p38 Molecular Targeting for Next-Generation Multiple Myeloma Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. p38 Molecular Signaling
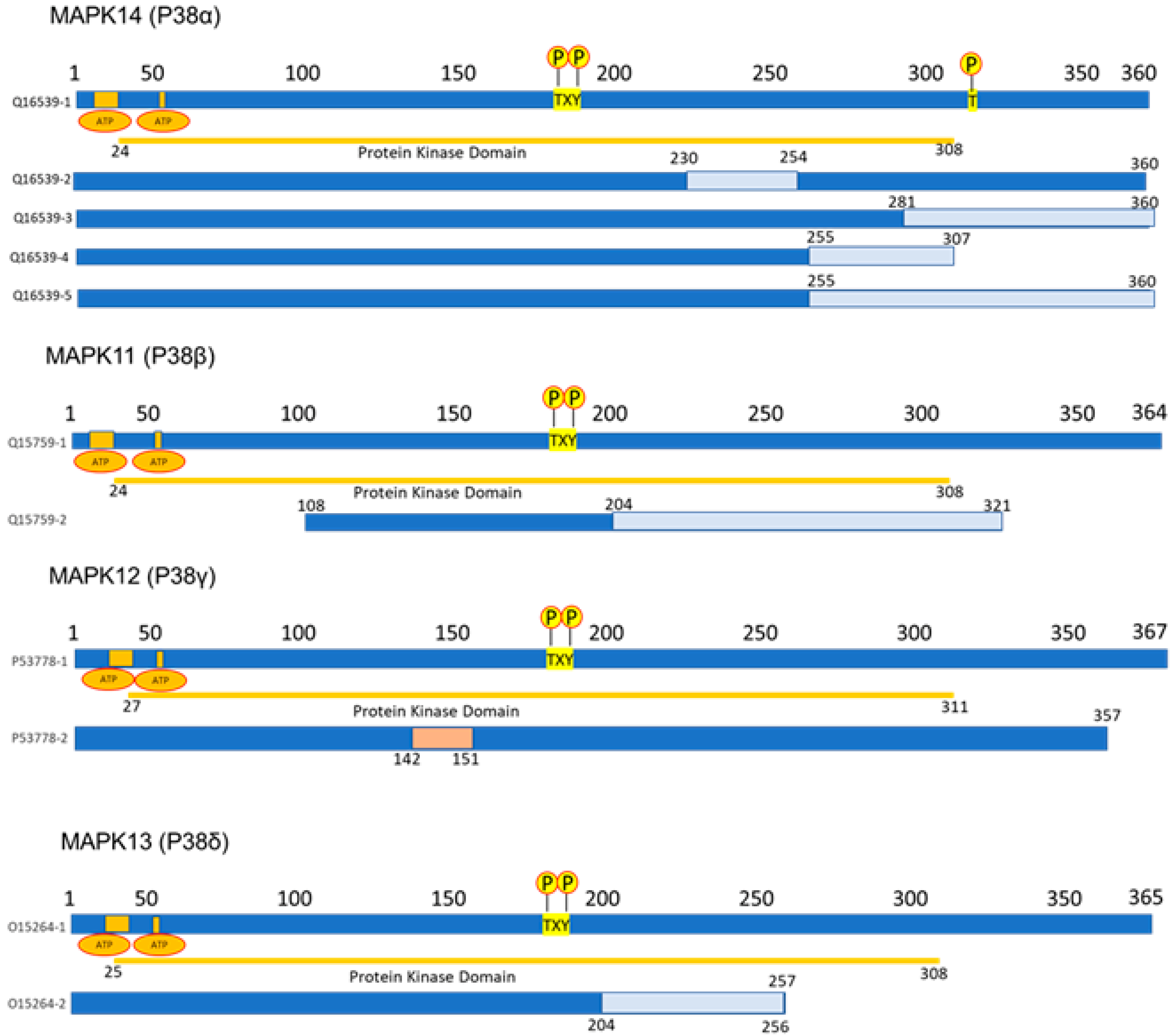
2.1. p38 Structure
2.2. p38 Regulation
2.2.1. Transcription Factors in the Regulation of p38
2.2.2. Non-Coding RNAs in the Regulation of p38
2.3. p38 Chemicals Inhibitors on Cancer
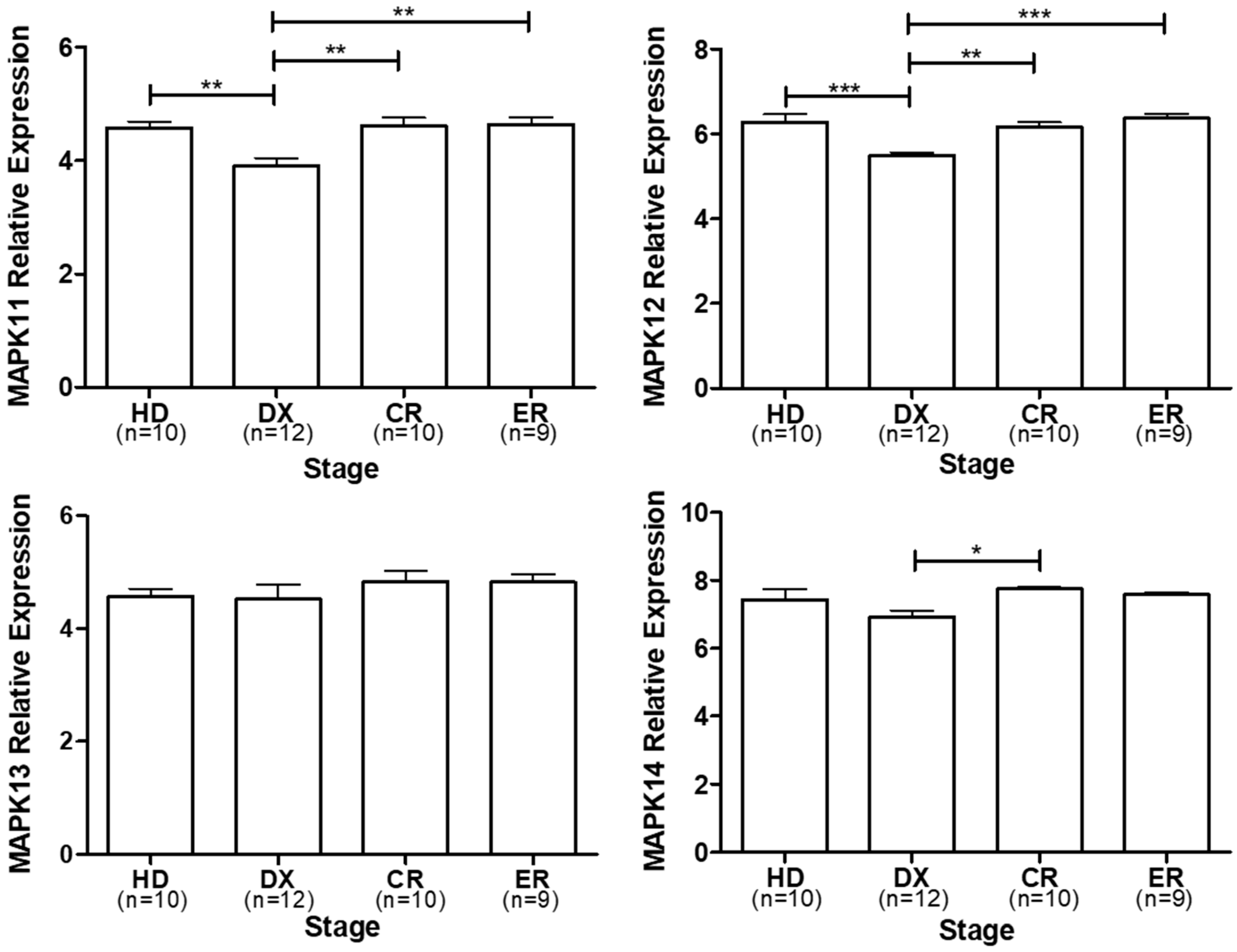
3. p38 Expression in Multiple Myeloma
4. p38 as a Molecular Target in Multiple Myeloma Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| ATRA | All-trans retinoic acid |
| ATO | Arsenic trioxide |
| ATF2 | Activating Transcription Factor 2 |
| ATF1 | Activating Transcription Factor 1 |
| AP-1 | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit |
| ASCL1 | Achaete-Scute Family BHLH Transcription Factor 1 |
| ATF4 | Activating Transcription Factor 4 |
| ATF7 | Activating Transcription Factor 7 |
| ARID3A | AT-Rich Interaction Domain 3A |
| ARID1A | AT-Rich Interaction Domain 1A |
| ASCL2 | Achaete-Scute Family BHLH Transcription Factor 2 |
| ARNT | Aryl Hydrocarbon Receptor Nuclear Translocator |
| ATRA | All-trans retinoic acid |
| ATO | Arsenic trioxide |
| AKT1 | AKT Serine/Threonine Kinase 1 |
| Ape/Ref-1 | apurinic endonuclease/redox factor-1 |
| BMSCs | Bone-marrow-derived mesenchymal stem cells |
| BCL6 | B-Cell Lymphoma 6 Protein |
| BACH2 | BTB Domain and CNC Homolog 2 |
| BM | Bone marrow |
| BMMNC | BM mononuclear cells. |
| BRAF | B-Raf Proto-Oncogene, Serine/Threonine Kinase |
| CML | Chronic myeloid leukemia |
| CRC | Colorectal cancer |
| CDR1-AS | CDR1 Antisense RNA |
| CEBPA | CCAAT/Enhancer-Binding Protein Alpha |
| CTCF | CCCTC-Binding Factor |
| CTSC | Cathepsin C |
| CHOP10 | DNA Damage-Inducible Transcript 3 |
| CDC25 | Cell Division Cycle 25 |
| CXCL14 | C-X-C Motif Chemokine Ligand 14 |
| CXCR4 | C-X-C Motif Chemokine Receptor 4 |
| C-JUN | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit |
| c-MYC | Myc Proto-Oncogene Protein |
| CREB1 | Cyclic AMP-Responsive Element-Binding Protein 1 |
| CDK | Cyclin-dependent kinase. |
| CDK7 | Cyclin-dependent kinase 7 |
| DUSP1 | Dual Specificity Protein Phosphatase 1 |
| DUSP10 | Dual Specificity Protein Phosphatase 10 |
| DUSP16 | Dual Specificity Protein Phosphatase 16 |
| DBA | Dibenzylideneacetone |
| DCs | Dendritic cells |
| DKK-1 | Dickkopf WNT Signaling Pathway Inhibitor 1 |
| DNAM1 | CD226 Antigen |
| EPD | Eukaryotic Promotor Database |
| EMT | Epithelial–mesenchymal transition |
| E2F1 | E2F Transcription Factor 1 |
| EGR1 | Early Growth Response 1 |
| EP300 | E1A-Binding Protein P300 |
| ELK1 | ETS Transcription Factor ELK1 |
| ESCC | Esophageal squamous cell carcinoma |
| EPD | Eukaryotic Promotor Database |
| EEF2K | Eukaryotic Elongation Factor 2 Kinase |
| ESCC | Esophageal squamous cell carcinoma |
| ERK1/2 | Extracellular Signal-Regulated Kinase 2 (MAPK1) |
| FOXP3 | Forkhead Box P3 |
| FGFR1 | Fibroblast Growth Factor Receptor 1 |
| FOXM1 | Forkhead Box M1 |
| GA | Gambogic acid |
| GDF4 | Growth Differentiation Factor 4 |
| GADD45A | Growth Arrest and DNA Damage-Inducible Alpha |
| G-CSF | Granulocyte colony-stimulating factor |
| HEK293 | Human Embryonic Kidney cell line |
| HIF1A | Hypoxia-Inducible Factor 1 Subunit Alpha |
| HDAC3 | Histone Deacetylase 3 |
| HDAC9 | Histone Deacetylase 9 |
| HCC | Hepatocellular carcinoma |
| HO-1 | Heme oxygenase-1 |
| Imp 7/3 | Importin-7 |
| Imp 9/3 | Importin-9 |
| LncRNAs | Long noncoding RNA |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-12B | Interleukin 12β |
| IAV | Influenza A virus |
| IKK | Inhibitor of Nuclear Factor Kappa-B Kinase |
| IRAK4 | Interleukin 1 Receptor-Associated Kinase 4 |
| IGF-1 | Insulin-like growth factor 1 |
| JUN | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit |
| JAK | Janus Kinase |
| KLF4 | Kruppel-Like Factor 4 |
| KAT2B | Lysine Acetyltransferase 2B |
| KRAS | KRAS Proto-Oncogene, GTPase |
| LINCRNA | Long intergenic non-coding RNA |
| LPS | Lipopolysaccharide |
| MM | Multiple myeloma |
| MDR | Multidrug Resistance Protein |
| MAPK | Mitogen-activated protein kinase |
| MGUS | Monoclonal gammopathy of unknown significance |
| MKKs | MAP kinase kinases |
| MW | Molecular weight |
| MEF2 | Myocyte Enhancer Factor 2 |
| MSK1 | Nuclear Mitogen- And Stress-Activated Protein Kinase 1 |
| MSK2 | Nuclear Mitogen- And Stress-Activated Protein Kinase 2 |
| MAPKAPK2 | MAPK-Activated Protein Kinase 2 |
| MART | Melanoma Antigen Recognized By T-Cells 1 |
| MAP3K71P1 | TGF-Beta-Activated Kinase 1 (MAP3K7)-Binding Protein 1 |
| MAP2K3 | Mitogen-Activated Protein Kinase Kinase 3 |
| MKK3 | MAP kinase kinases 3 |
| MAP2K4 | Mitogen-Activated Protein Kinase Kinase 4 |
| MKK4 | MAP kinase kinases 4 |
| MAP2K6 | Mitogen-Activated Protein Kinase Kinase 6 |
| MKK6 | MAP kinase kinases 6 |
| MYB | MYB Proto-Oncogene, Transcription Factor |
| MK2 | MAPK Activated Protein Kinase 2 |
| MK3 | MAPK Activated Protein Kinase 3 |
| mRNA | Messenger RNA |
| MMP-2 | Matrix Metallopeptidase 2 |
| MMP-9 | Matrix Metallopeptidase 9 |
| MYC | MYC Proto-Oncogene, BHLH Transcription Factor |
| MSCs | Mesenchymal stem cells |
| MKK6 | Mitogen-Activated Protein Kinase Kinase 6 |
| MDS | Myelodysplastic syndrome |
| MCP1 | C-C Motif Chemokine Ligand 2 |
| mTOR | Mechanistic Target Of Rapamycin Kinase |
| NHL | Non-Hodgkin’s lymphoma |
| NSPCs | Neural stem/progenitor cells |
| NFKB1 | Nuclear Factor Kappa B Subunit 1 |
| ND8/34 | Cell Line from mouse |
| NSCLC | Non-small-cell lung cancer |
| NOTCH3 | Notch Receptor 3 |
| NKG2D | NK Cell Receptor D |
| OSCs | Osteosarcoma cells |
| PINT | p53-induced noncoding transcript. |
| PCAT1 | Prostate Cancer-Associated Transcript 1 |
| POU1F1 | Pituitary Transcript Factor 1 |
| PPMID | Protein Phosphatase Magnesium-Dependent 1 Delta |
| P53 | Tumor Protein P53 |
| P38 | Mitogen-activated protein kinase p38 |
| PRKD1 | Protein Kinase D1 |
| PRAK | MAPK Activated Protein Kinase 5 |
| rRNA | Ribosomal RNA |
| RAC3 | Rac Family Small GTPase 3 |
| RAF | Raf-1 Proto-Oncogene, Serine/Threonine Kinase |
| SRF | Serum Response Factor |
| STMN1 | Stathmin 1 |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STAT4 | Signal Transducer and Activator of Transcription 4 |
| SMAD2 | SMAD Family Member 2 |
| SMAD3 | SMAD Family Member 3 |
| SMAD4 | SMAD Family Member 4 |
| SP1 | Specificity Protein 1 |
| SYK | Spleen-Associated Tyrosine Kinase |
| SNGH5 | Small Nucleolar RNA Host Gene 5 |
| SDF1 | C-X-C Motif Chemokine Ligand 12 |
| TCR | T-cell receptor |
| TAB1 | TAK1-Binding Protein 1 |
| TNF-α | Tumor necrosis Factor alfa |
| TP53 | Tumor Protein P53 |
| TGF-β | Transforming Growth Factor Beta 1 |
| TNBC | Triple-negative breast cancer |
| Tris DBA | Tris(dibenzylideneacetone)dipalladium |
| TAK1 | Mitogen-Activated Protein Kinase Kinase Kinase 7 |
| TOPK/PBK | Cell-derived protein kinase T-LAK/PDZ-binding kinase |
| TLR5 | Toll Like Receptor 5 |
| TLR7 | Toll Like Receptor 7 |
| TLR9 | Toll Like Receptor 9 |
| VEGF | Vascular endothelial growth factor |
| VDR | Vitamin D Receptor |
| XBP1 | X-Box-Binding Protein 1 |
| YY1 | YIN-YANG-1 |
| ZAP70 | 70 KDa Zeta-Chain-Associated Protein |
| ZCCHC14 | Zinc Finger CCHC Domain-Containing Protein 14 |
References
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Bortezomib or High-Dose Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2005, 352, 2487–2498. Available online: https://www.nejm.org (accessed on 27 September 2023). [CrossRef] [PubMed]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- de la Puente, P.; Azab, A. Contemporary drug therapies for multiple myeloma. Drugs Today 2013, 49, 563. [Google Scholar] [CrossRef]
- Gui, T.; Sun, Y.; Shimokado, A.; Muragaki, Y. The Roles of Mitogen-Activated Protein Kinase Pathways in TGF-β-Induced Epithelial-Mesenchymal Transition. J. Signal Transduct. 2012, 2012, 289243. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef]
- Sahu, V.; Mohan, A.; Dey, S. p38 MAP kinases: Plausible diagnostic and prognostic serum protein marker of non small cell lung cancer. Exp. Mol. Pathol. 2019, 107, 118–123. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Robidoux, J.; Cao, W.; Quan, H.; Daniel, K.W.; Moukdar, F.; Bai, X.; Floering, L.M.; Collins, S. Selective activation of mitogen-activated protein (MAP) kinase kinase 3 and p38α MAP kinase is essential for cyclic AMP-dependent UCP1 expression in adipocytes. Mol. Cell. Biol. 2005, 25, 5466–5479. [Google Scholar] [CrossRef]
- Kudaravalli, S.; Hollander, P.D.; Mani, S.A. Role of p38 MAP kinase in cancer stem cells and metastasis. Oncogene 2022, 41, 3177–3185. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef]
- He, J.; Liu, Z.; Zheng, Y.; Qian, J.; Li, H.; Lu, Y.; Xu, J.; Hong, B.; Zhang, M.; Lin, P.; et al. p38 MAPK in myeloma cells regulates osteoclast and osteoblast activity and induces bone destruction. Cancer Res. 2012, 72, 6393–6402. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Podar, K.; Chauhan, D.; Ishitsuka, K.; Mitsiades, C.; Tai, Y.-T.; Hamasaki, M.; Raje, N.; Hideshima, H.; Schreiner, G.; et al. p38 MAPK inhibition enhances PS-341 (bortezomib)-induced cytotoxicity against multiple myeloma cells. Oncogene 2004, 23, 8766–8776. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Yu, W.; Li, A.; Chen, X.; Chen, Y.; Chen, J. Trifluoperazine Synergistically Potentiates Bortezomib-Induced Anti-Cancer Effect in Multiple Myeloma via Inhibiting P38 MAPK/NUPR1. Tohoku J. Exp. Med. 2022, 257, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Zhang, E.; Chen, J.; Huang, X.; Yan, H.; Cao, W.; Qu, J.; Gu, H.; Xu, R.; et al. Dihydroartemisinin Modulates Apoptosis and Autophagy in Multiple Myeloma through the P38/MAPK and Wnt/β-Catenin Signaling Pathways. Oxidative Med. Cell Longev. 2020, 2020, 6096391. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, J.; Silke, J. An overview of mammalian p38 mitogen-activated protein kinases, central regulators of cell stress and receptor signaling. F1000Research 2020, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Ah-Kang, J.; Ulaszek, J.; Mahmud, D.; Wickrema, A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. Proc. Natl. Acad. Sci. USA 2004, 101, 147–152. [Google Scholar] [CrossRef]
- Deak, M.; Clifton, A.D.; Lucocq, J.M.; Alessi, D.R. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998, 17, 4426–4441. [Google Scholar] [CrossRef]
- Pierrat, B.; Correia, J.d.S.; Mary, J.-L.; Tomás-Zuber, M.; Lesslauer, W. RSK-B, a novel ribosomal S6 kinase family member, is a CREB kinase under dominant control of p38α mitogen-activated protein kinase (p38αMAPK). J. Biol. Chem. 1998, 273, 29661–29671. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.; Che, Y.; Han, P.-L.; Lee, J.-K. Constitutive Activity and Differential Localization of p38 and p38 MAPKs in Adult Mouse Brain. J. Neurosci. Res. 2000, 60, 623–631. [Google Scholar] [CrossRef]
- Enslen, H.; Raingeaud, J.; Davis, R.J. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 1998, 273, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pohl, N.M.; Loesch, M.; Hou, S.; Li, R.; Qin, J.-Z.; Cuenda, A.; Chen, G. p38α antagonizes p38γ activity through c-Jun-dependent ubiquitin-proteasome pathways in regulating Ras transformation and stress response. J. Biol. Chem. 2007, 282, 31398–31408. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Paris, F.; Huot, J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget 2017, 8, 55684–55714. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, N.; Nikolic, I.; Leiva, M.; Pulgarín-Alfaro, M.; Santamans, A.M.; Bernardo, E.; Mora, A.; Herrera-Melle, L.; Rodríguez, E.; Beiroa, D.; et al. p38α blocks brown adipose tissue thermogenesis through p38δ inhibition. PLoS Biol. 2018, 16, e2004455. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; McGowan, C.H.; Zhao, M.; He, L.; Downey, J.S.; Fearns, C.; Wang, Y.; Huang, S.; Han, J. Involvement of the MKK6-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 2000, 20, 4543–4552. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gaestel, M. In the cellular garden of forking paths: How p38 MAPKs signal for downstream assistance. Biol. Chem. 2002, 383, 1519–1536. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef]
- Robinson, K.S.; Toh, G.A.; Rozario, P.; Chua, R.; Bauernfried, S.; Sun, Z.; Firdaus, M.J.; Bayat, S.; Nadkarni, R.; Poh, Z.S.; et al. ZAKα-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science 2022, 377, 328–335. [Google Scholar] [CrossRef]
- Vega, G.; Aviles-Salas, A.; Chalapud, J.R.; Martínez-Paniagua, M.; Pelayo, R.; Mayani, H.; Hernández-Pando, R.; Martínez-Maza, O.; Huerta-Yepez, S.; Bonavida, B.; et al. P38 MAPK expression and activation predicts failure of response to CHOP in patients with Diffuse Large B-Cell Lymphoma. BMC Cancer 2015, 15, 722. [Google Scholar] [CrossRef]
- Feng, Y.; Wen, J.; Chang, C.-C. p38 Mitogen-activated protein kinase and hematologic malignancies. Arch. Pathol. Lab. Med. 2009, 133, 1850–1856. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Ghiassi, M.; Bakin, A.; Aakre, M.; Lundquist, C.A.; Engel, M.E.; Arteaga, C.L.; Moses, H.L. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 2001, 12, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yuan, L.; Slakey, L.M.; Jones, E.F.; Burow, E.M.; Hill, S.M. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010, 12, R107. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.T.; Truong, N.V.; Wu, W.-G.; Su, Y.-C.; Hsu, T.-S.; Lin, L.-Y. Tumor suppressor p53 mediates interleukin-6 expression to enable cancer cell evasion of genotoxic stress. Cell Death Discov. 2023, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Lu, X.; Liu, D.; Yarbrough, W.G. LZAP inhibits p38 MAPK (p38) phosphorylation and activity by facilitating p38 association with the wild-type p53 induced phosphatase 1 (WIP1). PLoS ONE 2011, 6, e16427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pillai, V.B.; Sundaresan, N.R.; Samant, S.A.; Wolfgeher, D.; Trivedi, C.M.; Gupta, M.P. Acetylation of a conserved lysine residue in the ATP binding pocket of p38 augments its kinase activity during hypertrophy of cardiomyocytes. Mol. Cell. Biol. 2011, 31, 2349–2363. [Google Scholar] [CrossRef]
- Lee, J.C.; Laydon, J.T.; McDonnell, P.C.; Gallagher, T.F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M.J.; Keys, J.R.; Vatter, S.W.L.; et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994, 372, 739–746. [Google Scholar] [CrossRef]
- Zervos, A.S.; Faccio, L.; Gatto, J.P.; Kyriakis, J.M.; Brent, R. Mxi2, a mitogen-activated protein kinase that recognizes and phosphorylates Max protein. Proc. Natl. Acad. Sci. USA 1995, 92, 10531–10534. [Google Scholar] [CrossRef]
- Sudo, T.; Yagasaki, Y.; Hama, H.; Watanabe, N.; Osada, H. Exip, a new alternative splicing variant of p38α, can induce an earlier onset of apoptosis in HeLa cells. Biochem. Biophys. Res. Commun. 2002, 291, 838–843. [Google Scholar] [CrossRef]
- Wang, P.; Yu, P.; Gao, P.; Shi, T.; Ma, D. Discovery of novel human transcript variants by analysis of intronic single-block EST with polyadenylation site. BMC Genom. 2009, 10, 518. [Google Scholar] [CrossRef]
- Lominadze, G.; Rane, M.J.; Merchant, M.; Cai, J.; Ward, R.A.; McLeish, K.R. Myeloid-related protein-14 is a p38 MAPK substrate in human neutrophils. J. Immunol. 2005, 174, 7257–7267. [Google Scholar] [CrossRef]
- Raingeaud, J.; Gupta, S.; Rogers, J.S.; Dickens, M.; Han, J.; Ulevitch, R.J.; Davis, R.J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995, 270, 7420–7426. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, U.; Will, J.; Varin, A.; Hoelzer, D.; Herbein, G. Histone deacetylase 3, a class I histone deacetylase, suppresses MAPK11-mediated activating transcription factor-2 activation and represses TNF gene expression. J. Immunol. 2004, 173, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Suzuki, Y.; Nishikawa, T.; Otsuki, T.; Sugiyama, T.; Irie, R.; Wakamatsu, A.; Hayashi, K.; Sato, H.; Nagai, K.; et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat. Genet. 2004, 36, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.E.; Wright, C.L.; Edwards, C.A.; Davis, M.P.; Grinham, J.A.; Cole, C.G.; Goward, M.E.; Aguado, B.; Mallya, M.; Mokrab, Y.; et al. A genome annotation-driven approach to cloning the human ORFeome. Genome Biol. 2004, 5, R84. [Google Scholar] [CrossRef] [PubMed]
- Court, N.W.; dos Remedios, C.G.; Cordell, J.; Bogoyevitch, M.A. Cardiac expression and subcellular localization of the p38 mitogen-activated protein kinase member, stress-activated protein kinase-3 (SAPK3). J. Mol. Cell. Cardiol. 2002, 34, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Lechner, C.; Zahalka, A.M.; Giot, J.F.; Møller, N.P.; Ullrich, A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc. Natl. Acad. Sci. USA 1996, 93, 4355–4359. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, D.S.; Wagner, L.; Feingold, A.E.; Shenmen, C.M.; Grouse, L.H.; Schuler, G.; Klein, S.L.; Old, S.; Rasooly, R.; Good, P.; et al. The status, quality, and expansion of the NIH full-length cDNA project: The Mammalian Gene Collection (MGC). Genome Res. 2004, 14, 2121–2127. [Google Scholar] [CrossRef]
- New, L.; Jiang, Y.; Zhao, M.; Liu, K.; Zhu, W.; Flood, L.J.; Kato, Y.; Parry, G.C.; Han, J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998, 17, 3372–3384. [Google Scholar] [CrossRef]
- Pantoja-Escobar, G.; Morales-Martínez, M.; Vega, G.G.; Castro-Escarpulli, G.; Vega, M.I. Cytotoxic effect caspase activation dependent of a genetically engineered fusion protein with a CD154 peptide mimetic (OmpC-CD154p) on B-NHL cell lines is mediated by the inhibition of bcl-6 and YY1 through MAPK p38 activation. Leuk. Lymphoma 2019, 60, 1062–1070. [Google Scholar] [CrossRef]
- Zhao, M.; New, L.; Kravchenko, V.V.; Kato, Y.; Gram, H.; di Padova, F.; Olson, E.N.; Ulevitch, R.J.; Han, J. Regulation of the MEF2 family of transcription factors by p38. Mol. Cell. Biol. 1999, 19, 21–30. [Google Scholar] [CrossRef]
- Yang, S.-H.; Galanis, A.; Sharrocks, A.D. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol. Cell. Biol. 1999, 19, 4028–4038. [Google Scholar] [CrossRef] [PubMed]
- Umasuthan, N.; Bathige, S.; Noh, J.K.; Lee, J. Gene structure, molecular characterization and transcriptional expression of two p38 isoforms (MAPK11 and MAPK14) from rock bream (Oplegnathus fasciatus). Fish Shellfish Immunol. 2015, 47, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, X.; Li, J.; Hao, D.; Wang, Z.; Shi, H.; Han, L.; Zhou, H.; Sun, J. Prioritizing candidate disease-related long non-coding RNAs by walking on the heterogeneous lncRNA and disease network. Mol. BioSyst. 2015, 11, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Shen, X.; Su, Z.; Ju, S. Emerging roles of noncoding RNAs in multiple myeloma: A review. J. Cell. Physiol. 2019, 234, 7957–7969. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gong, X.; Huang, M. YY1-Activated Long Noncoding RNA SNHG5 Promotes Glioblastoma Cell Proliferation through p38/MAPK Signaling Pathway. Cancer Biother. Radiopharm. 2019, 34, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Marín-Béjar, O.; Marchese, F.P.; Athie, A.; Sánchez, Y.; González, J.; Segura, V.; Huang, L.; Moreno, I.; Navarro, A.; Monzó, M.; et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex 2. Genome Biol. 2013, 14, R104. [Google Scholar] [CrossRef] [PubMed]
- Rocci, A.; Hofmeister, C.C.; Pichiorri, F. The potential of miRNAs as biomarkers for multiple myeloma. Expert Rev. Mol. Diagn. 2014, 14, 947–959. [Google Scholar] [CrossRef]
- Gu, C.; Li, T.; Yin, Z.; Chen, S.; Fei, J.; Shen, J.; Zhang, Y. Integrative analysis of signaling pathways and diseases associated with the miR-106b/25 cluster and their function study in berb erine-induced multiple myeloma cells. Funct. Integr. Genom. 2017, 17, 253–262. [Google Scholar] [CrossRef]
- Li, S.; Zhu, J.; Li, J.; Li, S.; Li, B. MicroRNA-141 inhibits proliferation of gastric cardia adenocarcinoma by targeting MACC1. Arch. Med. Sci. 2018, 14, 588–596. [Google Scholar] [CrossRef]
- Hui, L.; Bakiri, L.; Mairhorfer, A.; Schweifer, N.; Haslinger, C.; Kenner, L.; Komnenovic, V.; Scheuch, H.; Beug, H.; Wagner, E.F. p38α suppresses normal and cancer cell proliferation by antagonizing the JNK–c-Jun pathway. Nat. Genet. 2007, 39, 741–749. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Feng, B.; Han, S.; Zhang, K.; Chen, J.; Li, C.; Wang, R.; Chen, L. The Roles of MicroRNA-141 in Human Cancers: From Diagnosis to Treatment. Cell. Physiol. Biochem. 2016, 38, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.M. Potential of p38 MAP kinase inhibitors in the treatment of cancer. Prog. Drug Res. 2003, 60, 59–92. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.; Link, A.; Leost, M.; Zaharevitz, D.W.; Gussio, R.; Sausville, E.A.; Meijer, L.; Kunick, C. Paullones, a series of cyclin-dependent kinase inhibitors: Synthesis, evaluation of CDK1/cyclin B inhibition, and in vitro antitumor activity. J. Med. Chem. 1999, 42, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.I.; Huerta-Yepaz, S.; Garban, H.; Jazirehi, A.; Emmanouilides, C.; Bonavida, B. Rituximab inhibits p38 MAPK activity in 2F7 B NHL and decreases IL-10 transcription: Pivotal role of p38 MAPK in drug resistance. Oncogene 2004, 23, 3530–3540. [Google Scholar] [CrossRef] [PubMed]
- Gum, R.J.; McLaughlin, M.M.; Kumar, S.; Wang, Z.; Bower, M.J.; Lee, J.C.; Adams, J.L.; Livi, G.P.; Goldsmith, E.J.; Young, P.R. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J. Biol. Chem. 1998, 273, 15605–15610. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Imachi, H.; Yoshimoto, T.; Fukunaga, K.; Sato, S.; Ibata, T.; Kobayashi, T.; Dong, T.; Yonezaki, K.; Yamaji, N.; et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995, 364, 229–233. [Google Scholar] [CrossRef]
- Goedert, M. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997, 16, 3563–3571. [Google Scholar] [CrossRef]
- Zohn, I.E.; Li, Y.; Skolnik, E.Y.; Anderson, K.V.; Han, J.; Niswander, L. p38 and a p38-Interacting Protein Are Critical for Downregulation of E-Cadherin during Mouse Gastrulation. Cell 2006, 125, 957–969. [Google Scholar] [CrossRef]
- Wen, J.; Cheng, H.Y.; Feng, Y.; Rice, L.; Liu, S.; Mo, A.; Huang, J.; Zu, Y.; Ballon, D.J.; Chang, C. P38 MAPK inhibition enhancing ATO-induced cytotoxicity against multiple myeloma cells. Br. J. Haematol. 2008, 140, 169–180. [Google Scholar] [CrossRef]
- Koerber, R.-M.; Held, S.A.E.; Heine, A.; Kotthoff, P.; Daecke, S.N.; Bringmann, A.; Brossart, P. Analysis of the anti-proliferative and the pro-apoptotic efficacy of Syk inhibition in multiple myeloma. Exp. Hematol. Oncol. 2015, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jian, Q.; Lei, L.; Lv, N.; Williamson, R.A.; Chen, P.; Zhang, D.; Hu, J. Resistance of osteosarcoma cells to the proapoptotic effects of carfilzomib involves activation of mitogen activated protein kinase pathways. Exp. Physiol. 2021, 106, 438–449. [Google Scholar] [CrossRef]
- Dey, P.; Biswas, S.; Das, R.; Chatterjee, S.; Ghosh, U. p38 MAPK inhibitor SB203580 enhances anticancer activity of PARP inhibitor olaparib in a synergistic way on non-small cell lung carcinoma A549 cells. Biochem. Biophys. Res. Commun. 2023, 670, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.P.; Reddy, H.; Caivano, M.; Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000, 351 Pt 1, 95–105. [Google Scholar] [CrossRef]
- Saklatvala, J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004, 4, 372–377. [Google Scholar] [CrossRef]
- Sharma, H.S.; Muresanu, D.F.; Nozari, A.; Lafuente, J.V.; Tian, Z.R.; Sahib, S.; Bryukhovetskiy, I.; Bryukhovetskiy, A.; Buzoianu, A.D.; Patnaik, R.; et al. Pathophysiology of blood-brain barrier in brain tumor. Novel therapeutic advances using nanomedicine. Int. Rev. Neurobiol. 2020, 151, 1–66. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, X.; Gao, Z.; Yang, N.; Xue, F. AICAR attenuates postoperative abdominal adhesion formation by inhibiting oxidative stress and promoting mesothelial cell repair. PLoS ONE 2022, 17, e0272928. [Google Scholar] [CrossRef]
- Duffy, J.P.; Harrington, E.M.; Salituro, F.G.; Cochran, J.E.; Green, J.; Gao, H.; Bemis, G.W.; Evindar, G.; Galullo, V.P.; Ford, P.J.; et al. The Discovery of VX-745: A Novel and Selective p38α Kinase Inhibitor. ACS Med. Chem. Lett. 2011, 2, 758–763. [Google Scholar] [CrossRef]
- Hideshima, T.; Akiyama, M.; Hayashi, T.; Richardson, P.; Schlossman, R.; Chauhan, D.; Anderson, K.C. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood 2003, 101, 703–705. [Google Scholar] [CrossRef]
- Ding, C. Drug evaluation: VX-702, a MAP kinase inhibitor for rheumatoid arthritis and acute coronary syndrome. Curr. Opin. Investig. Drugs 2006, 7, 1020–1025. Available online: https://articles/journal_contribution/Drug_evaluation_VX-702_a_MAP_kinase_inhibitor_for_rheumatoid_arthritis_and_acute_coronary_syndrome/23215691/1 (accessed on 7 December 2023).
- Wydra, V.R.; Ditzinger, R.B.; Seidler, N.J.; Hacker, F.W.; Laufer, S.A. A patent review of MAPK inhibitors (2018—Present). Expert Opin. Ther. Pat. 2023, 33, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, V.; Plunkett, W.; Rodriguez, O.C.; Nowak, B.J.; Du, M.; Ayres, M.; Kisor, D.F.; Mitchell, B.S.; Kurtzberg, J.; Keating, M.J. Compound GW506U78 in refractory hematologic malignancies: Relationship between cellular pharmacokinetics and clinical response. J. Clin. Oncol. 1998, 16, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Ghias, K.; Ma, C.; Gandhi, V.; Platanias, L.C.; Krett, N.L.; Rosen, S.T. 8-Amino-adenosine induces loss of phosphorylation of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2, and Akt kinase: Role in induction of apoptosis in multiple myeloma. Mol. Cancer Ther. 2005, 4, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Medicherla, S.; Reddy, M.; Ma, J.Y.; Navas, T.A.; Li, L.; Nguyen, A.N.; Kerr, I.; Hanjarappa, N.; Protter, A.A.; Higgins, L.S. p38α-Selective MAP Kinase Inhibitor Reduces Tumor Growth in Mouse Xenograft Models of Multiple Myeloma. Anticancer Res. 2008, 28, 3827–3833. Available online: https://ar.iiarjournals.org/content/28/6A/3827 (accessed on 27 September 2023). [PubMed]
- Navas, A.T.; Nguyen, A.N.; Hideshima, T.; Reddy, M.; Ma, J.Y.; Haghnazari, E.; Henson, M.; Stebbins, E.G.; Kerr, I.; O’Young, G.; et al. Inhibition of p38α MAPK enhances proteasome inhibitor-induced apoptosis of myeloma cells by modulating Hsp27, Bcl-X(L), Mcl-1 and p53 levels in vitro and inhibits tumor growth in vivo. Leukemia 2006, 20, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Vanderkerken, K.; Medicherla, S.; Coulton, L.; De Raeve, H.; Willems, A.; Lawson, M.; Van Camp, B.; Protter, A.A.; Higgins, L.S.; Menu, E.; et al. Inhibition of p38α mitogen-activated protein kinase prevents the development of osteolytic bone disease, reduces tumor burden, and increases survival in murine models of multiple myeloma. Cancer Res. 2007, 67, 4572–4577. [Google Scholar] [CrossRef]
- Nguyen, A.N.; Stebbins, E.G.; Henson, M.; O’Young, G.; Choi, S.J.; Quon, D.; Damm, D.; Reddy, M.; Ma, J.Y.; Haghnazari, E.; et al. Normalizing the bone marrow microenvironment with p38 inhibitor reduces multiple myeloma cell proliferation and adhesion and suppresses osteoclast formation. Exp. Cell Res. 2006, 312, 1909–1923. [Google Scholar] [CrossRef]
- Siegel, D.S.; Krishnan, A.; Lonial, S.; Chatta, G.; Alsina, M.; Jagannath, S.; Richardson, P.; Hohl, R.; Lust, J.; Bensinger, W.; et al. Phase II Trial of SCIO-469 as Monotherapy (M) or in Combination with Bortezomib (MB) in Relapsed Refractory Multiple Myeloma (MM). Blood 2006, 108, 3580. [Google Scholar] [CrossRef]
- Yasui, H.; Hideshima, T.; Ikeda, H.; Jin, J.; Ocio, E.M.; Kiziltepe, T.; Okawa, Y.; Vallet, S.; Podar, K.; Ishitsuka, K.; et al. BIRB 796 enhances cytotoxicity triggered by bortezomib, heat shock protein (Hsp) 90 inhibitor, and dexamethasone via inhibition of p38 mitogen-activated protein kinase/Hsp27 pathway in multiple myeloma cell lines and inhibits paracrine tumour growth. Br. J. Haematol. 2007, 136, 414–423. [Google Scholar] [CrossRef]
- Holtzman, M.J.; Romero, A.G.; Gerovac, B.J.; Han, Z.; Keeler, S.P.; Wu, K. Mitogen-Activated Protein Kinase Inhibitors, Methods of Making, and Methods of Use Thereof. December 2019. Available online: https://patents.google.com/patent/US11407771B2/en (accessed on 20 November 2023).
- Campbell, R.M.; Anderson, B.D.; Brooks, N.A.; Brooks, H.B.; Chan, E.M.; De Dios, A.; Gilmour, R.; Graff, J.R.; Jambrina, E.; Mader, M.; et al. Characterization of LY2228820 dimesylate, a potent and selective inhibitor of p38 MAPK with antitumor activity. Mol. Cancer Ther. 2014, 13, 364–374. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Bakar, J.; Chitnis, S.P.; Sausville, E.L.; Ashtekar, K.D.; Mendelson, B.E.; Long, K.; Smith, J.C.; Heppner, D.E.; Sheltzer, J.M. Inhibition of a lower potency target drives the anticancer activity of a clinical p38 inhibitor. Cell Chem. Biol. 2023, 30, 1211–1222.e5. [Google Scholar] [CrossRef] [PubMed]
- Signorile, M.L.; Grossi, V.; Fasano, C.; Forte, G.; Disciglio, V.; Sanese, P.; De Marco, K.; La Rocca, F.; Armentano, R.; Valentini, A.M.; et al. c-MYC Protein Stability Is Sustained by MAPKs in Colorectal Cancer. Cancers 2022, 14, 4840. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Hideshima, T.; Neri, P.; Vallet, S.; Shiraishi, N.; Okawa, Y.; Shen, Z.; Raje, N.; Kiziltepe, T.; Ocio, E.M.; et al. p38 mitogen-activated protein kinase inhibitor LY2228820 enhances bortezomib-induced cytotoxicity and inhibits osteoclastogenesis in multiple myeloma; therapeutic implications. Br. J. Haematol. 2008, 141, 598–606. [Google Scholar] [CrossRef]
- Patnaik, A.; Appleman, L.J.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Weiss, G.J.; Sachdev, J.C.; Chadha, M.; Fulk, M.; et al. First-in-human Phase I Study of Copanlisib (BAY 80-6946), an Intravenous Pan-Class I Phosphatidylinositol 3-kinase Inhibitor, in Patients with Advanced Solid Tumors and non-Hodgkin’s Lymphomas. Ann. Oncol. 2016, 27, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, J.; Wang, J.; Cao, Y.; Ling, J.; Qian, J.; Lu, Y.; Li, H.; Zheng, Y.; Lan, Y.; et al. Constitutive activation of p38 MAPK in tumor cells contributes to osteolytic bone lesions in multiple myeloma. Leukemia 2012, 26, 2114–2123. [Google Scholar] [CrossRef]
- Peng, H.; Peng, T.; Wen, J.; Engler, D.A.; Matsunami, R.K.; Su, J.; Zhang, L.; Chang, C.-C.; Zhou, X. Characterization of p38 MAPK isoforms for drug resistance study using systems biology approach. Bioinformatics 2014, 30, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Bergsagel, P.L.; Anderson, K.C. Multiple Myeloma: Increasing Evidence for a Multistep Transformation Process. Blood 1998, 91, 3–21. [Google Scholar] [CrossRef]
- Pratt, G. Molecular aspects of multiple myeloma. Mol. Pathol. 2002, 55, 273–283. [Google Scholar] [CrossRef]
- Platanias, L.C. Map kinase signaling pathways and hematologic malignancies. Blood 2003, 101, 4667–4679. [Google Scholar] [CrossRef]
- Liu, P.; Leong, T.; Quam, L.; Billadeau, D.; Kay, N.; Greipp, P.; Kyle, R.; Oken, M.; Van Ness, B. Activating Mutations of N- and K-ras in Multiple Myeloma Show Different Clinical Associations: Analysis of the Eastern Cooperative Oncology Group Phase III Trial. Blood 1996, 88, 2699–2706. [Google Scholar] [CrossRef]
- Zhang, B.; Fenton, R.G. Proliferation of IL-6-independent multiple myeloma does not require the activity of extracellular signal-regulated kinases (ERK1/2). J. Cell. Physiol. 2002, 193, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Fang, Q.; Li, Y.; Wang, J.; Sun, J.; Zhang, Y.; Hu, X.; Wang, P.; Zhou, S. Crucial role of heme oxygenase-1 in the sensitivity of acute myeloid leukemia cell line Kasumi-1 to ursolic acid. Anticancer Drugs 2014, 25, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ma, D.; Wang, P.; Cao, L.; Lu, T.; Fang, Q.; Zhao, J.; Wang, J. Potential crosstalk of the interleukin-6-heme oxygenase-1-dependent mechanism involved in resistance to lenalidomide in multiple myeloma cells. FEBS J. 2016, 283, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; De Bruyne, E.; Van Valckenborgh, E.; Lahoutte, T.; De Wever, O.; Vanderkerken, K.; Menu, E. Bone marrow stromal cell–derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014, 124, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Håland, E.; Moen, I.N.; Veidal, E.; Hella, H.; Misund, K.; Slørdahl, T.S.; Starheim, K.K. TAK1-inhibitors are cytotoxic for multiple myeloma cells alone and in combination with melphalan. Oncotarget 2021, 12, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, B.; Wu, H.; Ou, J.; Wei, R.; Liu, J.; Cai, W.; Liu, X.; Zhao, S.; Yang, J.; et al. Synergistic action of 5Z-7-oxozeaenol and bortezomib in inducing apoptosis of Burkitt lymphoma cell line Daudi. Tumor Biol. 2016, 37, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, D.; Fan, Z.; Yuan, Y.; Shao, M.; Hou, J.; Zhu, Y.; Wei, R.; Zhu, Y.; Qian, J.; et al. Targeting MK2 Is a Novel Approach to Interfere in Multiple Myeloma. Front. Oncol. 2019, 9, 722. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, Z.; Chang, S.; Li, B.; Yu, D.; Wu, H.; Xie, Y.; Wang, Y.; Xie, B.; Sun, X.; et al. Rafoxanide, an organohalogen drug, triggers apoptosis and cell cycle arrest in multiple myeloma by enhancing DNA damage responses and suppressing the p38 MAPK pathway. Cancer Lett. 2019, 444, 45–59. [Google Scholar] [CrossRef]
- Soriani, A.; Borrelli, C.; Ricci, B.; Molfetta, R.; Zingoni, A.; Fionda, C.; Carnevale, S.; Abruzzese, M.P.; Petrucci, M.T.; Ricciardi, M.R.; et al. p38 MAPK differentially controls NK activating ligands at transcriptional and post-transcriptional level on multiple myeloma cells. Oncoimmunology 2016, 6, e1264564. [Google Scholar] [CrossRef]
- Li, A.; Chen, X.; Jing, Z.; Chen, J. Trifluoperazine induces cellular apoptosis by inhibiting autophagy and targeting NUPR1 in multiple myeloma. FEBS Open Bio 2020, 10, 2097–2106. [Google Scholar] [CrossRef]
- Tiedemann, R.E.; Schmidt, J.; Keats, J.J.; Shi, C.-X.; Zhu, Y.X.; Palmer, S.E.; Mao, X.; Schimmer, A.D.; Stewart, A.K. Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-κB with antimyeloma activity in vitro and in vivo. Blood 2009, 113, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Kang, D.; Zhu, X.-Y.; Kong, X.-T.; Yu, H.; Chen, X.-L.; Jiang, P.-J.; Ni, H.-W. Effect of Celastrol Based on IRAK4/ERK/p38 Signaling Pathway on Proliferation and Apoptosis of Multiple Myeloma Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2022, 30, 175–182. [Google Scholar] [PubMed]
- Bhandarkar, S.S.; Bromberg, J.; Carrillo, C.; Selvakumar, P.; Sharma, R.K.; Perry, B.N.; Govindarajan, B.; Fried, L.; Sohn, A.; Reddy, K.; et al. Tris (dibenzylideneacetone) dipalladium, a N-myristoyltransferase-1 inhibitor, is effective against melanoma growth in vitro and in vivo. Clin. Cancer Res. 2008, 14, 5743–5748. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, P.; Azab, F.; Muz, B.; Luderer, M.; Arbiser, J.; Azab, A.K. Tris DBA palladium overcomes hypoxia-mediated drug resistance in multiple myeloma. Leuk. Lymphoma 2016, 57, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Stefka, A.T.; Johnson, D.; Rosebeck, S.; Park, J.; Nakamura, Y.; Jakubowiak, A.J. Potent anti-myeloma activity of the TOPK inhibitor OTS514 in pre-clinical models. Cancer Med. 2020, 9, 324–334. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Q.; Gu, H.; Chen, J.; Zhang, E.; Guo, X.; Huang, X.; Yan, H.; He, D.; Yang, Y.; et al. Therapeutic effects of the novel subtype-selective histone deacetylase inhibitor chidamide on myeloma-associated bone disease. Haematologica 2018, 103, 1369–1379. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Yang, J. Tumor cell p38 MAPK: A trigger of cancer bone osteolysis. Cancer Cell Microenviron. 2015, 2, e464. [Google Scholar] [CrossRef][Green Version]
- Trentin, L.; Miorin, M.; Facco, M.; Baesso, I.; Carraro, S.; Cabrelle, A.; Maschio, N.; Bortoli, M.; Binotto, G.; Piazza, F.; et al. Multiple myeloma plasma cells show different chemokine receptor profiles at sites of disease activity. Br. J. Haematol. 2007, 138, 594–602. [Google Scholar] [CrossRef]
- Broussas, M.; Boute, N.; Akla, B.; Berger, S.; Beau-Larvor, C.; Champion, T.; Robert, A.; Beck, A.; Haeuw, J.-F.; Goetsch, L.; et al. A New Anti-CXCR4 Antibody That Blocks the CXCR4/SDF-1 Axis and Mobilizes Effector Cells. Mol. Cancer Ther. 2016, 15, 1890–1899. [Google Scholar] [CrossRef]
- Pandey, M.K.; Kale, V.P.; Song, C.; Sung, S.-S.; Sharma, A.K.; Talamo, G.; Dovat, S.; Amin, S.G. Gambogic acid inhibits multiple myeloma mediated osteoclastogenesis through suppression of chemokine receptor CXCR4 signaling pathways. Exp. Hematol. 2014, 42, 883–896. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Cao, B.; Mao, X. The mTOR signaling pathway is an emerging therapeutic target in multiple myeloma. Curr. Pharm. Des. 2014, 20, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Xu, X.; Xu, Z.; Chen, G.; Zeng, Y.; Zhang, Z.; Cao, B.; Kong, Y.; Tang, X.; Mao, X. SC06, a novel small molecule compound, displays preclinical activity against multiple myeloma by disrupting the mTOR signaling pathway. Sci. Rep. 2015, 5, 12809. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Lee, S.-W. TLR5 activation by flagellin induces doxorubicin resistance via interleukin-6 (IL-6) expression in two multiple myeloma cells. Cell. Immunol. 2014, 289, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Abdi, J.; Mutis, T.; Garssen, J.; Redegeld, F. Characterization of the Toll-like receptor expression profile in human multiple myeloma cells. PLoS ONE 2013, 8, e60671. [Google Scholar] [CrossRef] [PubMed]
- Jego, G. Modulation of normal and malignant plasma cells function by toll-like receptors. Front. Biosci. (Elite Ed.) 2012, 4, 2289–2301. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Li, T.; Jiang, K.; Huang, Q.; Chen, Y.; Qian, F. Induction of chemoresistance by all-trans retinoic acid via a noncanonical signaling in multiple myeloma cells. PLoS ONE 2014, 9, e85571. [Google Scholar] [CrossRef]
- Otsuki, T.; Sakaguchi, H.; Hatayama, T.; Wu, P.; Takata, A.; Hyodoh, F. Effects of all-trans retinoic acid (ATRA) on human myeloma cells. Leuk. Lymphoma 2003, 44, 1651–1656. [Google Scholar] [CrossRef]
- Simard, C.; Cloutier, M.; Néron, S. Feasibility study: Phosphospecific flow cytometry enabling rapid functional analysis of bone marrow samples from patients with multiple myeloma. Cytom. Part B Clin. Cytom. 2014, 86, 139–144. [Google Scholar] [CrossRef]

| Transcription Factor | Putative Binding Site Positions Relative to TSS | |||
|---|---|---|---|---|
| MAPK14_1 | MAPK11_1 | MAPK12_1 | MAPK13_1 | |
| ARNT: HIF1A | −466, −455, −260 | −93, | −40, −88 | −120, 32 |
| ASCL1 | −933, −901, −880, −878, −785, −603, −602, −392, −391, −248, −59, 22, 2 | 74, 73, −459, −506, −550, −575, −609, −610, −755, −830, −937 | 44, 42, −2, −3, −190, −214, −215, −309, −310, −410, −411, −518, −523, −666, −688, −810, −812, −882, −883, −968, −982 | −993, −810, −809, −551, −278, −264, −254, −185, −184, −103, −102, −87, −40, −17, 25, 26 |
| ATF4 | −823, −777, −774 | |||
| ATF7 | −824, −823 | −92 | ||
| Ahr: Arnt | −466, −455, −334, −260 | −93 | −120, 32 | |
| Arid3a | −860 | −929, −490, −485 | ||
| Ascl2 | −901, −900, −785, −392, −391, −381, −380, −249, −248, −59, 24, 25 | −380, −829 | −2, −214, −309, −523, −524, −882, −883 | −809, −580, −278, −185, −184, −87 |
| Atf1 | −823 | −579 | −92 | −697 |
| BACH2 | −633, −629 | −392 | −508, −504 | |
| Bcl6 | −874 | −356, −497, −672, −732, −884 | −382, −927 | |
| CEBPA | −979, −823, −549 | −548 | −947 | |
| CTCF | −768, −513, −275, −209, 1 | −178, −219, −221, −253, −383, −584, −848 | 27, −52, −76, −146, −270, −336, −601, −672, −799, −891 | −870, −87, −54, −14 |
| E2F1 | −597, −596, −270, −269, −218, −217 | −183, −184, −290, −543, −544, −660 | 35, 34 | 15 |
| EGR1 | −465, −394, −28 | 34, −8, −252, −783, −903, −949 | 30, −256, −335, −602, −744, −917 | −866, −835, −604, −384, −278, −246, −147, −61, −39, −14 |
| ELF1 | −62, −28, 33 | 82, −126, −154, −489, −880 | 24, −120, −438 | −155, 73, 76 |
| JUN | −887, −822 | −609, −460 | ||
| KLF4 | −770, −587, −453, −334 | −91, −684 | −94, −290, −906 | −380, −122, −58 |
| MYC | −942, −785, −603, −602, −444, −392, −391, −266 | −230, −506 | −3, −87, −88, −518, −523, −643, −644 | −984, −606, −199, −185, −184, −103, −102, −87 |
| SMAD2:SMAD3: SMAD4 | −896, −464, −251, −204 | −147, −211, −926 | −260, −266, −563, −625, −829, −865, −910 | |
| YY1 | −977, −962, −547, −98 | −949, −814, −672 | ||
| XBP1 | −943 | −578 | −738 | |
| TP53 | −759, −758 | −578 | −754 | −959, −958, −437, −125, −124 |
| Stat4 | −419 | −672, −676 | −395 | |
| SP1 | −925, −881, −659, −633, −499, −225, −186, −141, −114, 72 | 63, −12, −33, −46, −87, −141, −165, −192, −200, −279, −294, −347, −508, −527, −543, −598, −650, −683, −759, −905 | 78, 28, 16, −19, −47, −103, −147, −164, −195, −206, −267, −289, −317, −592, −670, −740, −803, −822, −853, −905 | −868, −837, −666, −522, −329, −290, −257, −205, −59, −44, −25, −9 |
| POU1F1 | −575 | −407 | −919, −490, −395 | |
| NFKB1 | −982, −938, −552, −506, −32 | −69, −101, −102, −788, −945 | 34, 5, −62, −314, −513, −586, −899, −961 | −988, −944, −467, −466, −232, −211 |
| MAPK11 | MAPK12 | MAPK13 | MAPK14 | |
|---|---|---|---|---|
| Predicted microRNAs (miRtarBase) | hsa-miR-122-5p, hsa-miR-124-3p, hsa-let-7a-5p | hsa-miR-18a-5p, hsa-miR-150-5p, hsa-miR-18b-5p, hsa-miR-3134, hsa-miR-3691-5p, hsa-miR-4434, hsa-miR-4516, hsa-miR-4525, hsa-miR-4531, hsa-miR-4534, hsa-miR-4690-3p, hsa-miR-4731-5p, hsa-miR-4735-3p, hsa-miR-4761-5p, hsa-miR-4773, hsa-miR-5010-5p, hsa-miR-5187-5p, hsa-miR-5589-5p, hsa-miR-5685, hsa-miR-5703, hsa-miR-6778-3p, hsa-miR-6795-5p, hsa-miR-6798-5p, hsa-miR-6814-5p, hsa-miR-6887-5p, hsa-miR-8082 | hsa-miR-124-3p, hsa-miR-24-3p, hsa-miR-199a-3p, hsa-miR-200a-3p, hsa-miR-141-3p, hsa-miR-125b-5p, hsa-miR-214-3p, hsa-miR-155-5p, hsa-miR-17-5p, hsa-miR-106a-5p | |
| Bio-predicted (TargetScan) | hsa-miR-325-3p | hsa-miR-125a-5p, has-miR-125b-5p, has-miR-4319 | hsa-miR-3681-3p, hsa-miR-128-3p, has-miR-216-3p |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Martínez, M.; Vega, M.I. p38 Molecular Targeting for Next-Generation Multiple Myeloma Therapy. Cancers 2024, 16, 256. https://doi.org/10.3390/cancers16020256
Morales-Martínez M, Vega MI. p38 Molecular Targeting for Next-Generation Multiple Myeloma Therapy. Cancers. 2024; 16(2):256. https://doi.org/10.3390/cancers16020256
Chicago/Turabian StyleMorales-Martínez, Mario, and Mario I. Vega. 2024. "p38 Molecular Targeting for Next-Generation Multiple Myeloma Therapy" Cancers 16, no. 2: 256. https://doi.org/10.3390/cancers16020256
APA StyleMorales-Martínez, M., & Vega, M. I. (2024). p38 Molecular Targeting for Next-Generation Multiple Myeloma Therapy. Cancers, 16(2), 256. https://doi.org/10.3390/cancers16020256







