The Role of Capsule Endoscopy in the Diagnosis and Management of Small Bowel Tumors: A Narrative Review
Simple Summary
Abstract
1. Background and Definitions
1.1. Capsule Endoscopy
1.2. Small Bowel Tumors
1.3. Malignant Small Bowel Tumors
1.4. Benign Small Bowel Tumors
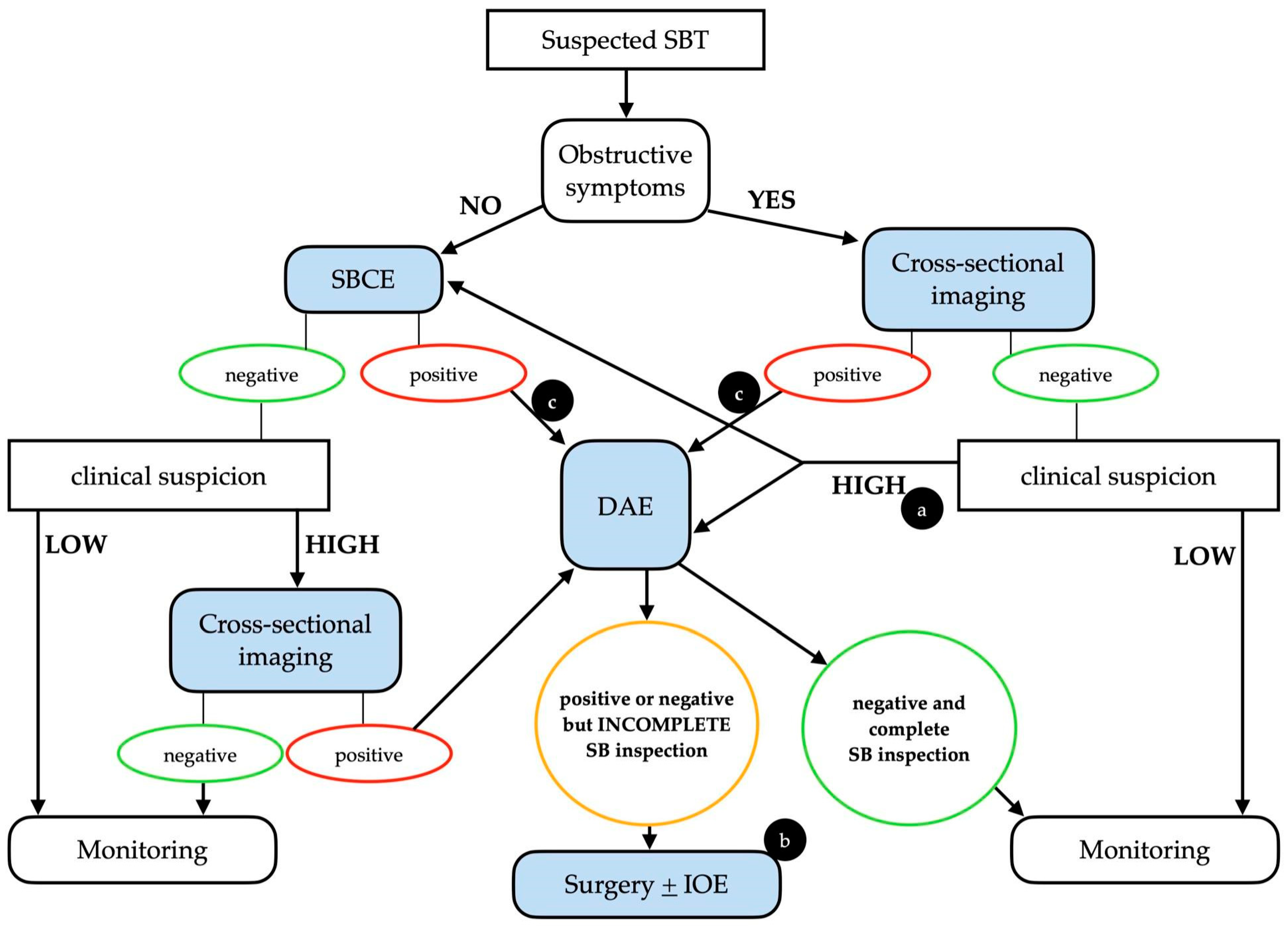
2. Diagnosis
2.1. When to Suspect a Small Bowel Tumor
2.2. Diagnostic Performance of Small Bowel Capsule Endoscopy
2.3. Small Bowel Capsule Endoscopy Limitations
2.4. Complementary Diagnostic Techniques
2.4.1. Device-Assisted Enteroscopy
2.4.2. Cross-Sectional Imaging
3. Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CTE | computed tomography enterography |
| DAE | device-assisted enteroscopy |
| DBE | double-balloon enteroscopy |
| DY | diagnostic yield |
| ESGE | European Society of Gastrointestinal Endoscopy |
| GI | gastrointestinal |
| GIST | gastrointestinal stromal tumor |
| HPS | hereditary polyposis syndromes |
| MRE | magnetic resonance enterography |
| NET | neuroendocrine tumor |
| PET | positron-emission tomography |
| SB | small bowel |
| SBCE | small bowel capsule endoscopy |
| SBE | single-balloon enteroscopy |
| SBT | small bowel tumor |
| SEL | subepithelial lesion |
| SPICE | smooth protruding lesion index on capsule endoscopy |
References
- Pennazio, M.; Venezia, L.; Cortegoso Valdivia, P.; Rondonotti, E. Device-assisted enteroscopy: An update on techniques, clinical indications and safety. Dig. Liver Dis. 2019, 51, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Morrison, H. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 2011, 3, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Rondonotti, E.; Koulaouzidis, A.; Georgiou, J.; Pennazio, M. Small bowel tumours: Update in diagnosis and management. Curr. Opin. Gastroenterol. 2018, 34, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, E.; Koffas, A.; Toumpanakis, C.; Keuchel, M. Updates in the diagnosis and management of small-bowel tumors. Best. Pract. Res. Clin. Gastroenterol. 2023, 64–65, 101860. [Google Scholar] [CrossRef] [PubMed]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 6785. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, M.; Rondonotti, E.; Pellicano, R.; Cortegoso Valdivia, P. Small bowel capsule endoscopy: Where do we stand after 20 years of clinical use? Minerva Gastroenterol. 2021, 67, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, M.; Spada, C.; Eliakim, R.; Keuchel, M.; May, A.; Mulder, C.J.; Rondonotti, E.; Adler, S.N.; Albert, J.; Baltes, P.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015, 47, 352–376. [Google Scholar] [CrossRef]
- Rondonotti, E.; Spada, C.; Adler, S.; May, A.; Despott, E.J.; Koulaouzidis, A.; Panter, S.; Domagk, D.; Fernandez-Urien, I.; Rahmi, G.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2018, 50, 423–446. [Google Scholar] [CrossRef]
- Lu, Y.; Fröbom, R.; Lagergren, J. Incidence patterns of small bowel cancer in a population-based study in Sweden: Increase in duodenal adenocarcinoma. Cancer Epidemiol. 2012, 36, e158–e163. [Google Scholar] [CrossRef]
- Bojesen, R.D.; Andersson, M.; Riis, L.B.; Nielsen, O.H.; Jess, T. Incidence of, phenotypes of and survival from small bowel cancer in Denmark, 1994-2010: A population-based study. J. Gastroenterol. 2016, 51, 891–899. [Google Scholar] [CrossRef]
- Cancer of the Small Intestine—Cancer Stat Facts. SEER. [Online]. Available online: https://seer.cancer.gov/statfacts/html/smint.html (accessed on 23 October 2023).
- Qubaiah, O.; Devesa, S.S.; Platz, C.E.; Huycke, M.M.; Dores, G.M. Small intestinal cancer: A population-based study of incidence and survival patterns in the United States, 1992 to 2006. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.A.; Yung, D.E.; Joshi, A.; Plevris, J.N.; Koulaouzidis, A. Small bowel malignancy in patients undergoing capsule endoscopy at a tertiary care academic center: Case series and review of the literature. Endosc. Int. Open 2017, 5, E463–E470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weiss, N.S.; Yang, C.P. Incidence of histologic types of cancer of the small intestine. J. Natl. Cancer Inst. 1987, 78, 653–656. [Google Scholar] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Rawla, P.; Barsouk, A.; Thandra, K.C. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med. Sci. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Sakae, H.; Kanzaki, H.; Nasu, J.; Akimoto, Y.; Matsueda, K.; Yoshioka, M.; Nakagawa, M.; Hori, S.; Inoue, M.; Inaba, T.; et al. The characteristics and outcomes of small bowel adenocarcinoma: A multicentre retrospective observational study. Br. J. Cancer 2017, 117, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Henriques, J.; Manfredi, S.; Tougeron, D.; Bouché, O.; Pezet, D.; Piessen, G.; Coriat, R.; Zaanan, A.; Legoux, J.L.; et al. Small bowel adenocarcinoma: Results from a nationwide prospective ARCAD-NADEGE cohort study of 347 patients. Int. J. Cancer 2020, 147, 967–977. [Google Scholar] [CrossRef]
- Legué, L.M.; Bernards, N.; Gerritse, S.L.; van Oudheusden, T.R.; de Hingh, I.H.; Creemers, G.M.; Ten Tije, A.J.; Lemmens, V.E. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: A population-based study in The Netherlands. Acta Oncol. 2016, 55, 1183–1189. [Google Scholar] [CrossRef]
- Hatzaras, I.; Palesty, J.A.; Abir, F.; Sullivan, P.; Kozol, R.A.; Dudrick, S.J.; Longo, W.E. Small-bowel tumors: Epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch. Surg. 2007, 142, 229–235. [Google Scholar] [CrossRef]
- Sidhu, P.S.; McAlindon, M.E.; Drew, K.; Sidhu, R. The Utility of Capsule Endoscopy in Patients under 50 Years of Age with Recurrent Iron Deficiency Anaemia: Is the Juice Worth the Squeeze? Gastroenterol. Res. Pract. 2015, 2015, 948574. [Google Scholar] [CrossRef]
- Koulaouzidis, A.; Yung, D.E.; Lam, J.H.P.; Smirnidis, A.; Douglas, S.; Plevris, J.N. The use of small-bowel capsule endoscopy in iron-deficiency anemia alone; be aware of the young anemic patient. Scand. J. Gastroenterol. 2012, 47, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Farmer, R.G.; Hawk, W.A. Metastatic tumors of the small bowel. Gastroenterology 1964, 47, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, S.C.; Parker, A.; Canales, L. Metastatic tumors to the upper gastrointestinal tract: Endoscopic experience. Am. J. Gastroenterol. 1992, 87, 1418–1423. [Google Scholar] [PubMed]
- Richie, R.E.; Reynolds, V.H.; Sawyers, J.L. Tumor metastases to the small bowel from extra-abdominal sites. South. Med. J. 1973, 66, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Accessed, W.H.C. 2023. [Online]. Available online: https://www.pathologyoutlines.com/topic/smallbowelWHOclassification.html (accessed on 23 October 2023).
- Williamson, J.M.L.; Williamson, R.C.N. Small bowel tumors: Pathology; management. J. Med. Assoc. Thai 2014, 97, 126–137. [Google Scholar]
- Herbsman, H.; Wetstein, L.; Rosen, Y.; Orces, H.; Alfonso, A.E.; Iyer, S.K.; Gardner, B. Tumors of the small intestine. Curr. Probl. Surg. 1980, 17, 121–182. [Google Scholar] [CrossRef]
- Pennazio, M.; Rondonotti, E.; Despott, E.J.; Dray, X.; Keuchel, M.; Moreels, T.; Sanders, D.S.; Spada, C.; Carretero, C.; Cortegoso Valdivia, P.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2022. Endoscopy 2023, 55, 58–95. [Google Scholar] [CrossRef]
- Barret, M.; Malamut, G.; Rahmi, G.; Samaha, E.; Edery, J.; Verkarre, V.; Macintyre, E.; Lenain, E.; Chatellier, G.; Cerf-Bensussan, N.; et al. Diagnostic yield of capsule endoscopy in refractory celiac disease. Am. J. Gastroenterol. 2012, 107, 1546–1553. [Google Scholar] [CrossRef]
- Hadithi, M.; Al-toma, A.; Oudejans, J.; van Bodegraven, A.A.; Mulder, C.J.; Jacobs, M. The value of double-balloon enteroscopy in patients with refractory celiac disease. Am. J. Gastroenterol. 2007, 102, 987–996. [Google Scholar] [CrossRef]
- Tomba, C.; Elli, L.; Bardella, M.T.; Soncini, M.; Contiero, P.; Roncoroni, L.; Locatelli, M.; Conte, D. Enteroscopy for the early detection of small bowel tumours in at-risk celiac patients. Dig. Liver Dis. 2014, 46, 400–404. [Google Scholar] [CrossRef]
- Zammit, S.C.; Elli, L.; Scaramella, L.; Sanders, D.S.; Tontini, G.E.; Sidhu, R. Small bowel capsule endoscopy in refractory celiac disease: A luxury or a necessity? Ann. Gastroenterol. 2021, 34, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zammit, S.C.; Sanders, D.S.; Cross, S.S.; Sidhu, R. Capsule endoscopy in the management of refractory coeliac disease. J. Gastrointest. Liver Dis. 2019, 28, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Branchi, F.; Orlando, S.; Roncoroni, L.; Barigelletti, G.; Fabiano, S.; Vecchi, M.; Penagini, R.; Doneda, L.; Elli, L. Effectiveness of Capsule Endoscopy and Double-Balloon Enteroscopy in Suspected Complicated Celiac Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 941–949.e3. [Google Scholar] [CrossRef] [PubMed]
- van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Dekker, E.; Jover, R.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Pellisé, M.; Saurin, J.C.; et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Valentin, M.; Haupt, S.; Seppälä, T.T.; Sampson, J.R.; Sunde, L.; Bernstein, I.; Jenkins, M.A.; Engel, C.; Aretz, S.; Nielsen, M.; et al. Mortality by age, gene and gender in carriers of pathogenic mismatch repair gene variants receiving surveillance for early cancer diagnosis and treatment: A report from the prospective Lynch syndrome database. eClinicalMedicine 2023, 58, 101909. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.S.; Eisen, G.M.; Friedman, S. A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy 2005, 37, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Lee, O.Y.; Jeen, Y.T.; Lim, C.Y.; Cheung, D.Y.; Cheon, J.H.; Ye, B.D.; Song, H.J.; Kim, J.S.; Do, J.H.; et al. Indications for Detection, Completion, and Retention Rates of Small Bowel Capsule Endoscopy Based on the 10-Year Data from the Korean Capsule Endoscopy Registry. Clin. Endosc. 2015, 48, 399–404. [Google Scholar] [CrossRef]
- Liao, Z.; Gao, R.; Xu, C.; Li, Z.-S. Indications; detection; completion, and retention rates of small-bowel capsule endoscopy: A systematic review. Gastrointest. Endosc. 2010, 71, 280–286. [Google Scholar] [CrossRef]
- Cortegoso Valdivia, P.; Skonieczna-Żydecka, K.; Elosua, A.; Sciberras, M.; Piccirelli, S.; Rullan, M.; Tabone, T.; Gawel, K.; Stachowski, A.; Lemiński, A.; et al. Indications, Detection, Completion and Retention Rates of Capsule Endoscopy in Two Decades of Use: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1105. [Google Scholar] [CrossRef]
- Rondonotti, E.; Pennazio, M.; Toth, E.; Menchen, P.; Riccioni, M.E.; De Palma, G.D.; Scotto, F.; De Looze, D.; Pachofsky, T.; Tacheci, I.; et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: A multicenter European study. Endoscopy 2008, 40, 488–495. [Google Scholar] [CrossRef]
- Yung, D.E.; Rondonotti, E.; Giannakou, A.; Avni, T.; Rosa, B.; Toth, E.; Lucendo, A.J.; Sidhu, R.; Beaumont, H.; Ellul, P.; et al. Capsule endoscopy in young patients with iron deficiency anaemia and negative bidirectional gastrointestinal endoscopy. United Eur. Gastroenterol. J. 2017, 5, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Gionchetti, P.; Calafiore, A.; Pagano, N.; Campieri, M.; Rizzello, F. Sporadic small bowel tumors detected by capsule endoscopy in patients with occult gastrointestinal bleeding. Intern. Emerg. Med. 2015, 10, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Segarajasingam, D.S.; Hanley, S.C.; Barkun, A.N.; Waschke, K.A.; Burtin, P.; Parent, J.; Mayrand, S.; Fallone, C.A.; Jobin, G.; Seidman, E.G.; et al. Randomized controlled trial comparing outcomes of video capsule endoscopy with push enteroscopy in obscure gastrointestinal bleeding. Can. J. Gastroenterol. Hepatol. 2015, 29, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ooka, S.; Kobayashi, K.; Kawagishi, K.; Kodo, M.; Yokoyama, K.; Sada, M.; Tanabe, S.; Koizumi, W. Roles of Capsule Endoscopy and Single-Balloon Enteroscopy in Diagnosing Unexplained Gastrointestinal Bleeding. Clin. Endosc. 2016, 49, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Kakiya, Y.; Shiba, M.; Okamoto, J.; Kato, K.; Minamino, H.; Ominami, M.; Fukunaga, S.; Nagami, Y.; Sugimori, S.; Tanigawa, T.; et al. A comparison between capsule endoscopy and double balloon enteroscopy using propensity score-matching analysis in patients with previous obscure gastrointestinal bleeding. Scand. J. Gastroenterol. 2017, 52, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cuadrado-Robles, E.; Esteban-Delgado, P.; Martínez-Andrés, B.; Zamora-Nava, L.E.; Rodrigo-Agudo, J.L.; Chacón-Martínez, S.; Torrella-Cortes, E.; Shanabo, J.; López-Higueras, A.; Muñoz-Bertrán, E.; et al. Diagnosis agreement between capsule endoscopy and double-balloon enteroscopy in obscure gastrointestinal bleeding at a referral center. Rev. Esp. Enferm. Dig. 2015, 107, 495–500. [Google Scholar] [CrossRef][Green Version]
- Limsrivilai, J.; Srisajjakul, S.; Pongprasobchai, S.; Leelakusolvong, S.; Tanwandee, T. A Prospective Blinded Comparison of Video Capsule Endoscopy Versus Computed Tomography Enterography in Potential Small Bowel Bleeding: Clinical Utility of Computed Tomography Enterography. J. Clin. Gastroenterol. 2017, 51, 611–618. [Google Scholar] [CrossRef]
- Chu, Y.; Wu, S.; Qian, Y.; Wang, Q.; Li, J.; Tang, Y.; Bai, T.; Wang, L. Complimentary Imaging Modalities for Investigating Obscure Gastrointestinal Bleeding: Capsule Endoscopy, Double-Balloon Enteroscopy, and Computed Tomographic Enterography. Gastroenterol. Res. Pract. 2016, 2016, 8367519. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, L.; Zhang, T.; Sun, P.; Sun, J.; Zhou, J.; Wang, L.; Fan, R.; Wang, Z.; Cheng, S.; et al. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: Experience from a Chinese tertiary hospital. Turk. J. Gastroenterol. 2020, 31, 30–35. [Google Scholar] [CrossRef]
- Gupta, A.; Postgate, A.J.; Burling, D.; Ilangovan, R.; Marshall, M.; Phillips, R.K.; Clark, S.K.; Fraser, C.H. A prospective study of MR enterography versus capsule endoscopy for the surveillance of adult patients with Peutz-Jeghers syndrome. AJR Am. J. Roentgenol. 2010, 195, 108–116. [Google Scholar] [CrossRef]
- Caspari, R.; von Falkenhausen, M.; Krautmacher, C.; Schild, H.; Heller, J.; Sauerbruch, T. Comparison of capsule endoscopy and magnetic resonance imaging for the detection of polyps of the small intestine in patients with familial adenomatous polyposis or with Peutz-Jeghers’ syndrome. Endoscopy 2004, 36, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Hong, S.N.; Jang, H.J.; Jeon, S.R.; Cha, J.M.; Park, S.J.; Byeon, J.S.; Ko, B.M.; Kim, E.R.; Choi, H.; et al. Clinical Efficacy of Various Diagnostic Tests for Small Bowel Tumors and Clinical Features of Tumors Missed by Capsule Endoscopy. Gastroenterol. Res. Pract. 2015, 2015, 623208. [Google Scholar] [CrossRef] [PubMed]
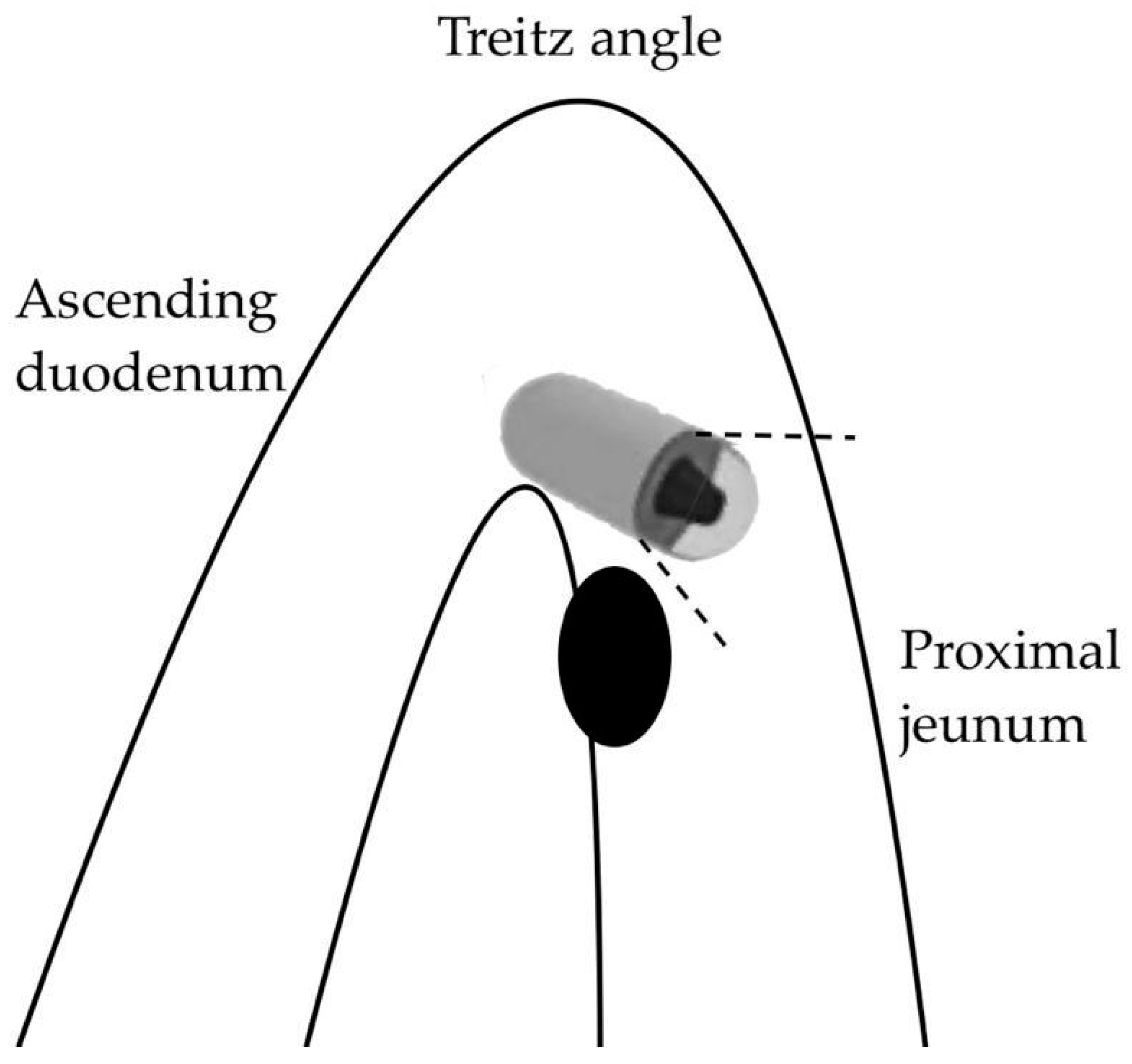
- Hirata, I.; Tsuboi, A.; Oka, S.; Sumioka, A.; Iio, S.; Hiyama, Y.; Kotachi, T.; Yuge, R.; Hayashi, R.; Urabe, Y.; et al. Diagnostic yield of proximal jejunal lesions with third-generation capsule endoscopy. DEN Open 2023, 3, e134. [Google Scholar] [CrossRef] [PubMed]
- Yung, D.; Robertson, A.R.; Davie, M.; Sidhu, R.; McAlindon, M.; Rahman, I.; Patel, P.; Sinha, L.; Mason, S.; Brzeszczynska, J.; et al. Double-headed small-bowel capsule endoscopy: Real-world experience from a multi-centre British study. Dig. Liver Dis. 2021, 53, 461–466. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.; Sihag, S.; Deane, C.; Walker, C.; Semenov, S.; Ryan, B.; Breslin, N.; O’Connor, A.; O’Donnell, S.; McNamara, D. Single or double headed capsules for the investigation of suspected small bowel bleeding: Are two heads better than one. Front. Gastroenterol. 2022, 1, 1071797. Available online: https://www.frontiersin.org/articles/10.3389/fgstr.2022.1071797 (accessed on 29 October 2023). [CrossRef]
- Yung, D.E.; Plevris, J.N.; Leenhardt, R.; Dray, X.; Koulaouzidis, A.; ESGE Small Bowel Research Working Group. Poor Quality of Small Bowel Capsule Endoscopy Images Has a Significant Negative Effect in the Diagnosis of Small Bowel Malignancy. Clin. Exp. Gastroenterol. 2020, 13, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, R.N.; Dolan, S.G.; Barlow, J.M.; Wells, M.L.; Sheedy, S.P.; Fidler, J.L.; Hansel, S.; Harmsen, S.; Fletcher, J.G. Impact of CT enterography on the diagnosis of small bowel gastrointestinal stromal tumors. Abdom. Radiol. 2017, 42, 1365–1373. [Google Scholar] [CrossRef]
- Gangi, A.; Siegel, E.; Barmparas, G.; Lo, S.; Jamil, L.H.; Hendifar, A.; Nissen, N.N.; Wolin, E.M.; Amersi, F. Multifocality in Small Bowel Neuroendocrine Tumors. J. Gastrointest. Surg. 2018, 22, 303–309. [Google Scholar] [CrossRef]
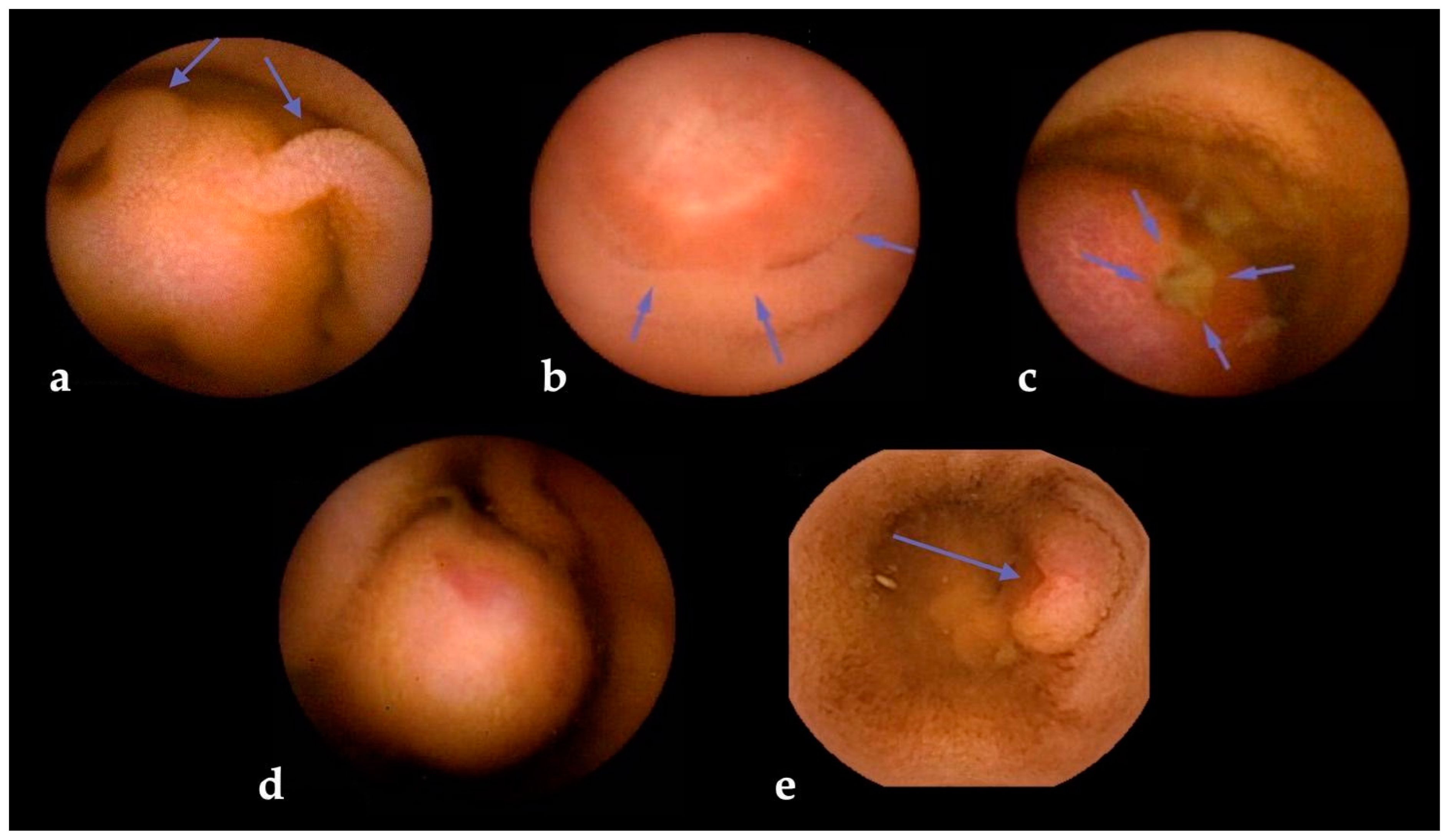
- Rosa, B.; Margalit-Yehuda, R.; Gatt, K.; Sciberras, M.; Girelli, C.; Saurin, J.C.; Cortegoso Valdivia, P.; Cotter, J.; Eliakim, R.; Caprioli, F.; et al. Scoring systems in clinical small-bowel capsule endoscopy: All you need to know! Endosc. Int. Open 2021, 9, E802–E823. [Google Scholar] [CrossRef]
- Girelli, C.M.; Porta, P.; Colombo, E.; Lesinigo, E.; Bernasconi, G. Development of a novel index to discriminate bulge from mass on small-bowel capsule endoscopy. Gastrointest. Endosc. 2011, 74, 1067–1074. [Google Scholar] [CrossRef]
- Sciberras, M.; Conti, K.; Elli, L.; Scaramella, L.; Riccioni, M.E.; Marmo, C.; Cadoni, S.; McAlindon, M.; Sidhu, R.; O’Hara, F.; et al. Score reproducibility and reliability in differentiating small bowel subepithelial masses from innocent bulges. Dig. Liver Dis. 2022, 54, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Amadi, C.; Gerson, L.B. Retention associated with video capsule endoscopy: Systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 1157–1168.e2. [Google Scholar] [CrossRef] [PubMed]
- Höög, C.M.; Bark, L.-Å.; Arkani, J.; Gorsetman, J.; Broström, O.; Sjöqvist, U. Capsule Retentions and Incomplete Capsule Endoscopy Examinations: An Analysis of 2300 Examinations. Gastroenterol. Res. Pract. 2012, 2012, 518718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Pan, J.; Liu, Y.W.; Sun, F.Y.; Qian, Y.Y.; Jiang, X.; Zou, W.B.; Xia, J.; Jiang, B.; Ru, N.; et al. Adverse events of video capsule endoscopy over the past two decades: A systematic review and proportion meta-analysis. BMC Gastroenterol. 2020, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xin, L.; Wang, Y.X.; Dong, Y.H.; Liao, Z.; Li, Z.S.; Du, Y.Q. Double-balloon enteroscopy for retrieving retained small-bowel video capsule endoscopes: A systematic review. Scand. J. Gastroenterol. 2020, 55, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Robles, E.P.; Delgado, P.E.; Conesa, P.B.; Andrés, B.M.; Guggiana, M.F.; Mateos, E.A.; Caballero, M.F.; Agudo, J.L.; Martínez, S.C.; Latorre, R.; et al. Role of double-balloon enteroscopy in malignant small bowel tumors. World J. Gastrointest. Endosc. 2015, 7, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Sekine, Y.; Sato, Y.; Higashizawa, T.; Miyata, T.; Iino, S.; Ido, K.; Sugano, K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest. Endosc. 2001, 53, 216–220. [Google Scholar] [CrossRef]
- Lee, B.I.; Choi, H.; Choi, K.Y.; Byeon, J.S.; Jang, H.J.; Eun, C.S.; Cheon, J.H.; Shin, S.J.; Kim, J.O.; Lee, M.S.; et al. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: KASID multi-center study. Dig. Dis. Sci. 2011, 56, 2920–2927. [Google Scholar] [CrossRef][Green Version]
- Murino, A.; Nakamura, M.; Watanabe, O.; Yamamura, T.; Nagura, A.; Yoshimura, T.; Nakano, A.; Goto, H.; Hirooka, Y. Effectiveness of Endoscopic Ultrasonography during Double Balloon Enteroscopy for characterization and management of small bowel submucosal tumours. Dig. Liver Dis. 2016, 48, 1187–1193. [Google Scholar] [CrossRef]
- Yamamoto, H.; Despott, E.J.; González-Suárez, B.; Pennazio, M.; Mönkemüller, K. The evolving role of device-assisted enteroscopy: The state of the art as of August 2023. Best Pract. Res. Clin. Gastroenterol. 2023, 64–65, 101858. [Google Scholar] [CrossRef]
- Pei-You, G.; Jun-Xia, L.; Feng-Li, L.; Liang-Ming, Z.; Hai-Zhu, X.; Yan-Bin, S. Retrospective comparison of Computed Tomography Enterography and Magnetic Resonance Enterography in diagnosing small intestine disease. J. Pak. Med. Assoc. 2015, 65, 710–714. [Google Scholar] [PubMed]
- Faggian, A.; Fracella, M.R.; D’Alesio, G.; Alabiso, M.E.; Berritto, D.; Feragalli, B.; Miele, V.; Iasiello, F.; Grassi, R. Small-Bowel Neoplasms: Role of MRI Enteroclysis. Gastroenterol. Res. Pract. 2015, 2016, e9686815. [Google Scholar] [CrossRef] [PubMed]
- Masselli, G.; Gualdi, G. CT and MR enterography in evaluating small bowel diseases: When to use which modality? Abdom. Imaging 2013, 38, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Soyer, P.; Aout, M.; Hoeffel, C.; Vicaut, E.; Placé, V.; Boudiaf, M. Helical CT-enteroclysis in the detection of small-bowel tumours: A meta-analysis. Eur. Radiol. 2013, 23, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, R.; Burling, D.; George, A.; Gupta, A.; Marshall, M.; Taylor, S.A. CT enterography: Review of technique and practical tips. Br. J. Radiol. 2012, 85, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.M.; Guglielmo, F.F.; Mitchell, D.G. How to perform and interpret cine MR enterography. J. Magn. Reson. Imaging 2015, 42, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Cronin, C.G.; Scott, J.; Kambadakone, A.; Catalano, O.A.; Sahani, D.; Blake, M.A.; McDermott, S. Utility of positron emission tomography/CT in the evaluation of small bowel pathology. BJR 2012, 85, 1211–1221. [Google Scholar] [CrossRef]
- Niederle, B.; Pape, U.F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef]
- Tziortziotis, I.; Laskaratos, F.-M.; Coda, S. Role of Artificial Intelligence in Video Capsule Endoscopy. Diagnostics 2021, 11, 1192. [Google Scholar] [CrossRef]
- ASGE Technology Committee; Wang, A.; Banerjee, S.; Barth, B.A.; Bhat, Y.M.; Chauhan, S.; Gottlieb, K.T.; Konda, V.; Maple, J.T.; Murad, F.; et al. Wireless capsule endoscopy. Gastrointest. Endosc. 2013, 78, 805–815. [Google Scholar] [CrossRef]
- Beg, S.; Card, T.; Sidhu, R.; Wronska, E.; Ragunath, K.; UK capsule endoscopy users’ group. The impact of reader fatigue on the accuracy of capsule endoscopy interpretation. Dig. Liver Dis. 2021, 53, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Dray, X.; Iakovidis, D.; Houdeville, C.; Jover, R.; Diamantis, D.; Histace, A.; Koulaouzidis, A. Artificial intelligence in small bowel capsule endoscopy—Current status, challenges and future promise. J. Gastroenterol. Hepatol. 2021, 36, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Aoki, T.; Aoyama, K.; Kato, Y.; Tsuboi, A.; Yamada, A.; Fujishiro, M.; Oka, S.; Ishihara, S.; Matsuda, T.; et al. Automatic detection and classification of protruding lesions in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest. Endosc. 2020, 92, 144–151.e1. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, R.; Koulaouzidis, A.; Histace, A.; Baatrup, G.; Beg, S.; Bourreille, A.; de Lange, T.; Eliakim, R.; Iakovidis, D.; Dam Jensen, M.; et al. Key research questions for implementation of artificial intelligence in capsule endoscopy. Therap Adv. Gastroenterol. 2022, 15, 17562848221132683. [Google Scholar] [CrossRef]
- Messmann, H.; Bisschops, R.; Antonelli, G.; Libânio, D.; Sinonquel, P.; Abdelrahim, M.; Ahmad, O.F.; Areia, M.; Bergman, J.J.G.H.M.; Bhandari, P.; et al. Expected value of artificial intelligence in gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2022, 54, 1211–1231. [Google Scholar] [CrossRef]
| Primary SB Malignant Tumors | Incidence Rate (/100,000 Person-Years) * | Mean Age at Diagnosis (Years) | Five-Year Relative Survival Rate (%) | Most Probable Location |
|---|---|---|---|---|
| NETs | 0.83 | 67–68 | 80 | Ileum |
| Carcinomas | 0.66 | 67–68 | 28 | Duodenum |
| Stromal tumors | 0.20 | 60–62 | 58 | All SB tracts |
| Lymphomas | 0.38 | 60–62 | 64 | All SB tracts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantasia, S.; Cortegoso Valdivia, P.; Kayali, S.; Koulaouzidis, G.; Pennazio, M.; Koulaouzidis, A. The Role of Capsule Endoscopy in the Diagnosis and Management of Small Bowel Tumors: A Narrative Review. Cancers 2024, 16, 262. https://doi.org/10.3390/cancers16020262
Fantasia S, Cortegoso Valdivia P, Kayali S, Koulaouzidis G, Pennazio M, Koulaouzidis A. The Role of Capsule Endoscopy in the Diagnosis and Management of Small Bowel Tumors: A Narrative Review. Cancers. 2024; 16(2):262. https://doi.org/10.3390/cancers16020262
Chicago/Turabian StyleFantasia, Stefano, Pablo Cortegoso Valdivia, Stefano Kayali, George Koulaouzidis, Marco Pennazio, and Anastasios Koulaouzidis. 2024. "The Role of Capsule Endoscopy in the Diagnosis and Management of Small Bowel Tumors: A Narrative Review" Cancers 16, no. 2: 262. https://doi.org/10.3390/cancers16020262
APA StyleFantasia, S., Cortegoso Valdivia, P., Kayali, S., Koulaouzidis, G., Pennazio, M., & Koulaouzidis, A. (2024). The Role of Capsule Endoscopy in the Diagnosis and Management of Small Bowel Tumors: A Narrative Review. Cancers, 16(2), 262. https://doi.org/10.3390/cancers16020262







