Proton Pump Inhibitors Worsen Colorectal Cancer Outcomes in Patients Treated with Bevacizumab
Abstract
Simple Summary
Abstract
1. Introduction
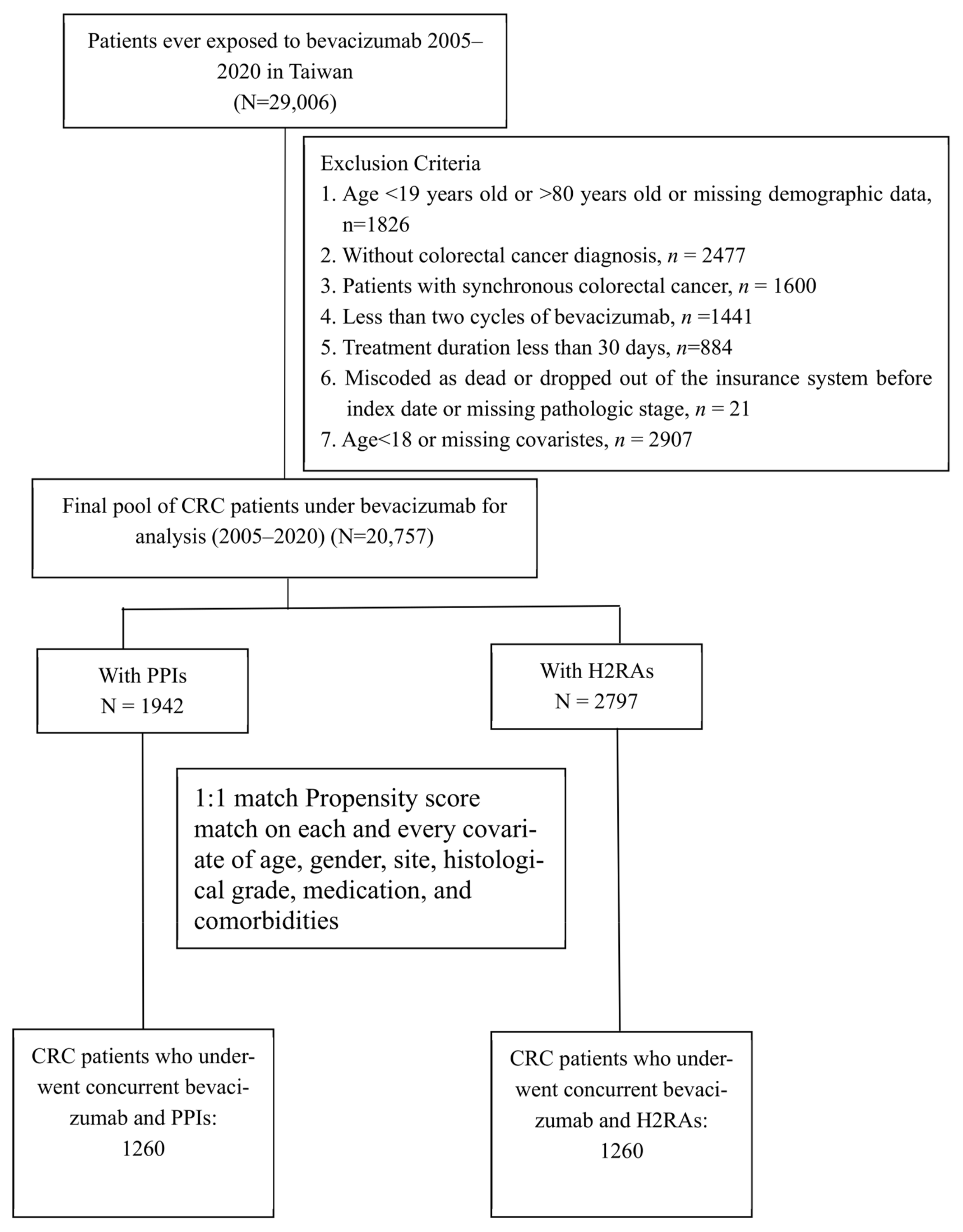
2. Materials and Methods
2.1. Ethics Statement
2.2. Data Source
2.3. Study Population and PPI/H2RA Exposure
2.4. Confounding Factors and Propensity Match
2.5. Study Outcomes
2.6. Statistical Methods
3. Results
3.1. Baseline Comparability
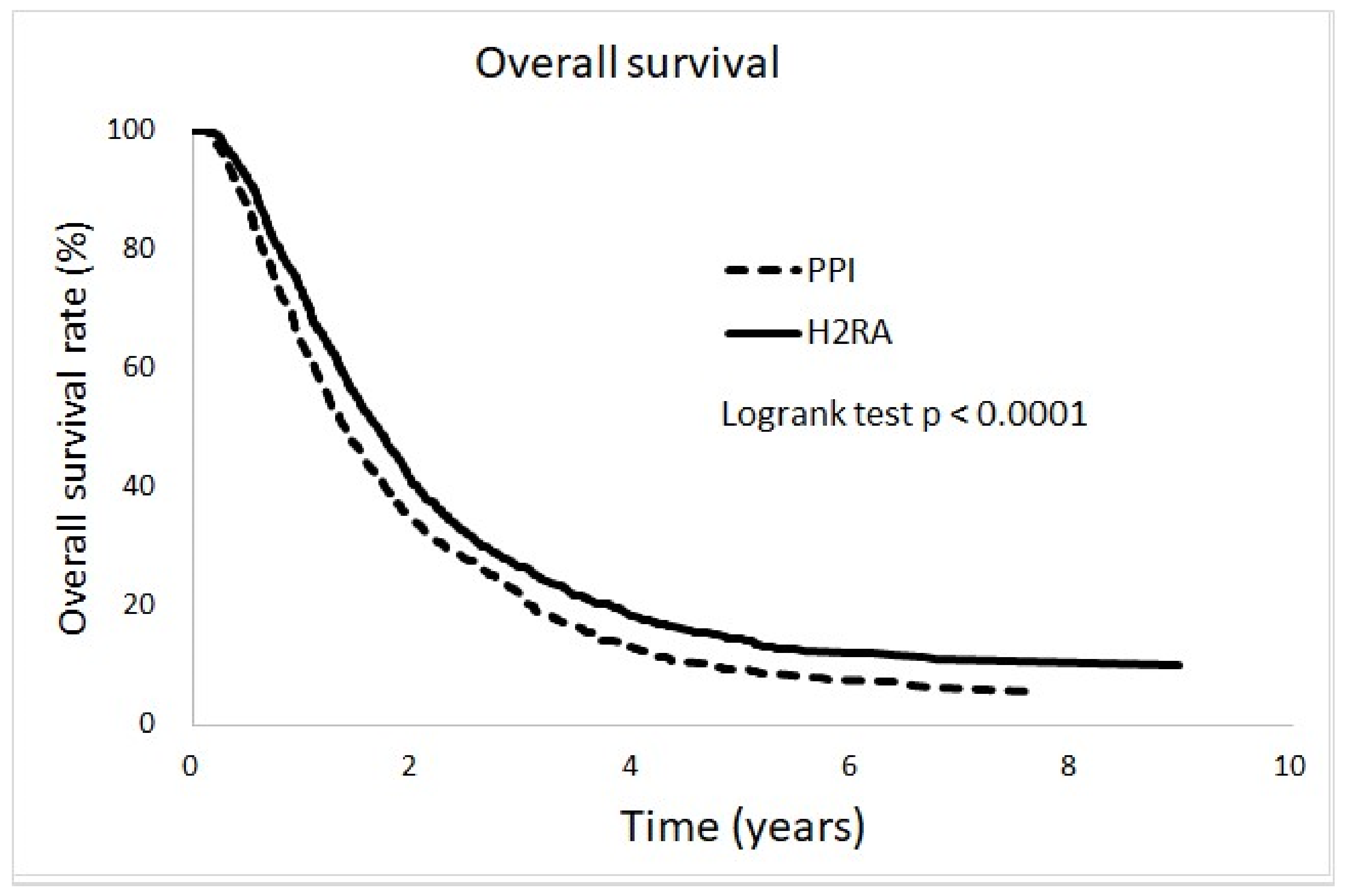
3.2. Incidence and Adjusted Hazard Ratio
3.3. Dose Response Relationship
4. Discussion
4.1. Strengths of the Study
4.2. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Ahmadizar, F.; Onland-Moret, N.C.; De Boer, A.; Liu, G.; Maitland-van der Zee, A.H. Efficacy and safety assessment of the addition of bevacizumab to adjuvant therapy agents in cancer patients: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0136324. [Google Scholar] [CrossRef] [PubMed]
- Hochster, H.S.; Hart, L.L.; Ramanathan, R.K.; Childs, B.H.; Hainsworth, J.D.; Cohn, A.L.; Wong, L.; Fehrenbacher, L.; Abubakr, Y.; Saif, M.W. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE Study. J. Clin. Oncol. 2008, 26, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Cleck, J.N. Adverse effects of anticancer agents that target the VEGF pathway. Nat. Rev. Clin. Oncol. 2009, 6, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Eser, K.; Önder, A.H.; Sezer, E.; Çil, T.; İnal, A.; Öztürk, B.; Erçolak, V.; Duman, B.B.; Çelik, H.; Köşeci, T. Proton pump inhibitors may reduce the efficacy of ribociclib and palbociclib in metastatic breast cancer patients based on an observational study. BMC Cancer 2022, 22, 516. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Malik, F.; Naglak, M.; Jafri, S.I.M.; Fidler, C.; Shahin, M.S.; Seidman, M.J.; Cohen, S.J. Bevacizumab-induced bleeding in colorectal and noncolorectal cancer patients in a community oncology setting: An Abington Memorial Hospital experience. J. Clin. Oncol. 2017, 35, e18250. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Scarpignato, C.; Gatta, L.; Zullo, A.; Blandizzi, C.; Group, S.-A.-F.; Italian Society of Pharmacology; the Italian Association of Hospital Gastroenterologists; the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases–a position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016, 14, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smelick, G.S.; Heffron, T.P.; Chu, L.; Dean, B.; West, D.A.; DuVall, S.L.; Lum, B.L.; Budha, N.; Holden, S.N.; Benet, L.Z. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug–drug interaction potential for molecular targeted agents in clinical development. Mol. Pharm. 2013, 10, 4055–4062. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.A.; Silva, P.A.; Lopes, M.S.; Yen, C.T.; Ricardo, E.D.; Mutão, T.; Pimenta, J.R.; Machado, L.M.; Shimba, D.S.; Peixoto, R.D. Proton pump inhibitors and oncologic treatment efficacy: A practical review of the literature for oncologists. Curr. Oncol. 2021, 28, 783–799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yagi, K.; Mitstui, M.; Zamami, Y.; Niimura, T.; Izawa-Ishizawa, Y.; Goda, M.; Chuma, M.; Fukunaga, K.; Shibata, T.; Ishida, S. Investigation of drugs affecting hypertension in bevacizumab-treated patients and examination of the impact on the therapeutic effect. Cancer Med. 2021, 10, 164–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welage, L.S. Overview of pharmacologic agents for acid suppression in critically ill patients. Am. J. Health Syst. Pharm. 2005, 62, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Hirasawa, N.; Ohuchi, K. Enhancement by histamine of vascular endothelial growth factor production in granulation tissue via H2 receptors. Br. J. Pharmacol. 2001, 134, 1419–1428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, J.-F.R.; Chiang, T.-L. Evolution of Taiwan’s health care system. Health Econ. Policy Law 2011, 6, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Su, C.-C.; Shao, S.-C.; Sung, S.-F.; Lin, S.-J.; Yang, Y.-H.K.; Lai, E.C.-C. Taiwan’s national health insurance research database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kao, C.-W.; Chiang, C.-J.; Lin, L.-J.; Huang, C.-W.; Lee, W.-C.; Lee, M.-Y.; Cheng-Yi, S.; Lin, H.-L.; Lin, M.-M.; Wang, Y.-P. Accuracy of long-form data in the Taiwan cancer registry. J. Formos. Med. Assoc. 2021, 120, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Maruo, A.; Ishida, S.; Aizawa, F.; Ushio, S.; Sakaguchi, S.; Kajizono, M.; Niimura, T.; Goda, M.; Hamano, H. Effects of vonoprazan and proton pump inhibitors on the efficacy of bevacizumab: A multicentre retrospective study. Clin. Exp. Med. 2023, 23, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.S.; Kang, J.; Morita, S.; Park, Y.S.; Sakamoto, J.; Muro, K.; Xu, R.-H.; Kim, T.W. Proton pump inhibitor use and the efficacy of chemotherapy in metastatic colorectal cancer: A post hoc analysis of a randomized phase III trial (AXEPT). Oncologist 2021, 26, e954–e962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colucci, R.; Blandizzi, C.; Tanini, M.; Vassalle, C.; Breschi, M.C.; Tacca, M.D. Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br. J. Pharmacol. 2005, 144, 338–348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasaki, T.; Mori, S.; Kishi, S.; Fujiwara-Tani, R.; Ohmori, H.; Nishiguchi, Y.; Hojo, Y.; Kawahara, I.; Nakashima, C.; Fujii, K. Effect of proton pump inhibitors on colorectal cancer. Int. J. Mol. Sci. 2020, 21, 3877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ando-Matsuoka, R.; Yagi, K.; Takaoka, M.; Sakajiri, Y.; Shibata, T.; Sawada, R.; Maruo, A.; Miyata, K.; Aizawa, F.; Hamano, H. Differential effects of proton pump inhibitors and vonoprazan on vascular endothelial growth factor expression in cancer cells. Drug Dev. Res. 2023, 84, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deva, S.; Jameson, M. Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer. Cochrane Database Syst. Rev. 2012, (6), CD007814. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Izumi, K.; Okabe, S. Roxatidine-and cimetidine-induced angiogenesis inhibition suppresses growth of colon cancer implants in syngeneic mice. J. Pharmacol. Sci. 2003, 93, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cao, F.; Zhu, Q.; Gan, M.; Wang, D. Perioperative cimetidine administration improves systematic immune response and tumor infiltrating lymphocytes in patients with colorectal cancer. Hepatogastroenterology 2013, 60, 244–247. [Google Scholar] [CrossRef] [PubMed]

| Covariate | PPI | H2RA | p Value |
|---|---|---|---|
| N (%), n = 1260 | N (%), n = 1260 | ||
| Age (year) | 0.9981 | ||
| 20–54 | 290 (23.02) | 292 (23.17) | |
| 55–62 | 326 (25.87) | 322 (25.56) | |
| 63–69 | 312 (24.76) | 312 (24.76) | |
| 70–80 | 332 (26.35) | 334 (26.51) | |
| Sex | 1.000 | ||
| Male | 770 (61.11) | 771 (61.19) | |
| Female | 490 (38.89) | 489 (38.81) | |
| Cancer site | 1.000 | ||
| Colon, left | 379 (30.08) | 379 (30.08) | |
| Colon, right | 354 (28.10) | 354 (28.10) | |
| Colon, unspecified | 70 (5.56) | 70 (5.56) | |
| Rectum | 457 (36.27) | 457 (36.27) | |
| Pathological grade | 0.9871 | ||
| 1 | 19 (1.51) | 19 (1.51) | |
| 2 | 675 (53.57) | 675 (53.57) | |
| 3 | 77 (6.11) | 76 (6.03) | |
| 4 and others | 489 (38.81) | 490 (38.89) | |
| Comorbidity | |||
| With PUD | 386(30.63) | 385 (30.56) | 0.9655 |
| With GI bleeding | 31 (2.46) | 28 (2.22) | 0.6927 |
| Medication | |||
| NSAID usage | 1054 (83.65) | 1054 (83.65) | 1.000 |
| Steroid usage | 1256 (99.68) | 1260 (100) | 0.1793 |
| CCI | 0.9998 | ||
| <8 | 64 (5.08) | 65 (5.16) | |
| 8 | 461 (36.59) | 461 (36.59) | |
| 9 | 417 (33.10) | 417 (33.10) | |
| >9 | 318(25.24) | 317(25.16) | |
| Urbanization | 0.9695 | ||
| High | 255 (20.24) | 250 (19.84) | |
| Median | 618 (49.05) | 621 (49.29) | |
| Low | 387 (30.71) | 389 (30.87) | |
| Region | 0.0048 | ||
| North | 452 (35.87) | 459 (36.43) | |
| Central | 360 (28.57) | 287 (22.78) | |
| East | 29 (2.30) | 36 (2.86) | |
| South | 419 (33.25) | 478 (37.94) | |
| SES (monthly income) | 0.2889 | ||
| ≤20.1 K | 444 (35.24) | 433 (34.37) | |
| 20.1–22.8 K | 205 (16.27) | 218 (17.30) | |
| 22.8–42 K | 325 (25.79) | 355 (28.17) | |
| ≥42 K | 286 (22.70) | 254 (20.16) |
| All-Cause Death | CRC-Specific Death | |||
|---|---|---|---|---|
| IP (95% CI) | Event (%) | IP (95% CI) | Event (%) | |
| PPIs vs. H2RAs | ||||
| PPIs | 464.1 (434.8, 495.0) | 928 (73.7) | 451.6 (422.7, 482.1) | 903 (71.7) |
| H2RAs | 388.6 (363.2, 415.3) | 870 (69.0) | 377.0 (352.0, 403.3) | 844 (67.0) |
| All-Cause Death | Cancer-Specific Death | |||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | |
| PPIs vs. H2RAs | 1.19 (1.09,1.31) | 0.0002 | 1.20 (1.09, 1.31) | 0.0002 |
| Dose Response | ||||
| cDDD 0–15 | 1.02 (0.87, 1.19) | 0.7931 | 1.03 (0.88, 1.20) | 0.7332 |
| cDDD 15–32 | 1.17 (1.01, 1.36) | 0.0316 | 1.18 (1.01, 1.36) | 0.0326 |
| cDDD 32–47 | 1.30 (1.12, 1.49) | 0.0004 | 1.29 (1.12, 1.49) | 0.0005 |
| cDDD ≥ 47 | 1.29 (1.12, 1.49) | 0.0005 | 1.30 (1.12, 1.50) | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Fang, C.-Y.; Chiou, W.-Y.; Chen, P.-T.; Hsu, T.-W.; Hung, S.-K.; Liao, Y.-T.; Hung, C.-S.; Tsai, J.-H. Proton Pump Inhibitors Worsen Colorectal Cancer Outcomes in Patients Treated with Bevacizumab. Cancers 2024, 16, 3378. https://doi.org/10.3390/cancers16193378
Wu C-C, Fang C-Y, Chiou W-Y, Chen P-T, Hsu T-W, Hung S-K, Liao Y-T, Hung C-S, Tsai J-H. Proton Pump Inhibitors Worsen Colorectal Cancer Outcomes in Patients Treated with Bevacizumab. Cancers. 2024; 16(19):3378. https://doi.org/10.3390/cancers16193378
Chicago/Turabian StyleWu, Chin-Chia, Chuan-Yin Fang, Wen-Yen Chiou, Pei-Tsen Chen, Ta-Wen Hsu, Shih-Kai Hung, Yu-Tso Liao, Chuan-Sheng Hung, and Jui-Hsiu Tsai. 2024. "Proton Pump Inhibitors Worsen Colorectal Cancer Outcomes in Patients Treated with Bevacizumab" Cancers 16, no. 19: 3378. https://doi.org/10.3390/cancers16193378
APA StyleWu, C.-C., Fang, C.-Y., Chiou, W.-Y., Chen, P.-T., Hsu, T.-W., Hung, S.-K., Liao, Y.-T., Hung, C.-S., & Tsai, J.-H. (2024). Proton Pump Inhibitors Worsen Colorectal Cancer Outcomes in Patients Treated with Bevacizumab. Cancers, 16(19), 3378. https://doi.org/10.3390/cancers16193378






