Efficacy and Safety of Fluorescence-Guided Surgery Compared to Conventional Surgery in the Management of Colorectal Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
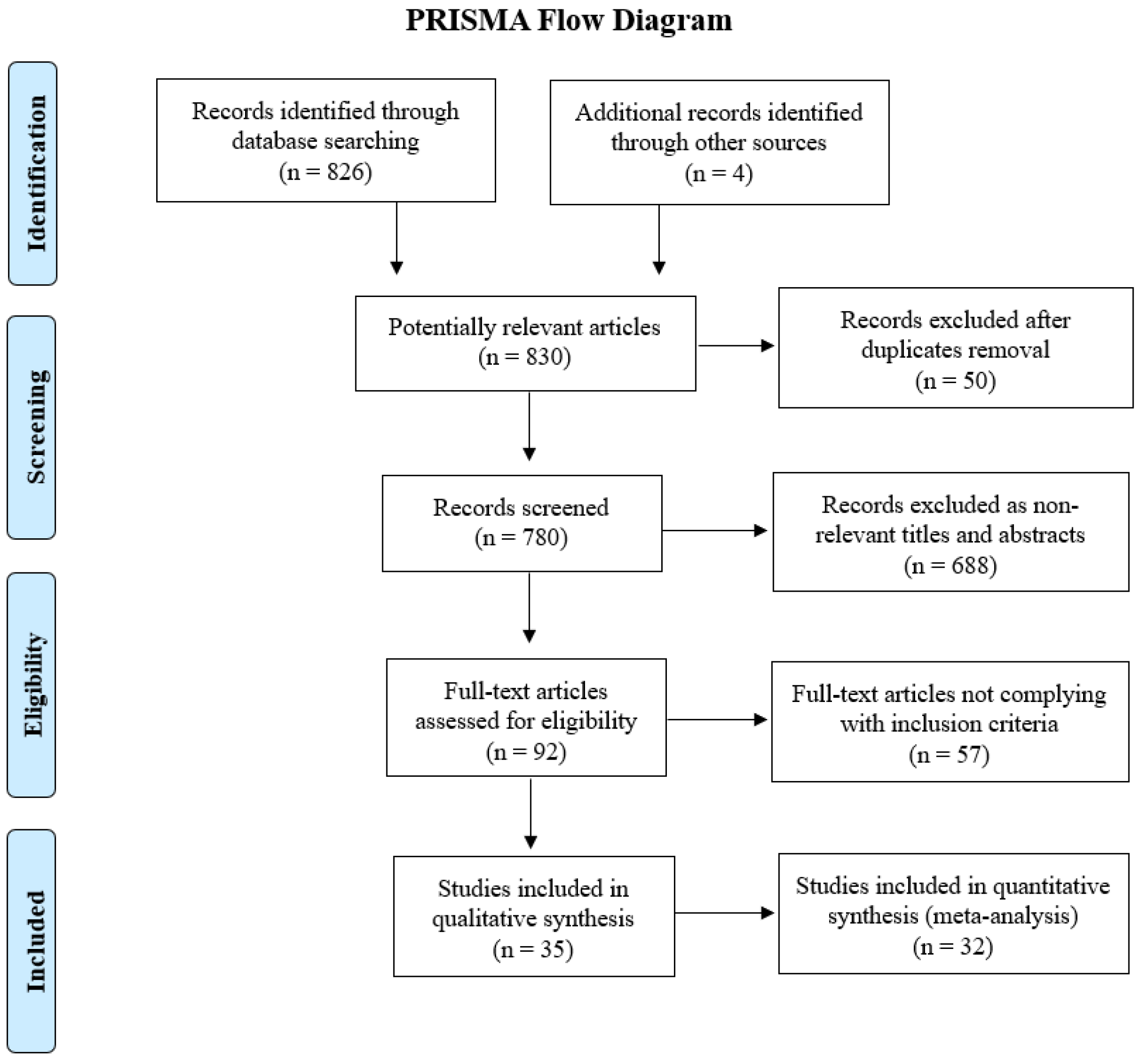
2. Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Quality Assessment of Studies
2.4. Statistical Analysis
3. Results
3.1. Baseline Study and Patient Characteristics
3.2. Fluorescence-Guided Surgery
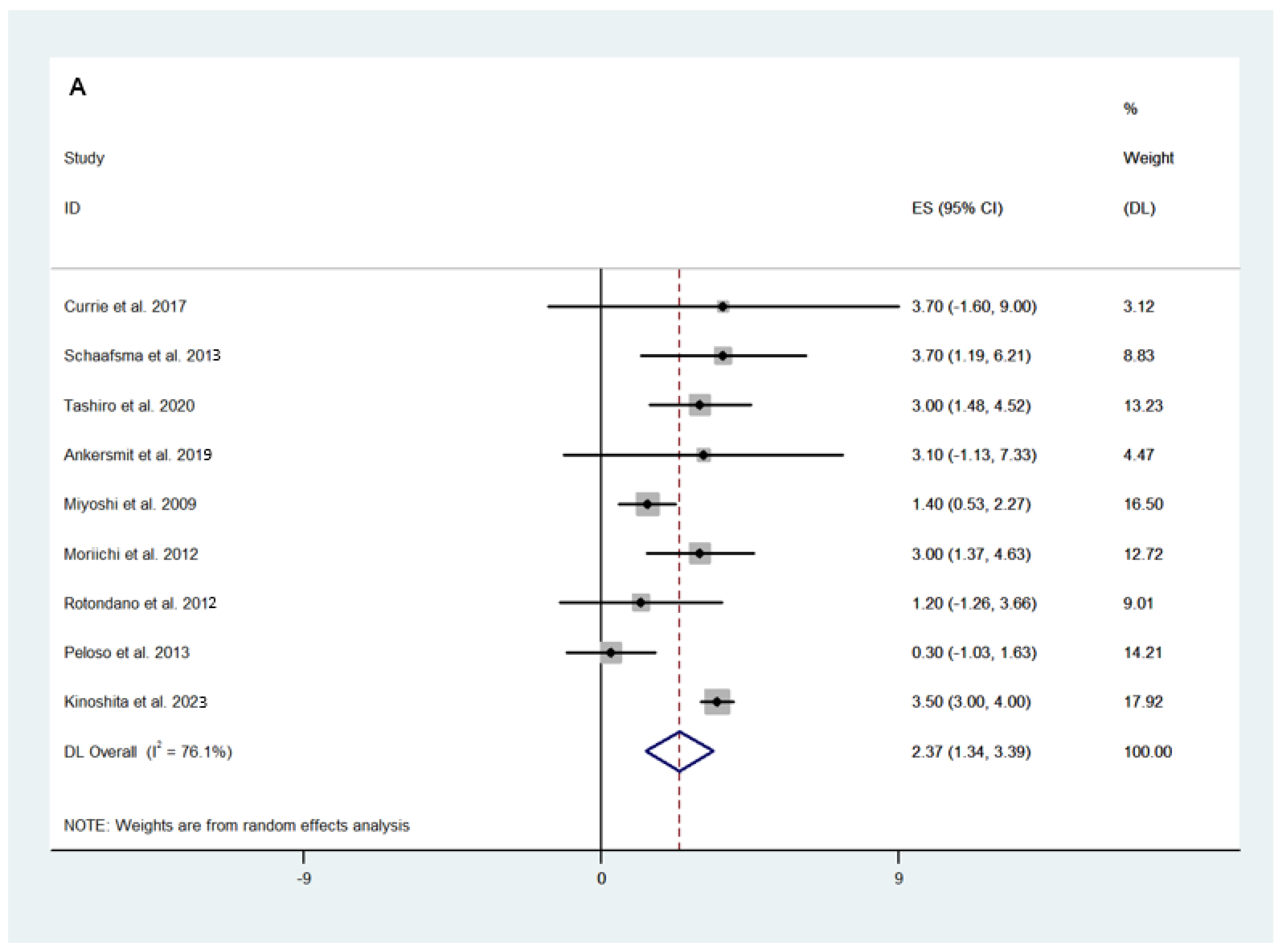
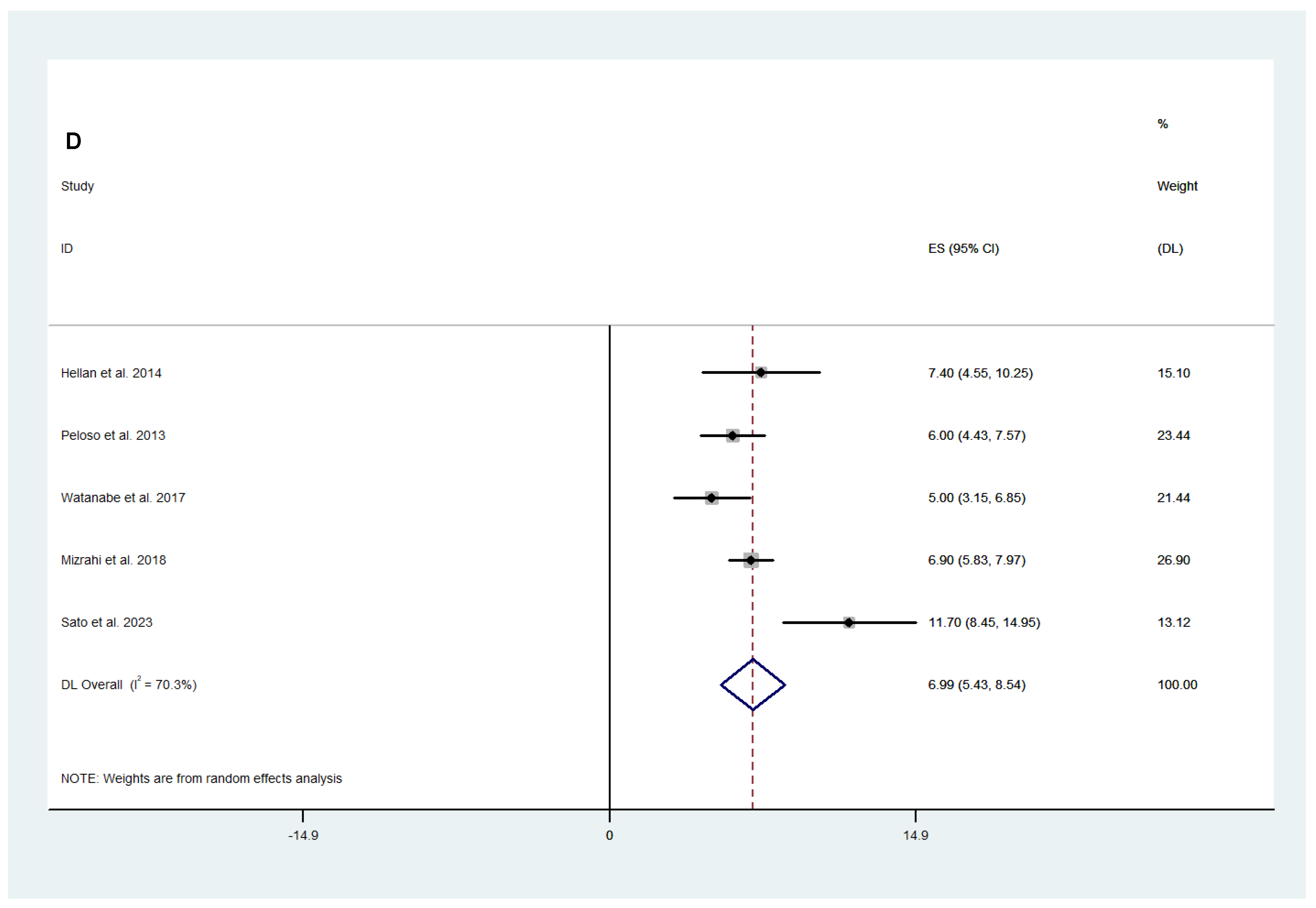
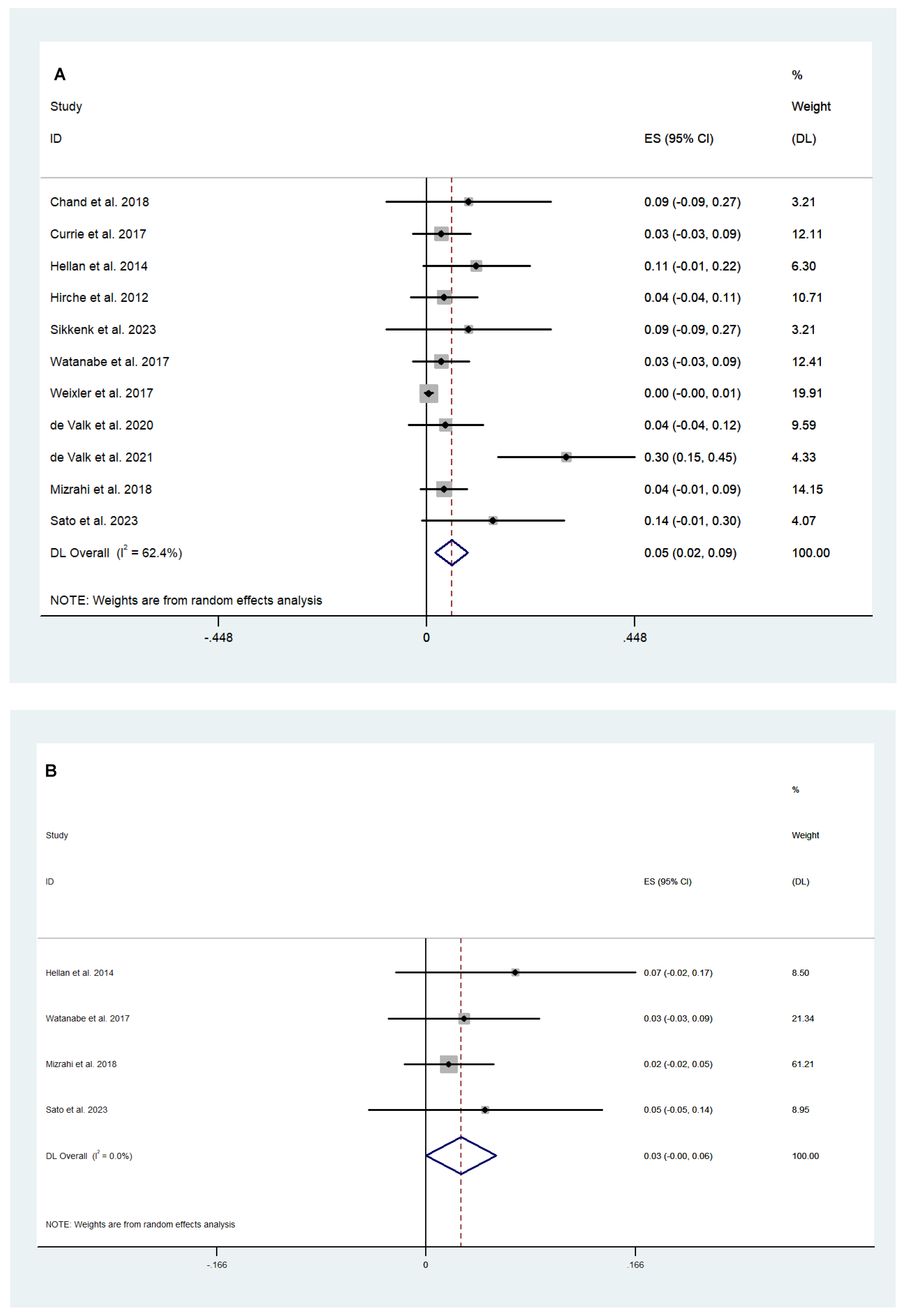
3.2.1. Surgical and Safety Outcomes
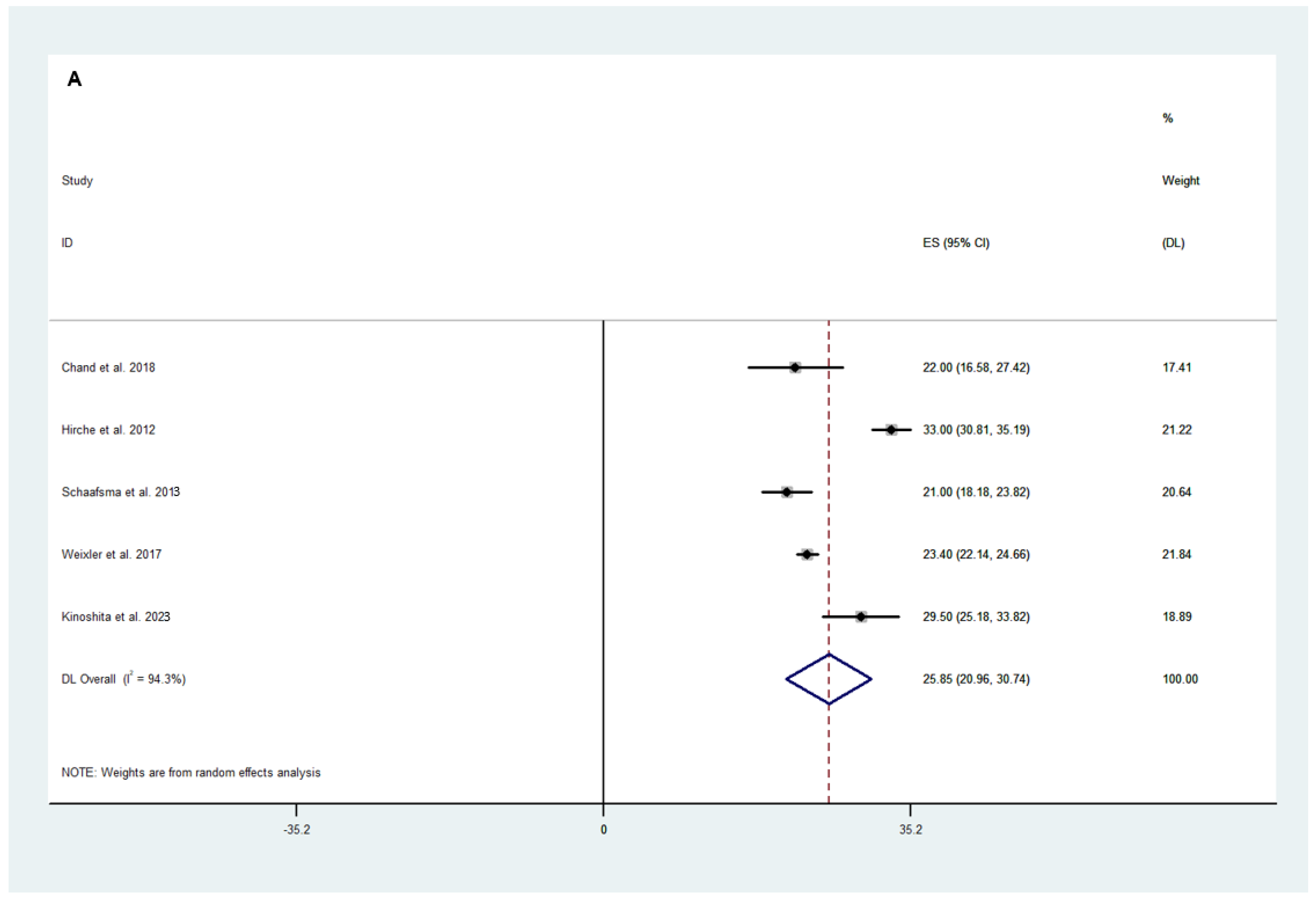
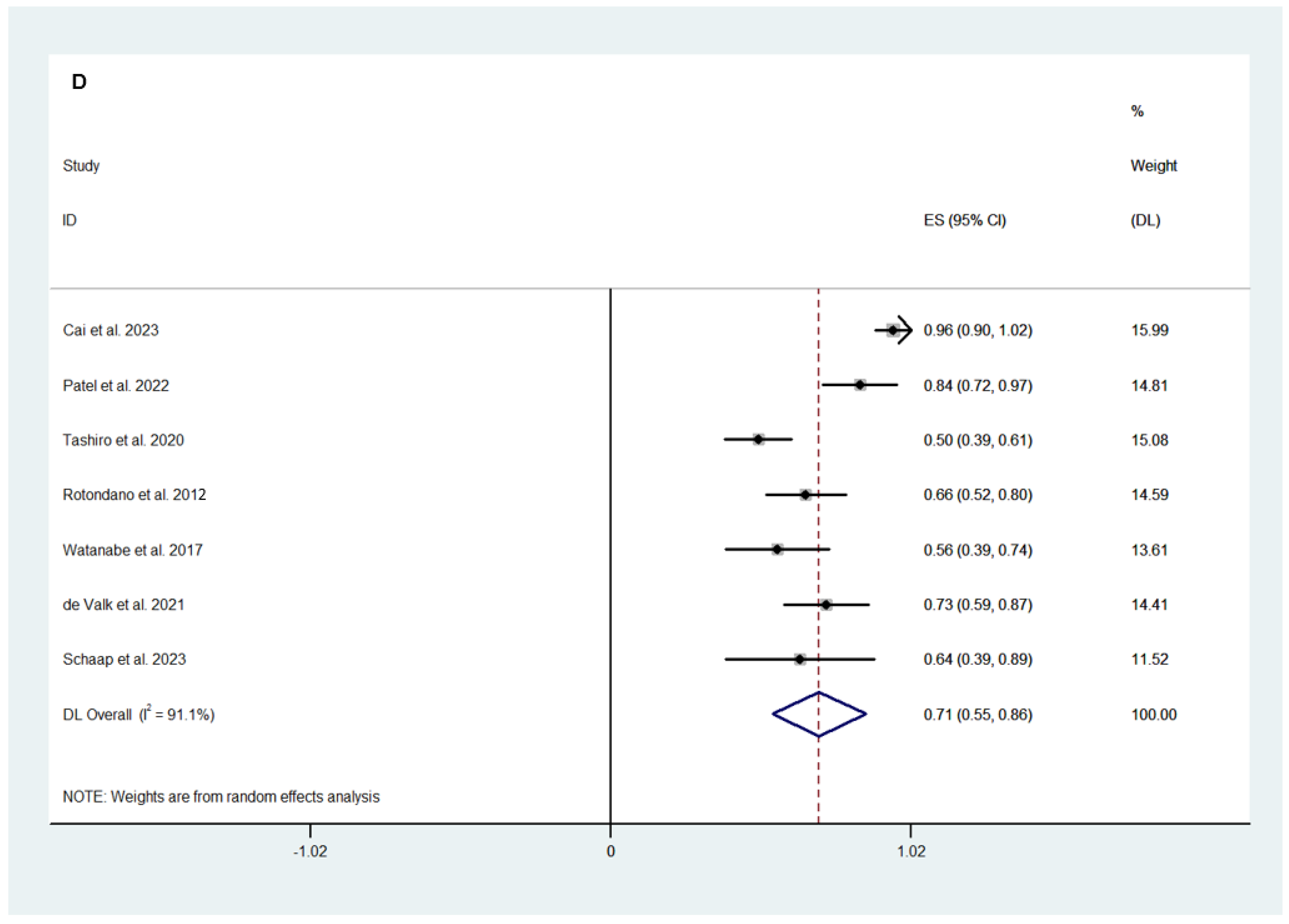
3.2.2. Efficacy Outcomes
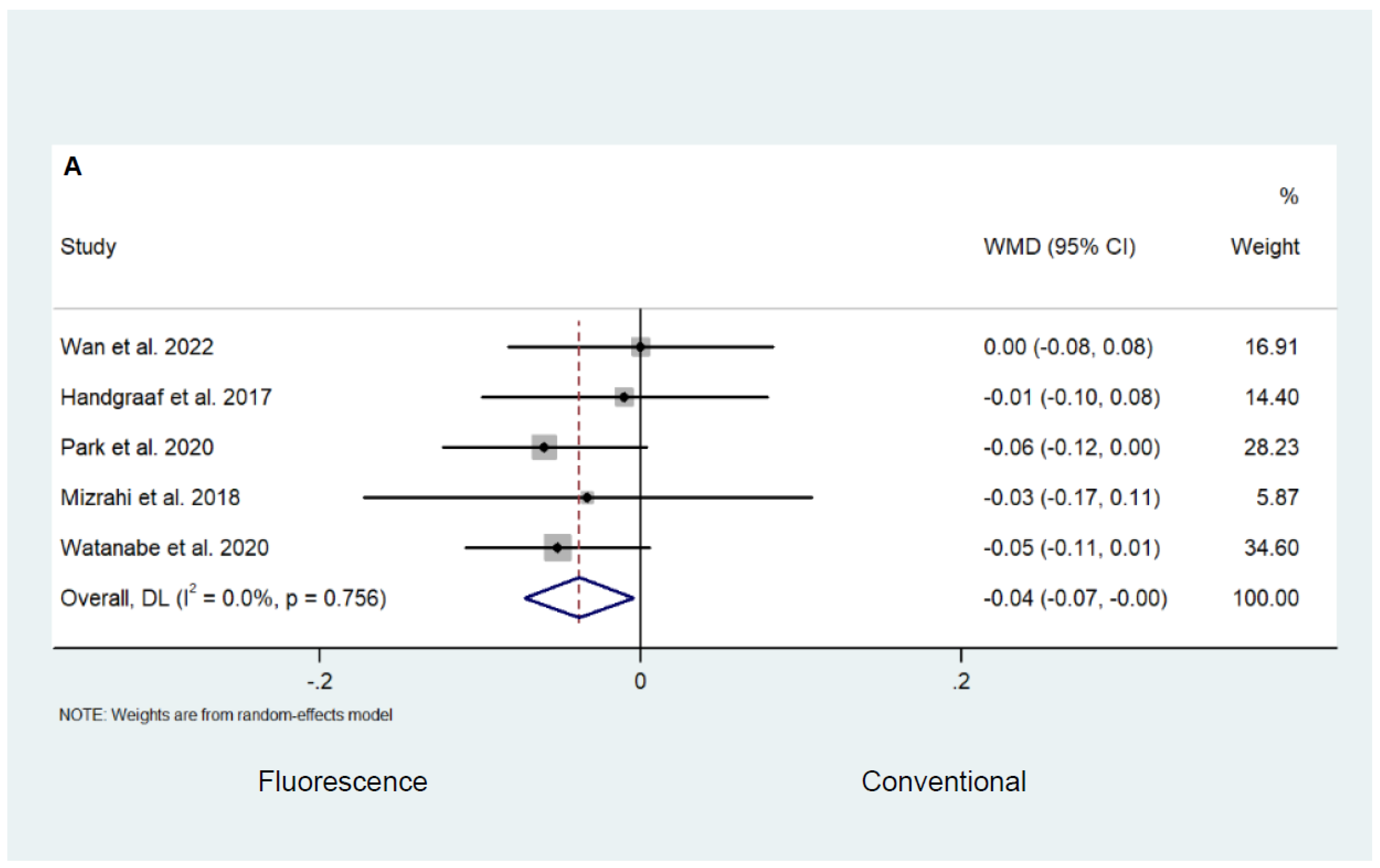
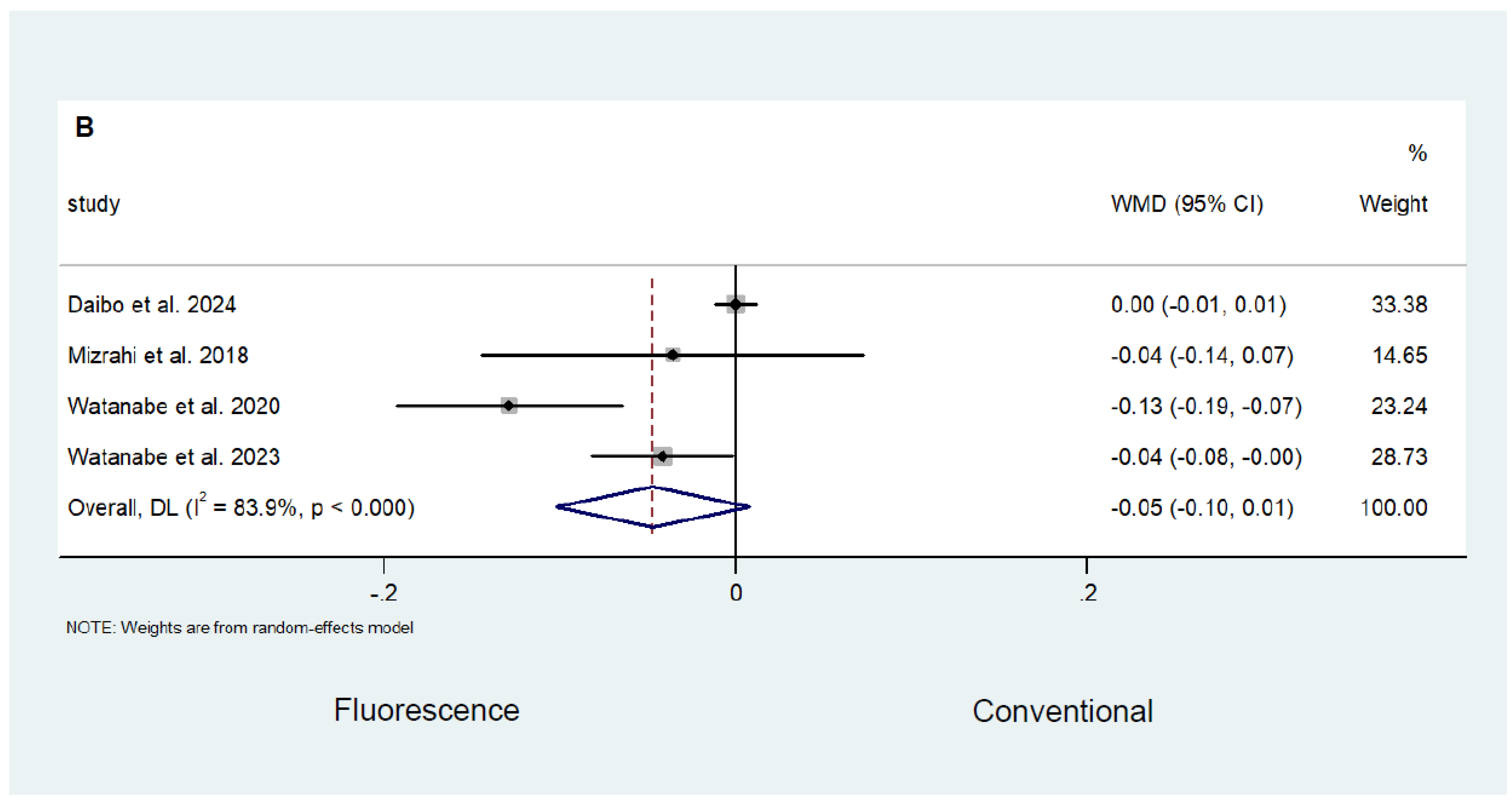
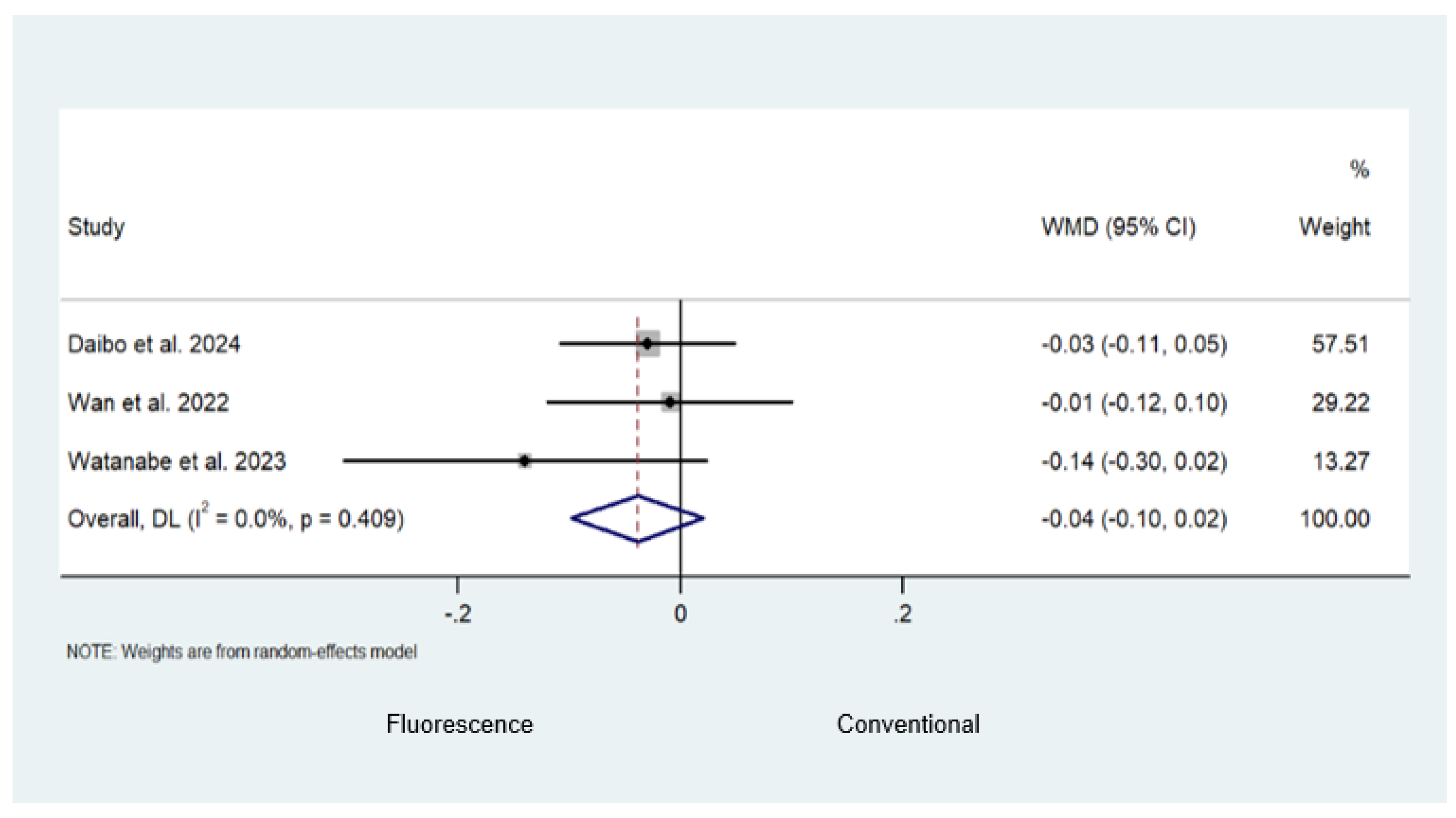
3.3. Fluorescence-Guided versus Conventional Surgery
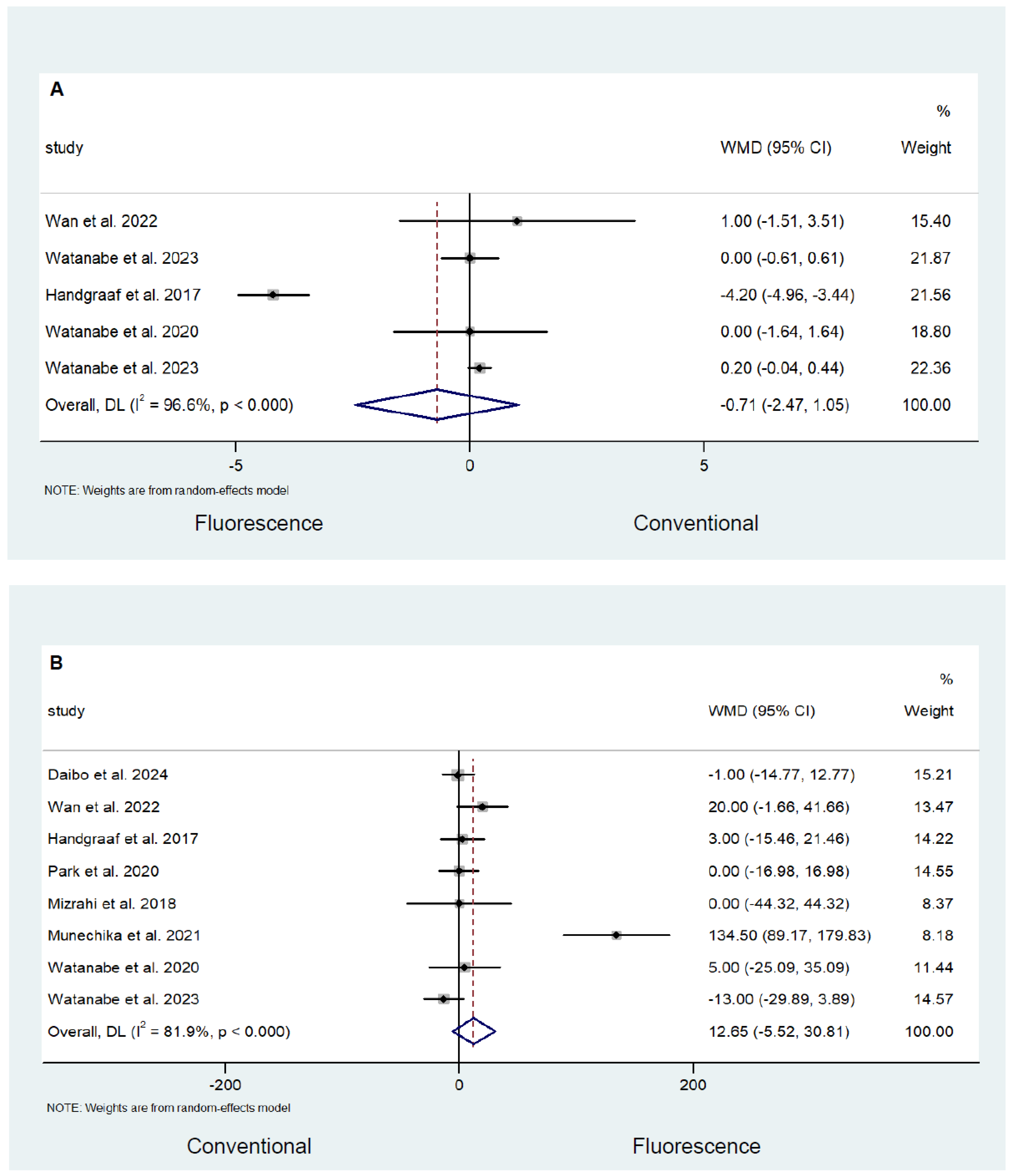
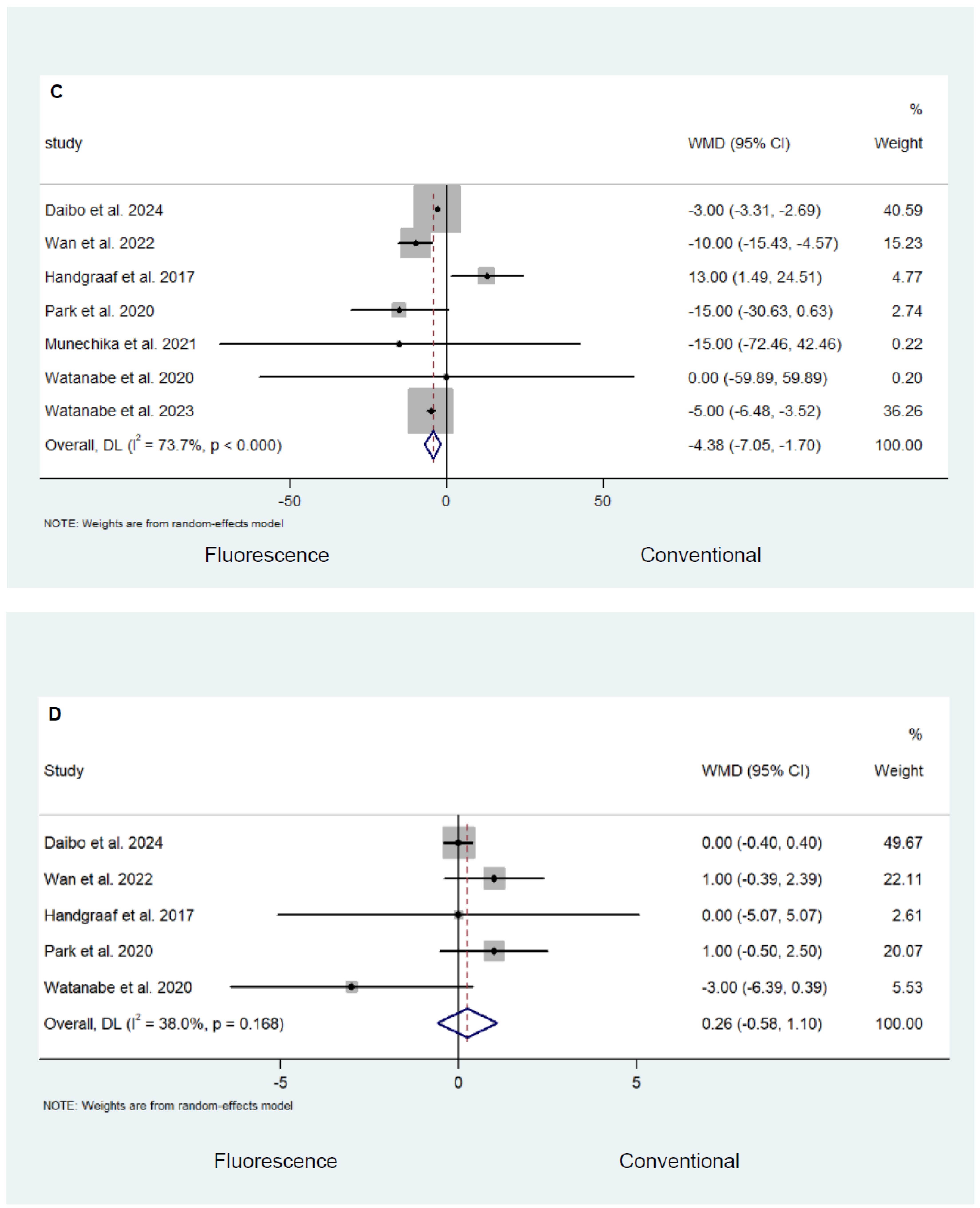
Safety and Efficacy Outcomes
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Zhang, N.; Wang, M.; Liu, Y.; Ma, L.; Wang, Q.; Yang, Q.; Liu, X.; Zhou, F.; Wei, Y. Distributions and Trends of the Global Burden of Colorectal Cancer Attributable to Dietary Risk Factors over the Past 30 Years. Nutrients 2023, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Beniwal, S.S.; Lamo, P.; Kaushik, A.; Lorenzo-Villegas, D.L.; Liu, Y.; MohanaSundaram, A. Current Status and Emerging Trends in Colorectal Cancer Screening and Diagnostics. Biosensors 2023, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Meng, L.; Liu, H.; Sun, L.; Nie, Y.; Wu, Q.; Fan, D.; Li, M. Let food be thy medicine: The role of diet in colorectal cancer: A narrative review. J. Gastrointest. Oncol. 2022, 13, 2020–2032. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome in Colorectal Cancer Progression. Front. Immunol. 2022, 12, 807648. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, S.M.; Galjart, B.; Verhoef, C.; Slooter, G.D.; Koopman, M.; Verhoeven, R.H.A.; de Wilt, J.H.W.; van Erning, F.N. Disease recurrence after colorectal cancer surgery in the modern era: A population-based study. Int. J. Color. Dis. 2021, 36, 2399–2410. [Google Scholar] [CrossRef]
- Zedan, A.; Salah, T. Total mesorectal excision for the treatment of rectal cancer. Electron. Physician 2015, 7, 1666–1772. [Google Scholar] [CrossRef]
- Maurer, C.A.; Renzulli, P.; Kull, C.; Käser, S.A.; Mazzucchelli, L.; Ulrich, A.; Büchler, M.W. The impact of the introduction of total mesorectal excision on local recurrence rate and survival in rectal cancer: Long-term results. Ann. Surg. Oncol. 2011, 18, 1899–1906. [Google Scholar] [CrossRef]
- Galema, H.A.; Meijer, R.P.J.; Lauwerends, L.J.; Verhoef, C.; Burggraaf, J.; Vahrmeijer, A.L.; Hutteman, M.; Keereweer, S.; Hilling, D.E. Fluorescence-guided surgery in colorectal cancer; A review on clinical results and future perspectives. Eur. J. Surg. Oncol. 2022, 48, 810–821. [Google Scholar] [CrossRef]
- Alius, C.; Tudor, C.; Badiu, C.D.; Dascalu, A.M.; Smarandache, C.G.; Sabau, A.D.; Tanasescu, C.; Balasescu, S.A.; Serban, D. Indocyanine Green-Enhanced Colorectal Surgery-between Being Superfluous and Being a Game-Changer. Diagnostics 2020, 10, 742. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef] [PubMed]
- De Muynck, L.D.A.N.; White, K.P.; Alseidi, A.; Bannone, E.; Boni, L.; Bouvet, M.; Falconi, M.; Fuchs, H.F.; Ghadimi, M.; Gockel, I.; et al. Consensus Statement on the Use of Near-Infrared Fluorescence Imaging during Pancreatic Cancer Surgery Based on a Delphi Study: Surgeons’ Perspectives on Current Use and Future Recommendations. Cancers 2023, 15, 652. [Google Scholar] [CrossRef]
- Garoufalia, Z.; Wexner, S.D. Indocyanine Green Fluorescence Guided Surgery in Colorectal Surgery. J. Clin. Med. 2023, 12, 494. [Google Scholar] [CrossRef]
- Belloni, E.; Muttillo, E.M.; di Saverio, S.; Gasparrini, M.; Brescia, A.; Nigri, G. The Role of Indocyanine Green Fluorescence in Rectal Cancer Robotic Surgery: A Narrative Review. Cancers 2022, 14, 2411. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3 (Updated February 2022); Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 20 April 2024).
- Wells, G.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 April 2024).
- Oxford Centre for Evidence-Based Medicine—Levels of Evidence (March 2009). Available online: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed on 20 April 2024).
- Chand, M.; Keller, D.S.; Joshi, H.M.; Devoto, L.; Rodriguez-Justo, M.; Cohen, R. Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech. Coloproctol. 2018, 22, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Currie, A.C.; Brigic, A.; Thomas-Gibson, S.; Suzuki, N.; Moorghen, M.; Jenkins, J.T.; Faiz, O.D.; Kennedy, R.H. A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur. J. Surg. Oncol. 2017, 43, 2044–2051. [Google Scholar] [CrossRef]
- Daibo, S.; Watanabe, J.; Suwa, H.; Sato, S.; Suwa, Y.; Ozawa, M.; Ishibe, A.; Endo, I. Short-term and Mid-term Outcomes of Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Right-Sided Colectomy: A Propensity Score-Matched Cohort Study. Dis. Colon. Rectum. 2024, 67, 82–89. [Google Scholar] [CrossRef]
- Hellan, M.; Spinoglio, G.; Pigazzi, A.; Lagares-Garcia, J.A. The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg. Endosc. 2014, 28, 1695–1702. [Google Scholar] [CrossRef]
- Hirche, C.; Mohr, Z.; Kneif, S.; Doniga, S.; Murawa, D.; Strik, M.; Hünerbein, M. Ultrastaging of colon cancer by sentinel node biopsy using fluorescence navigation with indocyanine green. Int. J. Color. Dis. 2012, 27, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E.; Verbeek, F.P.; van der Vorst, J.R.; Hutteman, M.; Kuppen, P.J.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Ex vivo sentinel node mapping in colon cancer combining blue dye staining and fluorescence imaging. J. Surg. Res. 2013, 183, 253–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sikkenk, D.J.; Sterkenburg, A.J.; Burghgraef, T.A.; Akol, H.; Schwartz, M.P.; Arensman, R.; Verheijen, P.M.; Nagengast, W.B.; Consten, E.C.J. Robot-assisted fluorescent sentinel lymph node identification in early-stage colon cancer. Surg. Endosc. 2023, 37, 8394–8403. [Google Scholar] [CrossRef]
- Wan, J.; Wang, S.; Yan, B.; Tang, Y.; Zheng, J.; Ji, H.; Hu, Y.; Zhuang, B.; Deng, H.; Yan, J. Indocyanine green for radical lymph node dissection in patients with sigmoid and rectal cancer: Randomized clinical trial. BJS Open 2022, 6, zrac151. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Ohya, H.; Sakai, J.; Suwa, Y.; Goto, K.; Nakagawa, K.; Ozawa, M.; Ishibe, A.; Suwa, H.; Kunisaki, C.; et al. Long-term outcomes of indocyanine green fluorescence imaging-guided laparoscopic lateral pelvic lymph node dissection for clinical stage II/III middle-lower rectal cancer: A propensity score-matched cohort study. Tech. Coloproctol. 2023, 27, 759–767. [Google Scholar] [CrossRef]
- Cai, X.; Hong, H.; Pan, W.; Chen, J.; Jiang, L.; Du, Q.; Li, G.; Lin, S.; Chen, Y. Does Using Indocyanine Green Fluorescence Imaging for Tumors Help in Determining the Safe Surgical Margin in Real-Time Navigation of Laparoscopic Hepatectomy? A Retrospective Study. Ann. Surg. Oncol. 2023, 30, 1981–1987. [Google Scholar] [CrossRef]
- De Gooyer, J.M.; Elekonawo, F.M.K.; Bremers, A.J.A.; Boerman, O.C.; Aarntzen, E.H.J.G.; de Reuver, P.R.; Nagtegaal, I.D.; Rijpkema, M.; de Wilt, J.H.W. Multimodal CEA-targeted fluorescence and radioguided cytoreductive surgery for peritoneal metastases of colorectal origin. Nat. Commun. 2022, 13, 2621. [Google Scholar] [CrossRef]
- Patel, I.; Bartlett, D.; Dasari, B.V.; Chatzizacharias, N.; Isaac, J.; Marudanayagam, R.; Mirza, D.F.; Roberts, J.K.; Sutcliffe, R.P. Detection of Colorectal Liver Metastases Using Near-Infrared Fluorescence Imaging During Hepatectomy: Prospective Single Centre UK Study. J. Gastrointest. Cancer 2023, 54, 574–579. [Google Scholar] [CrossRef]
- Tashiro, Y.; Aoki, T.; Hirai, T.; Koizumi, T.; Mansou, D.A.; Kusano, T.; Matsuda, K.; Yamada, K.; Nogaki, K.; Hakozaki, T.; et al. Pathological Validity of Using Near-infrared Fluorescence Imaging for Securing Surgical Margins During Liver Resection. Anticancer Res. 2020, 40, 3873–3882. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, G.S.; Park, J.S.; Park, S.Y.; Cho, S.H.; Seo, A.N.; Yoon, G.S. S122: Impact of fluorescence and 3D images to completeness of lateral pelvic node dissection. Surg. Endosc. 2020, 34, 469–476. [Google Scholar] [CrossRef]
- Ankersmit, M.; Hoekstra, O.S.; van Lingen, A.; Bloemena, E.; Jacobs, M.A.J.M.; Vugts, D.J.; Bonjer, H.J.; van Dongen, G.A.M.S.; Meijerink, W.J.H.J. Perioperative PET/CT lymphoscintigraphy and fluorescent real-time imaging for sentinel lymph node mapping in early staged colon cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Ohue, M.; Noura, S.; Yano, M.; Sasaki, Y.; Kishi, K.; Yamada, T.; Miyashiro, I.; Ohigashi, H.; Iishi, H.; et al. Surgical usefulness of indocyanine green as an alternative to India ink for endoscopic marking. Surg. Endosc. 2009, 23, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Moriichi, K.; Fujiya, M.; Sato, R.; Nata, T.; Nomura, Y.; Ueno, N.; Ishikawa, C.; Inaba, Y.; Ito, T.; Okamoto, K.; et al. Autofluorescence imaging and the quantitative intensity of fluorescence for evaluating the dysplastic grade of colonic neoplasms. Int. J. Color. Dis. 2012, 27, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Rotondano, G.; Bianco, M.A.; Sansone, S.; Prisco, A.; Meucci, C.; Garofano, M.L.; Cipolletta, L. Trimodal endoscopic imaging for the detection and differentiation of colorectal adenomas: A prospective single-centre clinical evaluation. Int. J. Color. Dis. 2012, 27, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.; Franchi, E.; Canepa, M.C.; Barbieri, L.; Briani, L.; Ferrario, J.; Bianco, C.; Quaretti, P.; Brugnatelli, S.; Dionigi, P.; et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB 2013, 15, 928–934. [Google Scholar] [CrossRef]
- Tanis, E.; Evers, D.J.; Spliethoff, J.W.; Pully, V.V.; Kuhlmann, K.; van Coevorden, F.; Hendriks, B.H.; Sanders, J.; Prevoo, W.; Ruers, T.J. In vivo tumor identification of colorectal liver metastases with diffuse reflectance and fluorescence spectroscopy. Lasers Surg. Med. 2016, 48, 820–827. [Google Scholar] [CrossRef]
- Handgraaf, H.J.M.; Boogerd, L.S.F.; Höppener, D.J.; Peloso, A.; Sibinga Mulder, B.G.; Hoogstins, C.E.S.; Hartgrink, H.H.; van de Velde, C.J.H.; Mieog, J.S.D.; Swijnenburg, R.J.; et al. Long-term follow-up after near-infrared fluorescence-guided resection of colorectal liver metastases: A retrospective multicenter analysis. Eur. J. Surg. Oncol. 2017, 43, 1463–1471. [Google Scholar] [CrossRef]
- Watanabe, J.; Ota, M.; Suwa, Y.; Ishibe, A.; Masui, H.; Nagahori, K. Evaluation of lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int. J. Color. Dis. 2017, 32, 201–207. [Google Scholar] [CrossRef]
- Weixler, B.; Rickenbacher, A.; Raptis, D.A.; Viehl, C.T.; Guller, U.; Rueff, J.; Zettl, A.; Zuber, M. Sentinel Lymph Node Mapping with Isosulfan Blue or Indocyanine Green in Colon Cancer Shows Comparable Results and Identifies Patients with Decreased Survival: A Prospective Single-Center Trial. World J. Surg. 2017, 41, 2378–2386. [Google Scholar] [CrossRef]
- De Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.P.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation Using Bevacizumab-800CW in Patients with Locally Advanced Rectal Cancer. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef]
- De Valk, K.S.; Deken, M.M.; Handgraaf, H.J.M.; Bhairosingh, S.S.; Bijlstra, O.D.; van Esdonk, M.J.; Terwisscha van Scheltinga, A.G.T.; Valentijn, A.R.P.M.; March, T.L.; Vuijk, J.; et al. First-in-Human Assessment of cRGD-ZW800-1, a Zwitterionic, Integrin-Targeted, Near-Infrared Fluorescent Peptide in Colon Carcinoma. Clin. Cancer Res. 2020, 26, 3990–3998. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.S.; Kim, H.J.; Woo, I.T.; Park, I.K.; Choi, G.S. Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Surgery Could Achieve Radical D3 Dissection in Patients With Advanced Right-Sided Colon Cancer. Dis. Colon. Rectum. 2020, 63, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Kawada, K.; Itatani, Y.; Okamura, R.; Oshima, N.; Okada, T.; Hida, K.; Obama, K. Timing of real-time indocyanine green fluorescence visualization for lymph node dissection during laparoscopic colon cancer surgery. Langenbecks Arch. Surg. 2023, 408, 38. [Google Scholar] [CrossRef] [PubMed]
- De Valk, K.S.; Deken, M.M.; Schaap, D.P.; Meijer, R.P.; Boogerd, L.S.; Hoogstins, C.E.; van der Valk, M.J.; Kamerling, I.M.; Bhairosingh, S.S.; Framery, B.; et al. Dose-Finding Study of a CEA-Targeting Agent, SGM-101, for Intraoperative Fluorescence Imaging of Colorectal Cancer. Ann. Surg. Oncol. 2021, 28, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, I.; de Lacy, F.B.; Abu-Gazala, M.; Fernandez, L.M.; Otero, A.; Sands, D.R.; Lacy, A.M.; Wexner, S.D. Transanal total mesorectal excision for rectal cancer with indocyanine green fluorescence angiography. Tech. Coloproctol. 2018, 22, 785–791. [Google Scholar] [CrossRef]
- Mizrahi, I.; Abu-Gazala, M.; Rickles, A.S.; Fernandez, L.M.; Petrucci, A.; Wolf, J.; Sands, D.R.; Wexner, S.D. Indocyanine green fluorescence angiography during low anterior resection for low rectal cancer: Results of a comparative cohort study. Tech. Coloproctol. 2018, 22, 535–540. [Google Scholar] [CrossRef]
- Munechika, T.; Kajitani, R.; Matsumoto, Y.; Nagano, H.; Komono, A.; Aisu, N.; Morimoto, M.; Yoshimatsu, G.; Yoshida, Y.; Hasegawa, S. Safety and effectiveness of high ligation of the inferior mesenteric artery for cancer of the descending colon under indocyanine green fluorescence imaging: A pilot study. Surg. Endosc. 2021, 35, 1696–1702. [Google Scholar] [CrossRef]
- Sato, K.; Kasajima, H.; Imaizumi, K.; Kurushima, M.; Umehara, M.; Tsuruga, Y.; Yamana, D.; Sato, A.; Ichimura, K.; Fukasawa, T.; et al. Visualization of Anorectal Lymphatic Flow Using Indocyanine Green Fluorescence Imaging: An Observational Study. Anticancer Res. 2023, 43, 1591–1598. [Google Scholar] [CrossRef]
- Schaap, D.P.; de Valk, K.S.; Deken, M.M.; Meijer, R.P.J.; Burggraaf, J.; Vahrmeijer, A.L.; Kusters, M.; SGM-101 Study Group. Carcinoembryonic antigen-specific, fluorescent image-guided cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer. Br. J. Surg. 2020, 107, 334–337. [Google Scholar] [CrossRef]
- Watanabe, J.; Ishibe, A.; Suwa, Y.; Suwa, H.; Ota, M.; Kunisaki, C.; Endo, I. Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: A propensity score-matched cohort study. Surg. Endosc. 2020, 34, 202–208. [Google Scholar] [CrossRef]
- Watanabe, J.; Takemasa, I.; Kotake, M.; Noura, S.; Kimura, K.; Suwa, H.; Tei, M.; Takano, Y.; Munakata, K.; Matoba, S.; et al. Blood Perfusion Assessment by Indocyanine Green Fluorescence Imaging for Minimally Invasive Rectal Cancer Surgery (EssentiAL trial): A Randomized Clinical Trial. Ann. Surg. 2023, 278, e688–e694. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, C.; Moussaron, A.; Stallivieri, A.; Mordon, S.; Frochot, C. Indocyanine green: Photosensitizer or chromophore? Still a debate. Curr. Med. Chem. 2014, 21, 1871–1897. [Google Scholar] [CrossRef] [PubMed]
- Skrivanová, K.; Skorpíková, J.; Svihálek, J.; Mornstein, V.; Janisch, R. Photochemical properties of a potential photosensitiser indocyanine green in vitro. J. Photochem. Photobiol. B 2006, 85, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, L.S.F.; Hoogstins, C.E.S.; Schaap, D.P.; Kusters, M.; Handgraaf, H.J.M.; van der Valk, M.J.M.; Hilling, D.E.; Holman, F.A.; Peeters, K.C.M.J.; Mieog, J.S.D.; et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: A dose-escalation pilot study. Lancet Gastroenterol. Hepatol. 2018, 3, 181–191. [Google Scholar] [CrossRef]
- Fujita, S.; Mizusawa, J.; Kanemitsu, Y.; Ito, M.; Kinugasa, Y.; Komori, K.; Ohue, M.; Ota, M.; Akazai, Y.; Shiozawa, M.; et al. Colorectal Cancer Study Group of Japan Clinical Oncology Group. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann. Surg. 2017, 266, 201–207. [Google Scholar] [CrossRef]
- Blanco-Colino, R.; Espin-Basany, E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: A systematic review and meta-analysis. Tech. Coloproctol. 2018, 22, 15–23. [Google Scholar] [CrossRef]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): A multi-institutional study. J. Am. Coll. Surg. 2015, 220, 82–92.e1. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.D.; Pigazzi, A.; McLemore, E.C.; Mutch, M.G.; Haas, E.; Rasheid, S.H.; Wait, A.D.; Paquette, I.M.; Bardakcioglu, O.; Safar, B.; et al. Perfusion Assessment in Left-Sided/Low Anterior Resection (PILLAR III): A Randomized, Controlled, Parallel, Multicenter Study Assessing Perfusion Outcomes with PINPOINT Near-Infrared Fluorescence Imaging in Low Anterior Resection. Dis. Colon. Rectum. 2021, 64, 995–1002. [Google Scholar] [CrossRef]
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Color. Dis. 2020, 22, 1147–1153. [Google Scholar] [CrossRef]
- Kudszus, S.; Roesel, C.; Schachtrupp, A.; Höer, J.J. Intraoperative laser fluorescence angiography in colorectal surgery: A noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch. Surg. 2010, 395, 1025–1030. [Google Scholar] [CrossRef]
- Armstrong, G.; Croft, J.; Corrigan, N.; Brown, J.M.; Goh, V.; Quirke, P.; Hulme, C.; Tolan, D.; Kirby, A.; Cahill, R.; et al. IntAct: Intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: A randomized controlled trial. Color. Dis. 2018, 20, O226–O234. [Google Scholar] [CrossRef] [PubMed]
- Cassinotti, E.; Al-Taher, M.; Antoniou, S.A.; Arezzo, A.; Baldari, L.; Boni, L.; Bonino, M.A.; Bouvy, N.D.; Brodie, R.; Carus, T.; et al. European Association for Endoscopic Surgery (EAES) consensus on Indocyanine Green (ICG) fluorescence-guided surgery. Surg. Endosc. 2023, 37, 1629–1648. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.P.J.; Faber, R.A.; Bijlstra, O.D.; Braak, J.P.B.M.; Meershoek-Klein Kranenbarg, E.; Putter, H.; Mieog, J.S.D.; Burggraaf, K.; Vahrmeijer, A.L.; Hilling, D.E.; et al. AVOID: A phase III, randomised controlled trial using indocyanine green for the prevention of anastomotic leakage in colorectal surgery. BMJ Open 2022, 12, e051144. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luna, M.R.; Okamoto, N.; Cinelli, L.; Baratelli, L.; Ségaud, S.; Rodríguez-Gómez, A.; Keller, D.S.; Zonoobi, E.; Bannone, E.; Marescaux, J.; et al. Quantification of bowel ischaemia using real-time multispectral Single Snapshot Imaging of Optical Properties (SSOP). Surg. Endosc. 2023, 37, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, A.; Agnus, V.; Barberio, M.; Seeliger, B.; Marchegiani, F.; Charles, A.L.; Geny, B.; Marescaux, J.; Mutter, D.; Diana, M. Computer-assisted quantification and visualization of bowel perfusion using fluorescence-based enhanced reality in left-sided colonic resections. Surg. Endosc. 2021, 35, 4321–4331. [Google Scholar] [CrossRef]
- Hren, R.; Sersa, G.; Simoncic, U.; Milanic, M. Imaging perfusion changes in oncological clinical applications by hyperspectral imaging: A literature review. Radiol. Oncol. 2022, 56, 420–429. [Google Scholar] [CrossRef]
- Jansen-Winkeln, B.; Germann, I.; Köhler, H.; Mehdorn, M.; Maktabi, M.; Sucher, R.; Barberio, M.; Chalopin, C.; Diana, M.; Moulla, Y.; et al. Comparison of hyperspectral imaging and fluorescence angiography for the determination of the transection margin in colorectal resections-a comparative study. Int. J. Color. Dis. 2021, 36, 283–291. [Google Scholar] [CrossRef]
- Pfahl, A.; Radmacher, G.K.; Köhler, H.; Maktabi, M.; Neumuth, T.; Melzer, A.; Gockel, I.; Chalopin, C.; Jansen-Winkeln, B. Combined indocyanine green and quantitative perfusion assessment with hyperspectral imaging during colorectal resections. Biomed. Opt. Express 2022, 13, 3145–3160. [Google Scholar] [CrossRef]
- Draijer, M.; Hondebrink, E.; van Leeuwen, T.; Steenbergen, W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med. Sci. 2009, 24, 639–651. [Google Scholar] [CrossRef]
- Nwaiwu, C.A.; McCulloh, C.J.; Skinner, G.; Shah, S.K.; Kim, P.C.W.; Schwaitzberg, S.D.; Wilson, E.B. Real-time First-In-Human Comparison of Laser Speckle Contrast Imaging and ICG in Minimally Invasive Colorectal & Bariatric Surgery. J. Gastrointest. Surg. 2023, 27, 3083–3085. [Google Scholar]
- Reeves, J.J.; Broderick, R.C.; Lee, A.M.; Blitzer, R.R.; Waterman, R.S.; Cheverie, J.N.; Jacobsen, G.R.; Sandler, B.J.; Bouvet, M.; Doucet, J.; et al. The price is right: Routine fluorescent cholangiography during laparoscopic cholecystectomy. Surgery 2022, 171, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Nijssen, D.J.; Wienholts, K.; Postma, M.J.; Tuynman, J.; Bemelman, W.A.; Laméris, W.; Hompes, R. The economic impact of anastomotic leakage after colorectal surgery: A systematic review. Tech. Coloproctol. 2024, 28, 55. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Country | Study Design (Level of Evidence) | Sample Size, n | Median/Mean Age (Range/SD) | Sex, Males | Median/Total Follow-up (Range) | T1, n (%) | T2, n (%) | T3, n (%) | T4, n (%) | Type of Fluorescence Agent | How Fluorescence Agent Was Administered | Fluorescence Dose | Molecular Target | Imaging Technique/Device | Time between Injection and Imaging | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| de Gooyer et al. [30], 2022 | Netherland | RCT (1) | 15 | - | 4 | - | - | - | - | - | 111In (In-DOTA-labetuzumab-IRDye800CW) | IV | 2 mg, 10 mg, 50 mg | CEA | NIR-FIS (Quest Medical Imaging Spectrum NIR FC)) | 5 or 6 days | 6 |
| de Valk et al. [32], 2020 | Netherlands | RCT (1) | 23 | 66.8 (49–80) | 10 (0.434) | - | - | - | - | - | cRGD-ZW800-1 | IV | 0.001 and 0.005 mg/kg | Integrins | Olympus Visera Elite II (CLV-S200-IR) | 2 to 4 h | N/A |
| Rotondano et al. [37], 2012 | Italy | RCT (1) | 47 | AFI: 54 (15) and HRE: 51 (12) | AFI: 24 (0.51) and HRE: 21 (0.446) | - | - | - | - | - | - | - | - | N/A | Olympus XCF-H260AZI | - | N/A |
| Wan et al. [27], 2022 | China | RCT (1) | 66 (33 ICG vs. 33 non-ICG) | 58 (39–79) | 45 | - | 0 | 6 (0.09) | 33 (0.5) | 27 (0.41) | ICG | Endoscopic submucosal injection | 2.5 mg/1 mL | N/A | FIS (Karl Storz) | 1 day | N/A |
| Watanabe et al. [41], 2017 | Japan | RCT (1) | 31 | 67.5 (±12.2) | 22 (0.80) | - | - | - | - | - | ICG | Laparoscopically injected | 2.5 mg | N/A | Laparoscopic NIR camera system (Karl Storz) | 30 min | N/A |
| Watanabe et al. [54], 2020 | Japan | RCT (1) | 839 (422 ICG vs. 417 non-ICG) | ICG: 66 (56–73) and non-ICG: 67 (58–74) | ICG: 266 (0.63) and non-ICG: 274 (0.66) | 1 month | 150 (0.36) | 117 (0.28) | 154 (0.37) | - | ICG | IV | 12.5 mg | N/A | Endoscopic camera system 1588 and 1688 (Stryker Corporation) | 1 min | N/A |
| Ankersmit et al. [34], 2019 | Netherlands | Prospective (2) | 10 | 71.5 (63–77) | 8 | - | - | - | - | - | [89Zr]Zr-Nanocoll (preoperative); ICG (intraoperative) | Endoscopically | “89Zr 0.4 mL | N/A | PET/CT; NIR laparoscopy (Olympus) | 46 (43–48) h | 5 |
| Cai et al. [29], 2023 | China | Prospective (2) | 34 | 55.7 (±9.6) | 22 | - | - | - | - | - | ICG | IV | 0.5 mg/kg | N/A | Laparoscopic FIS (Stryker Corporation) | 5–7 days | 6 |
| Chand et al. [20], 2018 | UK | Prospective (2) | 10 | 69.5 (±7.13) | 4 | 30 days | 1 (0.1) | 0 (0) | 3 (0.3) | 6 (0.6) | ICG | 1 mL subserosal ICG injection placed in four sites around the tumour | Varying concentrations (5 mg/10 mL, 5 mg/5 mL, 5 mg/3 mL) | N/A | Laparoscopic FC systems (Pinpoint and AIM 1588) | Immediate (intraoperative) | 7 * |
| Currie et al. [22], 2017 | UK | Prospective (2) | 30 | 68 (38–80) | - | - | 6 (0.2) | 8 (0.27) | 14 (0.47) | 2 (0.067) | ICG | Four 1 mL aliquots around the tumour | 5 mg/mL | N/A | Laparoscopic NIR FIS (Olympus) | Immediate (intraoperative) | 7 * |
| de Valk et al. [47], 2021 | Netherlands | Prospective (2) | 37 | 63 (±8.7, 43–79) | 23 (0.62) | Immediate (postoperative) | - | - | - | - | SGM-101 | IV over 30 min | 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg | CEA | Quest Spectrum Platform (Quest Medical Imaging) | At least 24 h | 7 * |
| Hellan et al. [23], 2014 | USA | Prospective (2) | 40 (28 with CRC) | 63.9 | 20 | 30 days | - | - | - | - | ICG | IV | Max dose 2 mg/kg | N/A | Fluorescence-capable da Vinci Si high-definition vision system (Firefly) | Mean 5.1 (+/−10) min | 7 * |
| Hirche et al. [24], 2012 | Germany | Prospective (2) | 26 | 67 (46–87) | - | - | 6 (0.23) | 5 (0.19) | 14 (0.54) | 1 (0.038) | ICG | Intraoperatively around the tumour | 5 mg/mL | N/A | FIS (IC-View) consisting of digital video camera with an integrated NIR light source | 3–10 min | 7 * |
| Kim et al. [33], 2020 | Korea | Prospective (2) | 10 | 60 (48–80) | 8 | - | - | - | - | - | ICG | Transanal | 2.5 mg/bodyweight | N/A | da Vinci Surgical System (Intuitive Surgical) | Immediate (intraoperative) | 5 |
| Kinoshita et al. [46], 2023 | Japan | Prospective (2) | 56 | 74 (65–77) | 35 (0.625) | - | 13 (0.232) | 10 (0.179) | 23 (0.411) | 10 (0.179) | ICG | Injection to subserosal layer at both proximal and distal points of the tumour | 0.2−0.5 mL at a concentration of 2.5 mg/mL | N/A | 1588 AIM camera system; infrared endoscopic camera system; D-Light P (Karl Storz) | 5 min, 30–60 min, and over 60 min | 7 * |
| Miyoshi et al. [35], 2009 | Japan | Prospective (2) | 40 | 63.5 (41–84) | 22 (0.55) | >9 days | - | - | - | - | ICG | - | - | N/A | CF-FH260AZI endoscope | - | 7 * |
| Moriichi et al. [36], 2012 | Japan | Prospective (2) | 67 | - | - | - | - | - | - | - | - | - | - | N/A | AFI | - | 6 |
| Munechika et al. [50], 2021 | Japan | Prospective (2) | 40 (20 ICG vs. 20 non-ICG) | 68.5 (±8.3, 47–83) | 36 (0.65) | 15 months (6–22) | 21 (0.525) | 19 (0.475) | ICG | IV | 5 mg | N/A | ICG NIRF (1588 AIM camera system, Stryker Corporation) | 24 s (3-61) | |||
| Patel et al. [31], 2022 | UK | Prospective (2) | 15 | - | 7 | - | - | - | - | - | ICG | IV | 10 mg/kg bodyweight | N/A | NIRF (IMAGE1 Spies, Karl Storz) | 1 day | 5 |
| Peloso et al. [38], 2013 | Italy | Prospective (2) | 25 | 61.5 (42–87) | 17 (0.68) | - | - | - | - | - | ICG | IV | 0.5 mg/kg bodyweight | N/A | NIR FC, the Photodynamic Eye (PDE) (Hamamatsu Photonics)) | 24 h | 7 * |
| Sato et al. [51], 2023 | Japan | Prospective (2) | 14 | 71.2 (±11.7,42–87) | 8 (0.57) | Immediate (postoperative) | 2 (0.14) | 3 (0.21) | 6 (0.43) | 3 (0.21) | ICG | 0.1 (0.25 mg) of ICG injected into submucosa at the dentate line 3 cm from the anal verge at the anterior, posterior, and bilateral walls | 2.5 mg/mL (25 mg in 10 mL distilled water) | N/A | Laparoscopic NIRF system (VISERA ELITE II, Olympus) | Immediate | 7 * |
| Schaafsma et al. [25], 2013 | USA | Prospective (2) | 22 | 69 (41–88) | 12 | - | 2 (0.09) | 7 (0.32) | 10 (0.45) | 3 (0.14) | HSA800 and blue dye | Injected submucosally circumferentially with a 5 mm margin around the tumour | 1 mL of 50 micromol/L HSA800 diluted in patent blue dye | N/A | Mini-FLARE camera system | 5 min | 7 * |
| Schaap et al. [52], 2020 | Netherlands | Prospective (2) | 14 | - | - | Immediate (postoperative) | - | - | - | - | SGM-101 | IV | - | CEA | Quest Spectrum Platform (Quest Medical Imaging) | 4–6 days before surgery | 7 * |
| Sikkenk et al. [26], 2023 | Netherlands | Prospective (2) | 10 | 70 (59–84) | 7 | - | 5 (0.5) | 0 | 0 | ICG | 1 mL ICG in four aliquots, injected submucosally around the tumour | 5 mg/ml | N/A | NIRF ‘firefly’ mode of da Vinci Xi | Immediate (intraoperative) | 7 * | |
| Tashiro et al. [32], 2020 | Japan | Prospective (2) | 72 | 67.5 (34–85) | 23 | - | - | - | - | - | ICG | IV | 0.5 mg/kg | N/A | SPY Portable Handheld Imaging System (Stryker Corporation) | 5 (2–34) days | 6 |
| Tanis et al. [39], 2016 | Netherlands | Prospective (2) | 17 | 62 (38–74) | 13 (0.764) | - | - | - | - | - | - | Optical needle | - | N/A | Diffuse reflectance spectroscopy and FS | - | 6 |
| Weixler et al. [42], 2017 | Switzerland | Prospective (2) | 220 | 70.5 (±11.2) | 126 (0.573) | 73.3 months (70.4–76.2) | 20 (0.91) | 37 (0.168) | 149 (0.677) | 14 (0.44) | ICG | Ex vivo injected with ICG subserosa around the tumour | - | N/A | ICG-based SLN-mapping | 5 min | 8 * |
| Daibo et al. [22], 2024 | Japan | Retrospective (3) | 462 (231 ICG vs. 231 non-ICG after propensity scoring) | 75 (68–79) | 216 | 36.9 months | 140 (0.30) | 92 (0.20) | 228 (0.49) | 0 | ICG | Subserosal submucosal layer injection around the tumour at two points | 2.5 mg/ml | N/A | ICG-FI systems (D-light, Karl Storz)) and endoscopic camera system (Stryker Corporation) | 30 min | 9* |
| de Jongh et al. [43], 2020 | Netherlands | Retrospective (3) | 25 | 56 (31–76) | 17 (0.68) | - | - | - | - | - | Bevacizumab-800CW | Back-table FGI | - | VEGF-A | High-resolution Odyssey CLx FIS (LI-COR Biosciences Inc.) | - | 7 * |
| Handgraaf et al. [40], 2017 | Netherlands | Retrospective (3) | 173 (106 fluorescence vs. 67 non-fluorescence) | Non-fluorescence: 63 (±9.4) vs. fluorescence: 62 (±9.2) | 94 (0.54) | 4 years | - | - | - | - | ICG | IV | 10 or 20 mg | N/A | Mini-FLARE® (Frangioni Laboratory), Artemis (Quest Innovations)), and laparoscope (Karl Storz) | 1 or 2 days | 9 * |
| Mizrahi et al. [48], 2018 | USA | Retrospective (3) | 54 | 63 (±12) | 31 (0.57) | Immediate (postoperative) | 5 (0.09) | 14 (0.26) | 28 (0.52) | 2 (0.04) | - | IV 3.5 mL ICG followed by a second and third bolus of same volume | 2.5 mg/mL (25 mg in 10 mL sterile water) | N/A | PINPOINT endoscopic FIS (Novadaq)) | Immediate (intraoperative) | 7 * |
| Mizrahi et al. [49], 2018 | USA | Retrospective (3) | 60 (30 fluorescence vs. 30 non-fluorescence) | 58 (±12) | 34 (0.57) | Immediate (postoperative) | 8 (0.13) | 10 (0.167) | 20 (0.33) | 2 (0.033) | ICG | IV 3.5 mL ICG followed by a second and third bolus of same volume | 2.5 mg/mL (25 mg in 10 mL sterile water) | N/A | PINPOINT endoscopic FIS (Novadaq)) | Immediate (intraoperative) | 8 * |
| Park et al. [45], 2020 | Korea | Retrospective (3) | 75 (25 ICG vs. 50 non-ICG) | ICG:71 (49–83) and non-ICG: 66 (42–84) | 42 (0.56) | Immediate (postoperative) | - | - | 58 (0.77) | 17 (0.23) | ICG | ICG solution was injected into the submucosa in the peritumoral area at one or two points | 0.2–0.3 mL of 2.5 mg/mL | N/A | D-light P (Karl Storz)and Firefly (Intuitive Surgical) | 3 to 24 h | 8 * |
| Watanabe et al. [53], 2020 | Japan | Retrospective (3) | 422 (211 ICG vs. 211 non-ICG after propensity scoring) | 66 (34–92) | 259 (0.61) | Immediate (postoperative) | - | - | - | - | ICG | IV | 0.25 mg/kg | N/A | NIR camera system | Immediate—just before proximal bowel resection | 8 * |
| Watanabe et al. [28], 2023 | Japan | Retrospective (3) | 116 (58 ICG vs. 58 non-ICG after propensity matching) | 65 (60–73) | 78 | 63.7 months (51.3–76.8) | 23 (0.2) | 40 (0.34) | 48 (0.41) | 0 | ICG | Injected locally into submucosal layer at four points (total 1 mL) on anal side of tumour using a flexisigmoidoscopy | 2.5 mg/1 mL | N/A | Laparoscopic NIRF system, 1588 AIM (Stryker Corporation) and D-light (Karl Storz) | Injected after GA induction | 9 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadel, M.G.; Zonoobi, E.; Rodríguez-Luna, M.R.; Mishima, K.; Ris, F.; Diana, M.; Vahrmeijer, A.L.; Perretta, S.; Ashrafian, H.; Fehervari, M. Efficacy and Safety of Fluorescence-Guided Surgery Compared to Conventional Surgery in the Management of Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 3377. https://doi.org/10.3390/cancers16193377
Fadel MG, Zonoobi E, Rodríguez-Luna MR, Mishima K, Ris F, Diana M, Vahrmeijer AL, Perretta S, Ashrafian H, Fehervari M. Efficacy and Safety of Fluorescence-Guided Surgery Compared to Conventional Surgery in the Management of Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(19):3377. https://doi.org/10.3390/cancers16193377
Chicago/Turabian StyleFadel, Michael G., Elham Zonoobi, María Rita Rodríguez-Luna, Kohei Mishima, Frédéric Ris, Michele Diana, Alexander L. Vahrmeijer, Silvana Perretta, Hutan Ashrafian, and Matyas Fehervari. 2024. "Efficacy and Safety of Fluorescence-Guided Surgery Compared to Conventional Surgery in the Management of Colorectal Cancer: A Systematic Review and Meta-Analysis" Cancers 16, no. 19: 3377. https://doi.org/10.3390/cancers16193377
APA StyleFadel, M. G., Zonoobi, E., Rodríguez-Luna, M. R., Mishima, K., Ris, F., Diana, M., Vahrmeijer, A. L., Perretta, S., Ashrafian, H., & Fehervari, M. (2024). Efficacy and Safety of Fluorescence-Guided Surgery Compared to Conventional Surgery in the Management of Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers, 16(19), 3377. https://doi.org/10.3390/cancers16193377






