Multiplex Immunofluorescence Captures Progressive Immune Exhaustion with Advancing Penile Squamous Cell Cancer Stage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Chahoud, J.; Kohli, M.; Spiess, P.E. Management of Advanced Penile Cancer. Mayo Clin. Proc. 2021, 96, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Chahoud, J.; Zacharias, N.M.; Pham, R.; Qiao, W.; Guo, M.; Lu, X.; Alaniz, A.; Segarra, L.; Martinez-Ferrer, M.; Gleber-Netto, F.O.; et al. Prognostic Significance of P16 and Its Relationship with Human Papillomavirus Status in Patients with Penile Squamous Cell Carcinoma: Results of 5 Years Follow-Up. Cancers 2022, 14, 6024. [Google Scholar] [CrossRef] [PubMed]
- Daling, J.R.; Madeleine, M.M.; Johnson, L.G.; Schwartz, S.M.; Shera, K.A.; Wurscher, M.A.; Carter, J.J.; Porter, P.L.; Galloway, D.A.; McDougall, J.K.; et al. Penile Cancer: Importance of Circumcision, Human Papillomavirus and Smoking in in Situ and Invasive Disease. Int. J. Cancer 2005, 116, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Kidd, L.C.; Chaing, S.; Chipollini, J.; Giuliano, A.R.; Spiess, P.E.; Sharma, P. Relationship between Human Papillomavirus and Penile Cancer—Implications for Prevention and Treatment. Transl. Androl. Urol. 2017, 6, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Sand, F.L.; Rasmussen, C.L.; Albieri, V.; Toft, B.G.; Norrild, B.; Munk, C.; Kjær, S.K. Prevalence of Human Papillomavirus DNA and p16INK4a in Penile Cancer and Penile Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis. Lancet Oncol. 2019, 20, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Zhuang, T.; Wu, S.; Brown, J.T.; Magee, D.; Carthon, B.C.; Kucuk, O.; Nabhan, C.; Barata, P.C.; Heath, E.I.; et al. Comprehensive Genomic Profiling of Penile Squamous Cell Carcinoma and the Impact of Human Papillomavirus Status on Immune-Checkpoint Inhibitor-Related Biomarkers. Cancer 2023, 129, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Cilio, S.; Tufano, A.; Pezone, G.; Alvino, P.; Spena, G.; Pandolfo, S.D.; Del Prete, P.; Amato, C.; Damiano, R.; Salonia, A.; et al. Sexual Outcomes after Conservative Management for Patients with Localized Penile Cancer. Curr. Oncol. 2023, 30, 10501–10508. [Google Scholar] [CrossRef]
- Chahoud, J.; Pham, R.; Sonpavde, G. Innovative Systemic Therapies for Penile Cancer. Curr. Opin. Urol. 2022, 32, 8. [Google Scholar] [CrossRef]
- Chadha, J.; Chahoud, J.; Spiess, P.E. An Update on Treatment of Penile Cancer. Ther. Adv. Med. Oncol. 2022, 14, 17588359221127254. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Chadha, J.; Chahoud, J. Penile Cancer: Updates in Systemic Therapy. Asian J. Urol. 2022, 9, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Chahoud, J.; Tamil, M.; Necchi, A. Second Line Salvage Systemic Therapy for Advanced Penile Cancer. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Chahoud, J.; Campbell, M.T.; Karp, D.D.; Wang, J.; Stephen, B.; Tu, S.-M.; Pettaway, C.A.; Naing, A. Pembrolizumab for Advanced Penile Cancer: A Case Series from a Phase II Basket Trial. Investig. New Drugs 2021, 39, 1405–1410. [Google Scholar] [CrossRef]
- Chahoud, J.; Skelton, W.P.; Spiess, P.E.; Walko, C.; Dhillon, J.; Gage, K.L.; Johnstone, P.A.S.; Jain, R.K. Case Report: Two Cases of Chemotherapy Refractory Metastatic Penile Squamous Cell Carcinoma With Extreme Durable Response to Pembrolizumab. Front. Oncol. 2020, 10, 615298. [Google Scholar] [CrossRef]
- de Vries, H.M.; Rafael, T.S.; Gil-Jimenez, A.; de Feijter, J.M.; Bekers, E.; van der Laan, E.; Lopez-Yurda, M.; Hooijberg, E.; Broeks, A.; Peters, D.; et al. Atezolizumab With or Without Radiotherapy for Advanced Squamous Cell Carcinoma of the Penis (The PERICLES Study): A Phase II Trial. JCO 2023, 41, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Spiess, P.E.; Necchi, A.; Pettaway, C.A.; Chahoud, J. Immune-Based Therapies in Penile Cancer. Nat. Rev. Urol. 2022, 19, 457–474. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Falasiri, S.; Hajiran, A.; Chahoud, J.; Spiess, P.E. The Immune Microenvironment in Penile Cancer and Rationale for Immunotherapy. J. Clin. Med. 2020, 9, 3334. [Google Scholar] [CrossRef]
- Aydin, A.M.; Chahoud, J.; Adashek, J.J.; Azizi, M.; Magliocco, A.; Ross, J.S.; Necchi, A.; Spiess, P.E. Understanding Genomics and the Immune Environment of Penile Cancer to Improve Therapy. Nat. Rev. Urol. 2020, 17, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-Analysis. JAMA Oncol. 2019, 5, 1195–1204. [Google Scholar] [CrossRef]
- Zargar-Shoshtari, K.; Spiess, P.E.; Berglund, A.E.; Sharma, P.; Powsang, J.M.; Giuliano, A.; Magliocco, A.M.; Dhillon, J. Clinical Significance of P53 and P16(Ink4a) Status in a Contemporary North American Penile Carcinoma Cohort. Clin. Genitourin. Cancer 2016, 14, 346–351. [Google Scholar] [CrossRef]
- Nuovo, G.J.; Hochman, H.A.; Eliezri, Y.D.; Lastarria, D.; Comite, S.L.; Silvers, D.N. Detection of Human Papillomavirus DNA in Penile Lesions Histologically Negative for Condylomata. Analysis by in Situ Hybridization and the Polymerase Chain Reaction. Am. J. Surg. Pathol. 1990, 14, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Uraoka, N.; Jiang, M.; Cook, P.; Gibbons, D.; Forget, M.-A.; Bernatchez, C.; Haymaker, C.; Wistuba, I.I.; Rodriguez-Canales, J. Validation of Multiplex Immunofluorescence Panels Using Multispectral Microscopy for Immune-Profiling of Formalin-Fixed and Paraffin-Embedded Human Tumor Tissues. Sci. Rep. 2017, 7, 13380. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Choi, J.; Mani, N.; Sanmamed, M.F.; Datar, I.; Sowell, R.; Du, V.Y.; Kaftan, E.; Goldberg, S.; Dong, W.; et al. A Dormant TIL Phenotype Defines Non-Small Cell Lung Carcinomas Sensitive to Immune Checkpoint Blockers. Nat. Commun. 2018, 9, 3196. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Nguyen, P.; Engle, E.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Green, B.; Soni, A.; Cuda, J.D.; Stein, J.E.; et al. Multidimensional, Quantitative Assessment of PD-1/PD-L1 Expression in Patients with Merkel Cell Carcinoma and Association with Response to Pembrolizumab. J. Immunother. Cancer 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.M.; Harris, W.H.; Smith, P.; Millis, R.R.; Rubens, R.D. Immunohistochemical Determination of Oestrogen Receptor: Comparison of Different Methods of Assessment of Staining and Correlation with Clinical Outcome of Breast Cancer Patients. Br. J. Cancer 1996, 74, 1445–1451. [Google Scholar] [CrossRef]
- Parra, E.R. Methods to Determine and Analyze the Cellular Spatial Distribution Extracted From Multiplex Immunofluorescence Data to Understand the Tumor Microenvironment. Front. Mol. Biosci. 2021, 8, 668340. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New Insights into M1/M2 Macrophages: Key Modulators in Cancer Progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next Generation of Immune Checkpoint Therapy in Cancer: New Developments and Challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef]
- Van den Eynde, B.J.; van Baren, N.; Baurain, J.-F. Is There a Clinical Future for IDO1 Inhibitors After the Failure of Epacadostat in Melanoma? Annu. Rev. Cancer Biol. 2020, 4, 241–256. [Google Scholar] [CrossRef]
- Mantovani, A.; Longo, D.L. Macrophage Checkpoint Blockade in Cancer—Back to the Future. New Engl. J. Med. 2018, 379, 1777–1779. [Google Scholar] [CrossRef]
- Weiskopf, K.; Jahchan, N.S.; Schnorr, P.J.; Cristea, S.; Ring, A.M.; Maute, R.L.; Volkmer, A.K.; Volkmer, J.-P.; Liu, J.; Lim, J.S.; et al. CD47-Blocking Immunotherapies Stimulate Macrophage-Mediated Destruction of Small-Cell Lung Cancer. J. Clin. Investig. 2016, 126, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- de la Iglesia, J.V.; Slebos, R.J.C.; Martin-Gomez, L.; Wang, X.; Teer, J.K.; Tan, A.C.; Gerke, T.A.; Aden-Buie, G.; van Veen, T.; Masannat, J.; et al. Effects of Tobacco Smoking on the Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2020, 26, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Zheng, W.; Zhang, L.; Yao, N. CD8+ T-Cell Exhaustion in the Tumor Microenvironment of Head and Neck Squamous Cell Carcinoma Determines Poor Prognosis. Ann. Transl. Med. 2022, 10, 273. [Google Scholar] [CrossRef]
- Haist, M.; Kaufmann, J.; Kur, I.-M.; Zimmer, S.; Grabbe, S.; Schmidberger, H.; Weigert, A.; Mayer, A. Response to Primary Chemoradiotherapy of Locally Advanced Oropharyngeal Carcinoma Is Determined by the Degree of Cytotoxic T Cell Infiltration within Tumor Cell Aggregates. Front. Immunol. 2023, 14, 1070203. [Google Scholar] [CrossRef]
- Tosi, A.; Parisatto, B.; Menegaldo, A.; Spinato, G.; Guido, M.; Del Mistro, A.; Bussani, R.; Zanconati, F.; Tofanelli, M.; Tirelli, G.; et al. The Immune Microenvironment of HPV-Positive and HPV-Negative Oropharyngeal Squamous Cell Carcinoma: A Multiparametric Quantitative and Spatial Analysis Unveils a Rationale to Target Treatment-Naïve Tumors with Immune Checkpoint Inhibitors. J. Exp. Clin. Cancer Res. 2022, 41, 279. [Google Scholar] [CrossRef]
- Wu, J.; Guo, Q.; Zhu, J.; Wu, Y.; Wang, S.; Liang, S.; Ju, X.; Wu, X. Developing a Nomogram for Preoperative Prediction of Cervical Cancer Lymph Node Metastasis by Multiplex Immunofluorescence. BMC Cancer 2023, 23, 485. [Google Scholar] [CrossRef] [PubMed]
- Lopez de Rodas, M.; Nagineni, V.; Ravi, A.; Datar, I.J.; Mino-Kenudson, M.; Corredor, G.; Barrera, C.; Behlman, L.; Rimm, D.L.; Herbst, R.S.; et al. Role of Tumor Infiltrating Lymphocytes and Spatial Immune Heterogeneity in Sensitivity to PD-1 Axis Blockers in Non-Small Cell Lung Cancer. J. Immunother. Cancer 2022, 10, e004440. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Jiang, T.; Chen, G.; Huang, Y.; Zhou, J.; Lin, L.; Feng, J.; Wang, Z.; Shu, Y.; Shi, J.; et al. Multiplexed Imaging of Tumor Immune Microenvironmental Markers in Locally Advanced or Metastatic Non-Small-Cell Lung Cancer Characterizes the Features of Response to PD-1 Blockade plus Chemotherapy. Cancer Commun. 2022, 42, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Zhang, J.; Jiang, M.; Tamegnon, A.; Pandurengan, R.K.; Behrens, C.; Solis, L.; Haymaker, C.; Heymach, J.V.; Moran, C.; et al. Immune Cellular Patterns of Distribution Affect Outcomes of Patients with Non-Small Cell Lung Cancer. Nat. Commun. 2023, 14, 2364. [Google Scholar] [CrossRef]
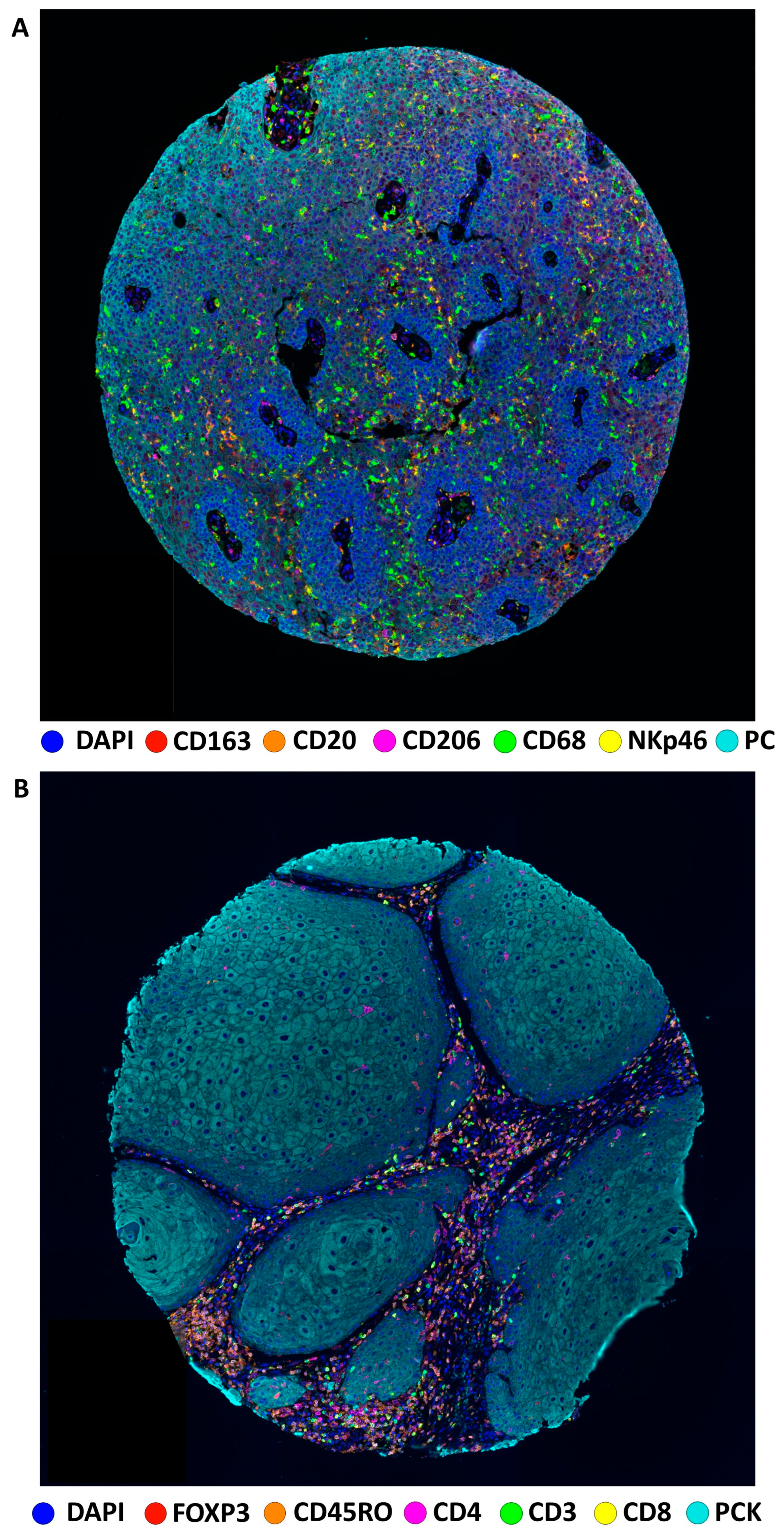
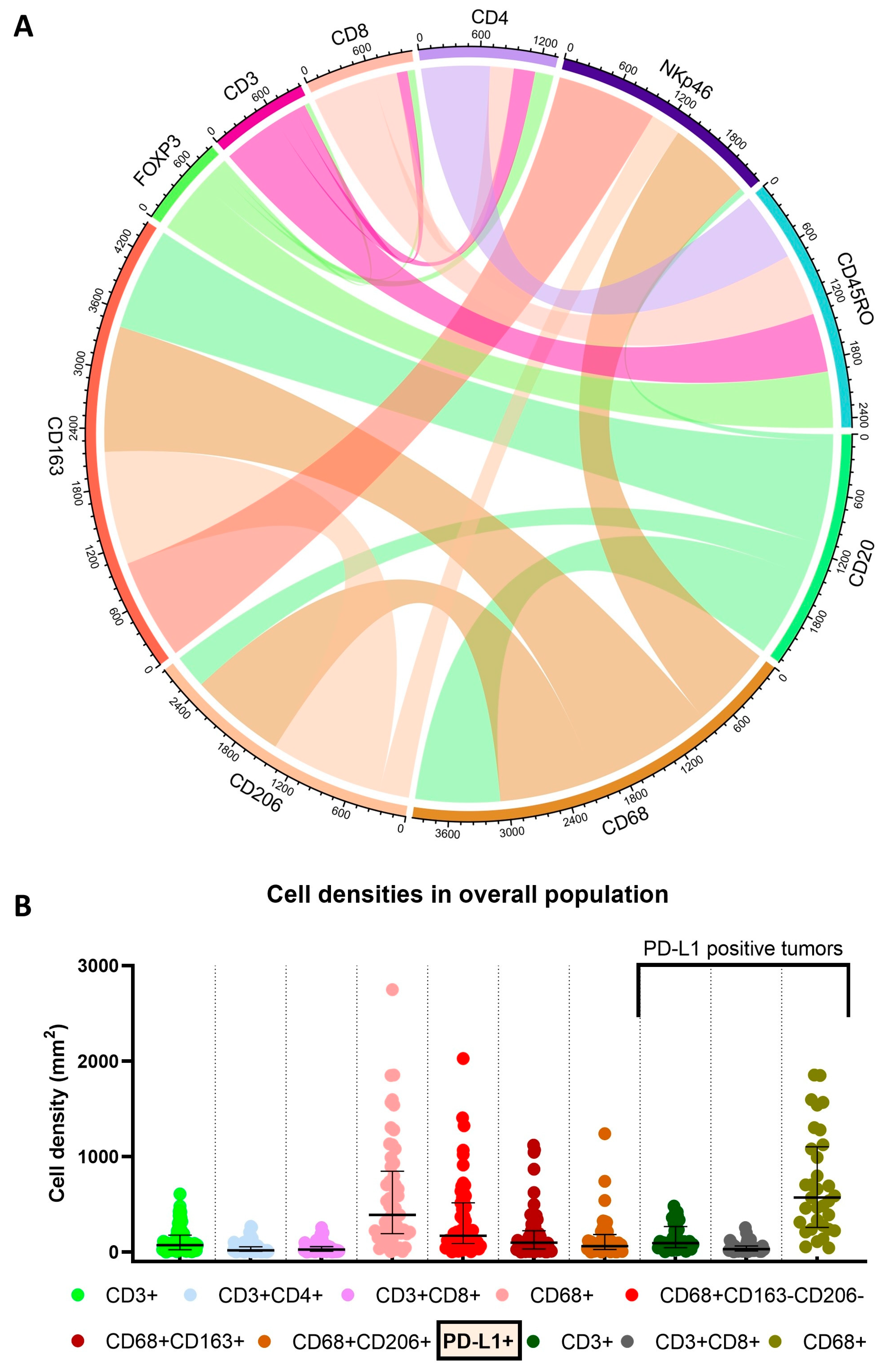
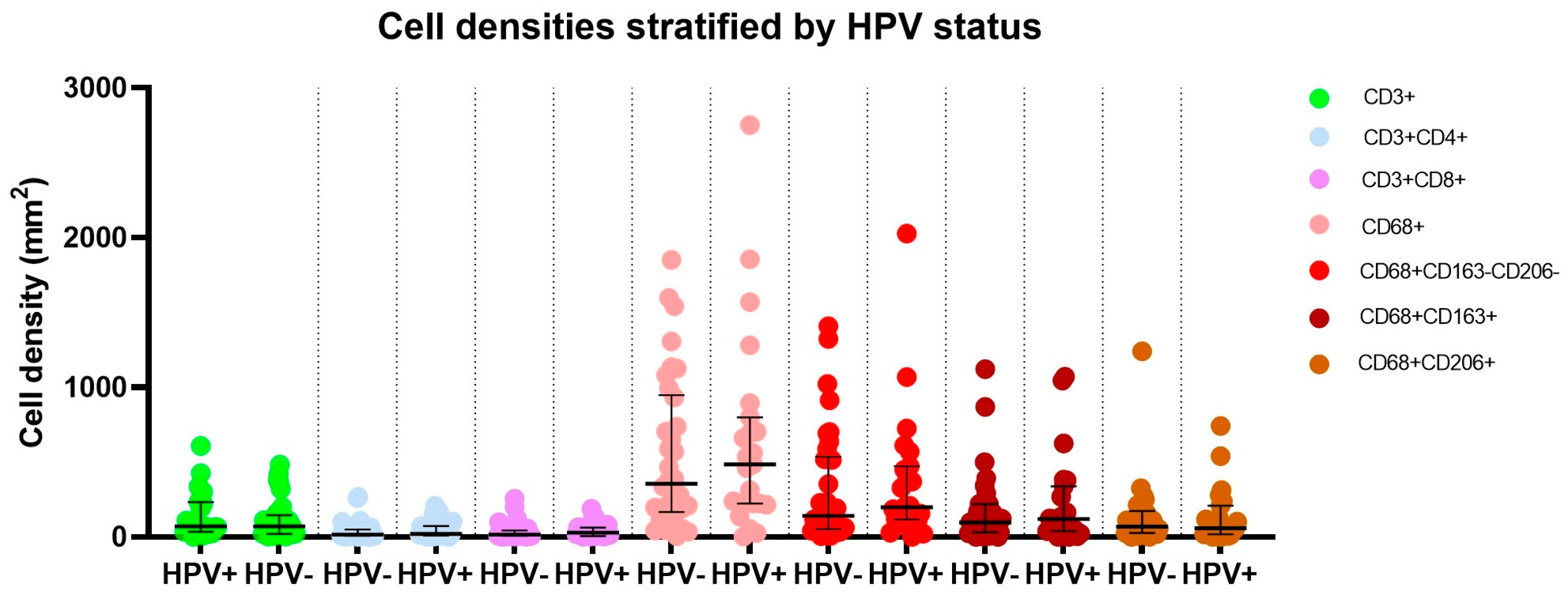
| Median age in years at surgery (IQR, range) | 59.9 (53.1–73.4, 30.5–92.0) |
| White race | 44 (77%) |
| HPV positive | 23 (40%) |
| P16 positive | 24 (42%) |
| Primary surgery | |
| Partial penectomy ± scrotectomy | 40 (70%) |
| Total penectomy | 12 (21%) |
| Wide local excision | 4 (7%) |
| Circumcision | 1 (2%) |
| Grade | |
| 1 | 38 (67%) |
| 2 | 16 (28%) |
| 3 | 3 (5%) |
| Lymphovascular invasion present | 32 (56%) |
| pT stage | |
| 1 | 17 (30%) |
| 2 | 16 (28%) |
| 3 | 22 (39%) |
| 4 | 2 (3%) |
| pN stage | |
| 0 | 26 (46%) |
| 1 | 6 (10%) |
| 2 | 20 (35%) |
| 3 | 5 (9%) |
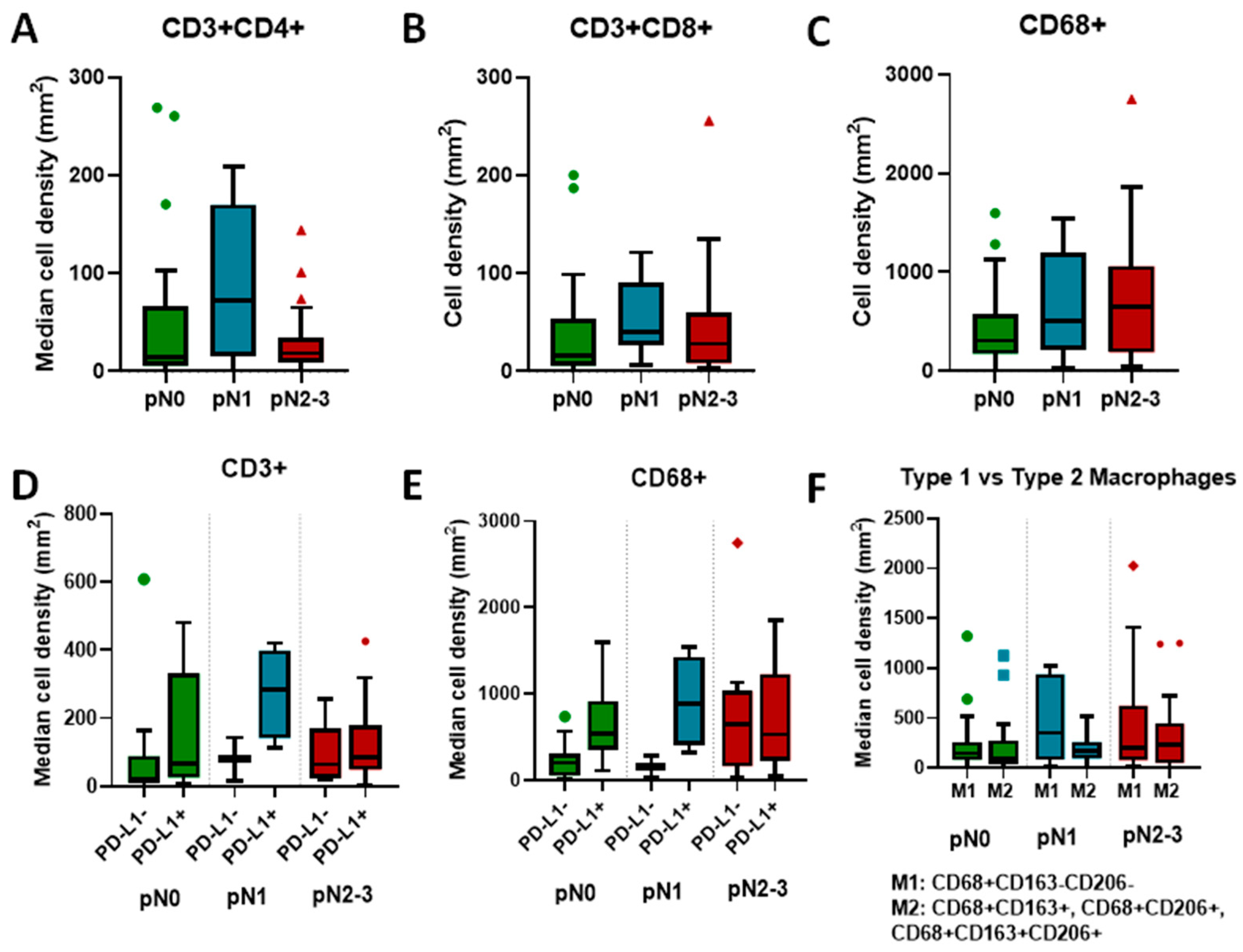
| Median Marker Density/mm2 (Range) | pN0 | pN1 | pN2-3 | p-Value |
|---|---|---|---|---|
| CD3+ | 44.2 | 188.4 | 77.9 | 0.15 |
| CD3+CD4+ | 14.8 | 71.7 | 18.5 | 0.29 |
| CD3+CD8+ | 16.2 | 40.2 | 28.2 | 0.19 |
| CD68+ | 306.4 | 502.5 | 648.3 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, F.; Nguyen, J.; Segura, C.M.; Paravathaneni, M.; Grass, G.D.; Johnstone, P.; Zacharias, N.M.; Pettaway, C.A.; Lu, X.; Kim, Y.; et al. Multiplex Immunofluorescence Captures Progressive Immune Exhaustion with Advancing Penile Squamous Cell Cancer Stage. Cancers 2024, 16, 303. https://doi.org/10.3390/cancers16020303
Ionescu F, Nguyen J, Segura CM, Paravathaneni M, Grass GD, Johnstone P, Zacharias NM, Pettaway CA, Lu X, Kim Y, et al. Multiplex Immunofluorescence Captures Progressive Immune Exhaustion with Advancing Penile Squamous Cell Cancer Stage. Cancers. 2024; 16(2):303. https://doi.org/10.3390/cancers16020303
Chicago/Turabian StyleIonescu, Filip, Jonathan Nguyen, Carlos Moran Segura, Mahati Paravathaneni, G. Daniel Grass, Peter Johnstone, Niki M. Zacharias, Curtis A. Pettaway, Xin Lu, Youngchul Kim, and et al. 2024. "Multiplex Immunofluorescence Captures Progressive Immune Exhaustion with Advancing Penile Squamous Cell Cancer Stage" Cancers 16, no. 2: 303. https://doi.org/10.3390/cancers16020303
APA StyleIonescu, F., Nguyen, J., Segura, C. M., Paravathaneni, M., Grass, G. D., Johnstone, P., Zacharias, N. M., Pettaway, C. A., Lu, X., Kim, Y., Whiting, J., Dhillon, J., Eschrich, S. A., Chadha, J., Gullapalli, K., Roman Souza, G., Miyagi, H., Manley, B. J., Spiess, P. E., & Chahoud, J. (2024). Multiplex Immunofluorescence Captures Progressive Immune Exhaustion with Advancing Penile Squamous Cell Cancer Stage. Cancers, 16(2), 303. https://doi.org/10.3390/cancers16020303











