follicular nodule (includes adenomatoid nodule, colloid nodule, etc.). Consistent with lymphocytic (Hashimoto) thyroiditis in the proper clinical context Consistent with granulomatous (subacute) thyroiditis | TIR2 Non-malignant |
| Thy3a Neoplasm possible—atypia/non-diagnostic | III. Atypia of undetermined significance or follicular lesion of undetermined significance | TIR3A Low-risk indeterminate lesion (LRIL) |
| Thy3f Neoplasm possible, suggesting follicular neoplasm | IV. Follicular neoplasm or suspicious for a follicular neoplasm Specify if Hürthle cell (oncocytic) type | TIR3B High-risk indeterminate lesion (HRIL) |
| Thy4 Suspicious of malignancy | V. Suspicious for malignancy | TIR4 Suspicious of malignancy |
| Thy5 Malignant | VI. Malignant | TIR5 Malignant |
| 2014 Italian SIAPEC-AIT | 2017 Bethesda | 2016 RCPath Classification | |||
|---|---|---|---|---|---|
| Diagnostic category (ROM %) | Management | Diagnostic category (ROM %) | Management | Diagnostic category (ROM %) | Management |
| TIR 3A Low-risk indeterminate lesion (12–22%) | Clinical follow up/Repeat FNA | III. AUS/FLUS (10–30%) | Repeat FNA/Molecular testing or lobectomy | Thy 3a Neoplasm possible – atypia/non-diagnostic (25%) | Multidisciplinary assessment |
| TIR 3B High-risk indeterminate lesion (30–55%) | Surgery | IV. Follicular neoplasm/suspicious follicular neoplasm (25–40%) | Molecular testing, lobectomy | Thy 3f Neoplasm possible, suggesting follicular neoplasm (31%) | Multidisciplinary assessment |
| History |
|
| Clinical examination |
|
| Clinical investigations |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanella, L.; Campennì, A.; Tuncel, M.; Petranović Ovčariček, P. Integrated Diagnostics of Thyroid Nodules. Cancers 2024, 16, 311. https://doi.org/10.3390/cancers16020311
Giovanella L, Campennì A, Tuncel M, Petranović Ovčariček P. Integrated Diagnostics of Thyroid Nodules. Cancers. 2024; 16(2):311. https://doi.org/10.3390/cancers16020311
Chicago/Turabian StyleGiovanella, Luca, Alfredo Campennì, Murat Tuncel, and Petra Petranović Ovčariček. 2024. "Integrated Diagnostics of Thyroid Nodules" Cancers 16, no. 2: 311. https://doi.org/10.3390/cancers16020311
APA StyleGiovanella, L., Campennì, A., Tuncel, M., & Petranović Ovčariček, P. (2024). Integrated Diagnostics of Thyroid Nodules. Cancers, 16(2), 311. https://doi.org/10.3390/cancers16020311






