C-Reactive Protein-to-Albumin Ratio as a Predictive Indicator for Evaluating Tolerability in S-1 Adjuvant Chemotherapy after Curative Surgery for Pancreatic Cancer: An External Validation Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
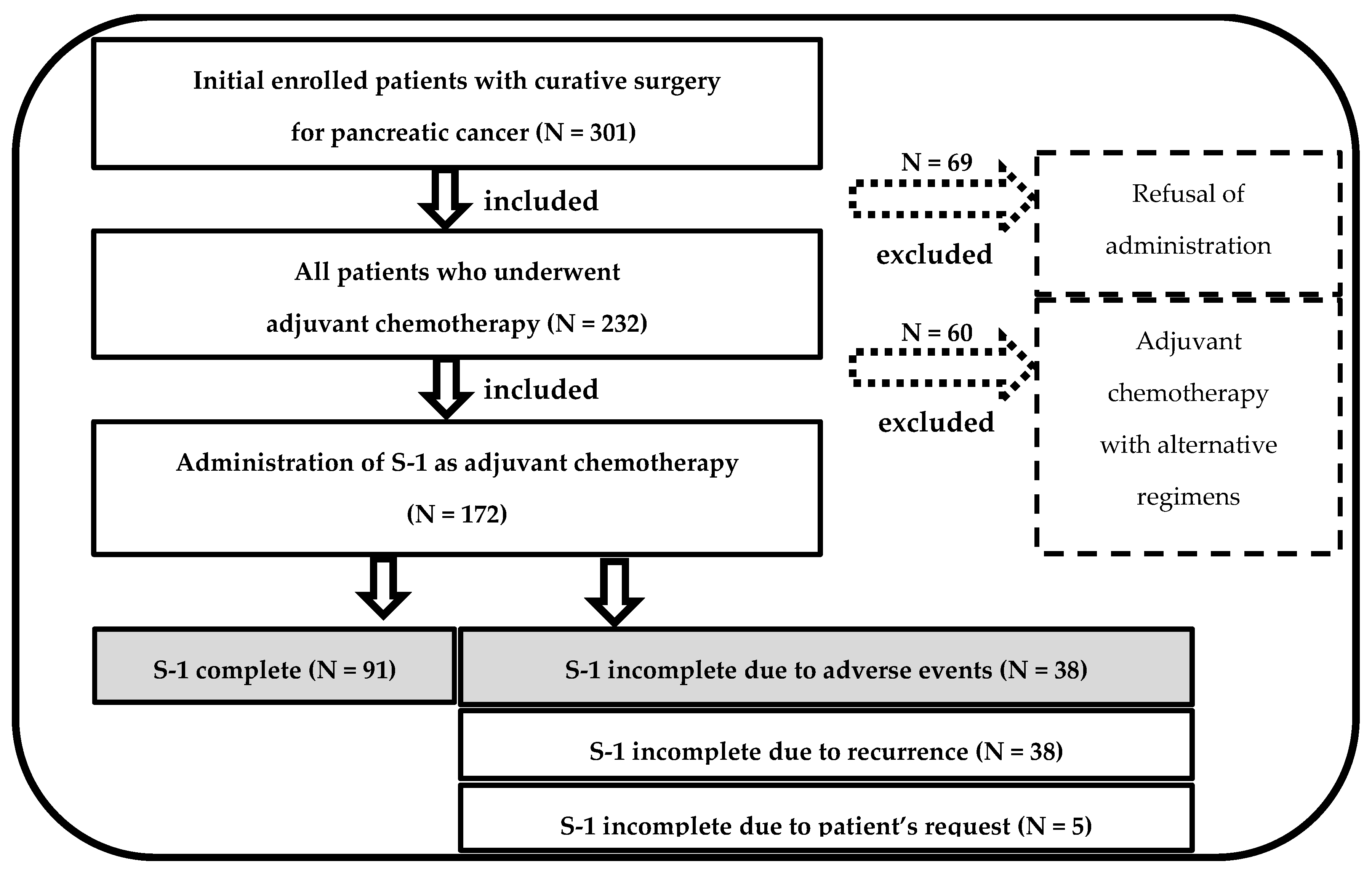
2.1. Patients
2.2. Operative Procedures and Perioperative Management in Dokkyo Medical University Hospital
2.3. AC Regimens and Postoperative Surveillance
2.4. Definition of CAR
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics with or without S-1 Completion in the Dokkyo Cohort
3.2. Calculation of an Optimal CAR
3.3. Multivariate Analysis for S-1 Completion
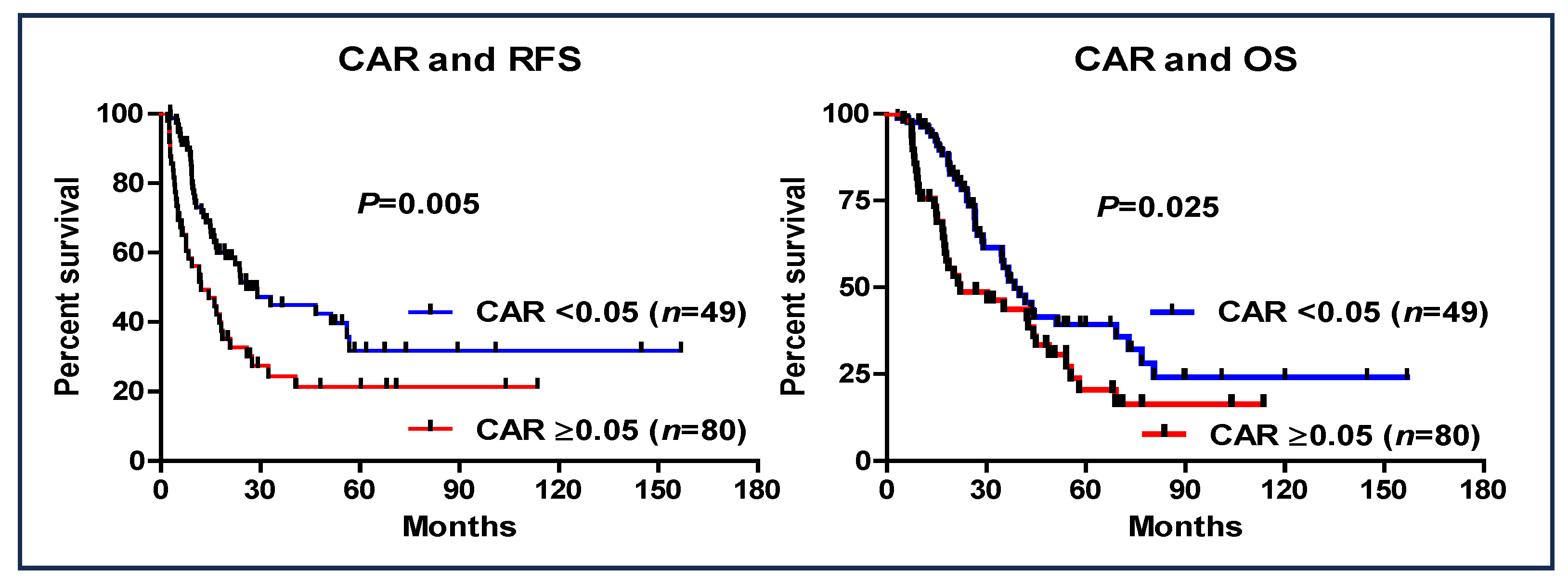
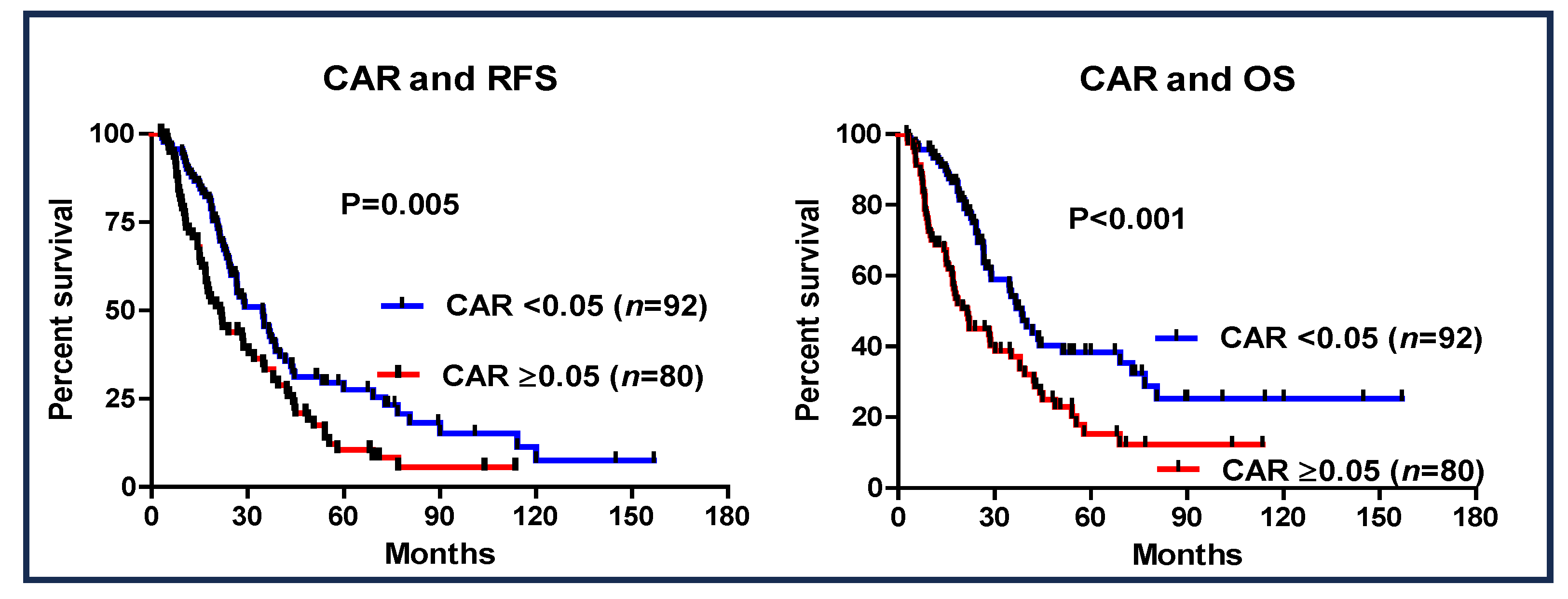
3.4. CAR and Patient Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRP | C-reactive protein |
| CAR | C-reactive protein-to-albumin ratio |
| AC | adjuvant chemotherapy |
| AEs | adverse events |
| PC | pancreatic cancer |
| POCs | postoperative complications |
| CD Classification | Clavien–Dindo classification |
| ROC | receiver operating characteristic |
| OS | overall survival |
| RFS | recurrence-free survival |
| RDI | relative dose intensity |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Japan Pancreas Society. Clinical Practice Guidelines for Pancreatic Cancer. Available online: http://www.suizou.org/pdf/pancreatic_cancer_cpg-2019.pdf (accessed on 5 March 2024). (In Japanese).
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Ueno, H.; Kosuge, T.; Matsuyama, Y.; Yamamoto, J.; Nakao, A.; Egawa, S.; Doi, R.; Monden, M.; Hatori, T.; Tanaka, M.; et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br. J. Cancer 2009, 101, 908–915. [Google Scholar] [CrossRef]
- Sakamoto, A.; Funamizu, N.; Shine, M.; Uraoka, M.; Nagaoka, T.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Takada, Y. Geriatric nutritional risk index predicts tolerability of S-1 as adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Pancreas 2023, 52, e196–e202. [Google Scholar] [CrossRef]
- Funamizu, N.; Utsunomiya, T.; Honjo, M.; Ito, C.; Shine, M.; Uraoka, M.; Nagaoka, T.; Tamura, K.; Sakamoto, K.; Ogawa, K.; et al. Preoperative C-reactive protein-to-albumin ratio predicts postoperative pancreatic fistula following pancreatoduodenectomy: A single-center, retrospective study. Curr. Oncol. 2022, 29, 9867–9874. [Google Scholar] [CrossRef]
- Funamizu, N.; Sogabe, K.; Shine, M.; Honjo, M.; Sakamoto, A.; Nishi, Y.; Matsui, T.; Uraoka, M.; Nagaoka, T.; Iwata, M.; et al. Association between the preoperative C-reactive protein-to-albumin ratio and the risk for postoperative pancreatic fistula following distal pancreatectomy for pancreatic cancer. Nutrients 2022, 14, 5277. [Google Scholar] [CrossRef]
- Murakawa, M.; Yamamoto, N.; Kamioka, Y.; Kamiya, M.; Kobayashi, S.; Ueno, M.; Morimoto, M.; Atsumi, Y.; Aoyama, T.; Tamagawa, H.; et al. Clinical implication of preoperative C-reactive protein-albumin ratio as a prognostic factor of patients with pancreatic ductal adenocarcinoma: A single-institutional retrospective study. In Vivo 2020, 34, 347–353. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, G.L. C-reactive protein to albumin ratio predicts the outcome in renal cell carcinoma: A meta-analysis. PLoS ONE 2019, 14, e0224266. [Google Scholar] [CrossRef]
- Funamizu, N.; Sakamoto, A.; Hikida, T.; Ito, C.; Shine, M.; Nishi, Y.; Uraoka, M.; Nagaoka, T.; Honjo, M.; Tamura, K.; et al. C-reactive protein-to-albumin ratio to predict tolerability of S-1 as an adjuvant chemotherapy in pancreatic cancer. Cancers 2024, 16, 922. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Palmer, D.; Jackson, R.; Cox, T.; Neoptolemos, J.P.; Ghaneh, P.; Rawcliffe, C.L.; Bassi, C.; Stocken, D.D.; Cunningham, D.; et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: Ongoing lessons from the ESPAC-3 study. J. Clin. Oncol. 2014, 32, 504–512. [Google Scholar] [CrossRef]
- Qi, W.; Wang, X.; Gan, L.; Li, Y.; Li, H.; Cheng, Q. The effect of reduced RDI of chemotherapy on the outcome of breast cancer patients. Sci. Rep. 2020, 10, 13241. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE); National Cancer Institute: Bethesda, MD, USA, 2017. [Google Scholar]
- Fairclough, E.; Cairns, E.; Hamilton, J.; Kelly, C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin. Med. 2009, 9, 30–33. [Google Scholar] [CrossRef]
- Wang, E.Y.; Chen, M.K.; Hsieh, M.Y.; Kor, C.T.; Liu, Y.T. Relationship between Preoperative Nutritional Status and Clinical Outcomes in Patients with Head and Neck Cancer. Nutrients 2022, 14, 5331. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Omura, K.; Takada, Y.; Ozaki, T.; Mishima, K.; Igarashi, K.; Wakabayashi, G. Geriatric nutritional risk index less than 92 is a predictor for late postpancreatectomy hemorrhage following pancreatoduodenectomy: A retrospective cohort study. Cancers 2020, 12, 2779. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Yang, J.; Wu, H.; Wen, T.; Wang, W.; Li, B.; Yan, L. Early postoperative controlling nutritional status (CONUT) score is associated with complication III-V after hepatectomy in hepatocellular carcinoma: A retrospective cohort study of 1334 patients. Sci. Rep. 2018, 8, 13406. [Google Scholar] [CrossRef]
- Hayama, T.; Hashiguchi, Y.; Ozawa, T.; Watanabe, M.; Fukushima, Y.; Shimada, R.; Nozawa, K.; Matsuda, K.; Fujii, S.; Fukagawa, T. The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci. Rep. 2022, 12, 3682. [Google Scholar] [CrossRef]
- Ishikawa, M.; Iwasaki, M.; Namizato, D.; Yamamoto, M.; Morita, T.; Ishii, Y.; Sakamoto, A. The neutrophil to lymphocyte ratio and serum albumin as predictors of acute kidney injury after coronary artery bypass grafting. Sci. Rep. 2022, 12, 15438. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Suzuki, H.; Takayama, H.; Higashi, S.; Hirano, Y.; Tezuka, M.; Ishida, T.; Ishihata, K.; Nishi, Y.; Nakamura, Y.; et al. Impact of preoperative low prognostic nutritional index and high intramuscular adipose tissue content on outcomes of patients with oral squamous cell carcinoma. Cancers 2020, 12, 3167. [Google Scholar] [CrossRef] [PubMed]
- Sanft, T.; Harrigan, M.; McGowan, C.; Cartmel, B.; Zupa, M.; Li, F.Y.; Ferrucci, L.M.; Puklin, L.; Cao, A.; Nguyen, T.H.; et al. Randomized Trial of Exercise and Nutrition on Chemotherapy Completion and Pathologic Complete Response in Women With Breast Cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis Study. J. Clin. Oncol. 2023, 41, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.P.S.; Silva, L.C.D.; Fayh, A.P.T. Nutritional intervention contributes to the improvement of symptoms related to quality of life in breast cancer patients undergoing neoadjuvant chemotherapy: A randomized clinical trial. Nutrients 2021, 13, 589. [Google Scholar] [CrossRef]
- Lieto, E.; Auricchio, A.; Tirino, G.; Pompella, L.; Panarese, I.; Del Sorbo, G.; Ferraraccio, F.; De Vita, F.; Galizia, G.; Cardella, F. Naples prognostic score predicts tumor regression grade in resectable gastric cancer treated with preoperative chemotherapy. Cancers 2021, 13, 4676. [Google Scholar] [CrossRef]
- Fanetti, G.; Polesel, J.; Fratta, E.; Muraro, E.; Lupato, V.; Alfieri, S.; Gobitti, C.; Minatel, E.; Matrone, F.; Caroli, A.; et al. Prognostic nutritional index predicts toxicity in head and neck cancer patients treated with definitive radiotherapy in association with chemotherapy. Nutrients 2021, 13, 1277. [Google Scholar] [CrossRef]
- Nomoto, N.; Tate, S.; Arai, M.; Iizaka, S.; Mori, C.; Sakurai, K. Pretreatment nutritional status in combination with inflammation affects chemotherapy interruption in women with ovarian, Fallopian tube, and peritoneal cancer. Nutrients 2022, 14, 5183. [Google Scholar] [CrossRef]
- Gouez, M.; Delrieu, L.; Bouleuc, C.; Girard, N.; Raynard, B.; Marchal, T. Association between nutritional status and treatment response and survival in patients treated with immunotherapy for lung cancer: A retrospective French study. Cancers 2022, 14, 3439. [Google Scholar] [CrossRef]
- Mękal, D.; Sobocki, J.; Badowska-Kozakiewicz, A.; Sygit, K.; Cipora, E.; Bandurska, E.; Czerw, A.; Deptała, A. Evaluation of Nutritional Status and the Impact of Nutritional Treatment in Patients with Pancreatic Cancer. Cancers 2023, 15, 3816. [Google Scholar] [CrossRef]
- Álvaro Sanz, E.; Abilés, J.; Garrido Siles, M.; Rivas Ruíz, F.; Tortajada Goitia, B.; Domínguez, A.R. Evaluation of a protocol to detect malnutrition and provide nutritional care for cancer patients undergoing chemotherapy. Sci. Rep. 2020, 10, 21186. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yeom, S.S.; Kim, C.H.; Kim, H.R. Nutritional risk screening score is associated with omission of adjuvant chemotherapy for stage III colon cancer. Am. J. Surg. 2020, 220, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Ihara, K.; Yamaguchi, S.; Shida, Y.; Fujita, J.; Matsudera, S.; Kikuchi, M.; Muroi, H.; Nakajima, M.; Sasaki, K.; Tsuchioka, T.; et al. Nutritional status predicts adjuvant chemotherapy outcomes for stage III colorectal cancer. J. Anus Rectum Colon. 2019, 3, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.T.; Tong, Y.X.; Xu, X.S.; Zhou, Y.; Zhang, S. Preoperative nutritional status contributes to the development of neutropenia event in patients with gastric cancer receiving CAPEOX adjuvant chemotherapy. Front. Oncol. 2020, 10, 692. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Fukuda, S.; Tsujie, M.; Kitani, K.; Inoue, K.; Hayashi, T.; Ishikawa, H.; Yukawa, M.; Inoue, M. Outcome predictors for patients with stage II/III gastric cancer who undergo gastrectomy and S-1 adjuvant chemotherapy. Oncol. Lett. 2017, 14, 1621–1627. [Google Scholar] [CrossRef]
- Gonda, K.; Shibata, M.; Sato, Y.; Washio, M.; Takeshita, H.; Shigeta, H.; Ogura, M.; Oka, S.; Sakuramoto, S. Elevated neutrophil-to-lymphocyte ratio is associated with nutritional impairment, immune suppression, resistance to S-1 plus cisplatin, and poor prognosis in patients with stage IV gastric cancer. Mol. Clin. Oncol. 2017, 7, 1073–1078. [Google Scholar] [CrossRef]
- Shirasaka, T.; Nakano, K.; Takechi, T.; Satake, H.; Uchida, J.; Fujioka, A.; Saito, H.; Okabe, H.; Oyama, K.; Takeda, S.; et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996, 56, 2602–2606. [Google Scholar]
- Nakachi, K.; Ikeda, M.; Konishi, M.; Nomura, S.; Katayama, H.; Kataoka, T.; Todaka, A.; Yanagimoto, H.; Morinaga, S.; Kobayashi, S.; et al. Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet 2023, 401, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.; Pathak, S.; Nunes, Q.M.; Pandanaboyana, S.; Macutkiewicz, C.; Smart, N.; Smith, A.M. Prognostic significance of pre-operative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: A systematic review. HPB 2015, 17, 285–291. [Google Scholar] [CrossRef]
- Li, N.; Tian, G.W.; Wang, Y.; Zhang, H.; Wang, Z.H.; Li, G. Prognostic role of the pretreatment C-reactive protein/albumin ratio in solid cancers: A meta-analysis. Sci. Rep. 2017, 7, 41298. [Google Scholar] [CrossRef]
- Tominaga, T.; Nonaka, T.; Sumida, Y.; Hidaka, S.; Sawai, T.; Nagayasu, T. The C-reactive protein to albumin ratio as a predictor of severe side effects of adjuvant chemotherapy in Stage III colorectal cancer patients. PLoS ONE 2016, 11, e0167967. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, Y.; Gao, Z. Pretreatment C-reactive protein/albumin ratio for predicting overall survival in pancreatic cancer: A meta-analysis. Medicine 2020, 99, e20595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X. Controlling nutritional status score, a promising prognostic marker in patients with gastrointestinal cancers after surgery: A systematic review and meta-analysis. Int. J. Surg. 2018, 55, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Pawlicki, K.; Mrowiec, S. Associations between nutritional and immune status and clinicopathologic factors in patients with pancreatic cancer: A comprehensive analysis. Cancers 2021, 13, 5041. [Google Scholar] [CrossRef] [PubMed]
- Ziętarska, M.; Krawczyk-Lipiec, J.; Kraj, L.; Zaucha, R.; Małgorzewicz, S. Chemotherapy-related toxicity, nutritional status and quality of life in Precachectic oncologic patients with, or without, high protein nutritional support. A prospective, randomized study. Nutrients 2017, 9, 1108. [Google Scholar] [CrossRef]
- Cintoni, M.; Grassi, F.; Palombaro, M.; Rinninella, E.; Pulcini, G.; Di Donato, A.; Salvatore, L.; Quero, G.; Tortora, G.; Alfieri, S.; et al. Nutritional interventions during chemotherapy for pancreatic cancer: A systematic review of prospective studies. Nutrients 2023, 15, 727. [Google Scholar] [CrossRef]

| Patient Characteristics | S-1 Complete Group(n = 91) | S-1 Non-Complete Group (n = 38) | p-Value |
|---|---|---|---|
| Sex (male rate%) | 52 (57.1%) | 17 (44.7%) | 0.198 |
| Age (year) | 65.8 (43–81) | 68.7 (47–84) | 0.127 |
| Body mass index (kg/m2) | 22.9 ± 0.4 | 22.6 ± 0.7 | 0.736 |
| Neoadjuvant chemotherapy, n (%) | 48 (52.7%) | 21 (55.3%) | 0.794 |
| Operation methods | |||
| DP | 32 (35.2%) | 9 (23.7%) | |
| PD | 54 (59.3%) | 27 (71.1%) | |
| TP | 5 (5.5%) | 2 (5.3%) | |
| Operation time (min) | 484.8 ± 15.1 | 525.9 ± 22.8 | 0.14 |
| Estimated blood loss (mL) | 746.7 ± 50.7 | 883.6 ± 109.6 | 0.197 |
| CD classification over grade 3 | 23 (25.3%) | 13 (34.2%) | 0.302 |
| Postoperative hospital stays (days) | 39.1 ± 2.0 | 31.7 ± 3.4 | 0.499 |
| Pathological Stage | |||
| 1 | 12 (13.2%) | 2 (5.3%) | |
| 2 | 77 (84.6%) | 29 (76.3%) | |
| 3 | 2 (2.2%) | 7 (18.4%) |
| Variables | S-1 Complete Group (n = 91) | S-1 Non-Complete Group (n = 38) | p-Value |
|---|---|---|---|
| Duration to AC initiation (day) | 63.1 ± 3.8 | 67.4 ± 8.7 | 0.422 |
| Data at the initiation of AC | |||
| Body mass index (kg/m2) | 20.9 ± 0.4 | 20.4 ± 0.7 | 0.536 |
| Alb (mg/dL) | 3.5 ± 0.1 | 3.0 ± 0.1 | <0.001 |
| CRP (mg/dL) | 0.2 ± 0.1 | 1.0 ± 0.2 | <0.001 |
| CEA (ng/mL) | 2.9 ± 0.3 | 4.4 ± 1.0 | 0.062 |
| CA19-9 (U/mL) | 69.2 ± 18.4 | 403.3 ± 431.6 | 0.123 |
| CAR | 0.07 ± 0.02 | 0.25 ± 0.04 | <0.001 |
| Severe AEs | 6 (6.6%) | 19 (50.0%) | <0.001 |
| Multivariate Analysis for S-1 Non-Completion | ||
|---|---|---|
| Hazard Ratio (95% CI) | p-Value | |
| CEA > 2.4 | 1.64 (0.65–4.11) | 0.295 |
| Severe AEs | 7.20 (2.45–21.10) | <0.001 |
| CAR ≥ 0.05 | 5.18 (2.01–13.30) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funamizu, N.; Mori, S.; Sakamoto, A.; Iwata, M.; Shine, M.; Ito, C.; Uraoka, M.; Ueno, Y.; Tamura, K.; Umeda, Y.; et al. C-Reactive Protein-to-Albumin Ratio as a Predictive Indicator for Evaluating Tolerability in S-1 Adjuvant Chemotherapy after Curative Surgery for Pancreatic Cancer: An External Validation Cohort Study. Cancers 2024, 16, 3372. https://doi.org/10.3390/cancers16193372
Funamizu N, Mori S, Sakamoto A, Iwata M, Shine M, Ito C, Uraoka M, Ueno Y, Tamura K, Umeda Y, et al. C-Reactive Protein-to-Albumin Ratio as a Predictive Indicator for Evaluating Tolerability in S-1 Adjuvant Chemotherapy after Curative Surgery for Pancreatic Cancer: An External Validation Cohort Study. Cancers. 2024; 16(19):3372. https://doi.org/10.3390/cancers16193372
Chicago/Turabian StyleFunamizu, Naotake, Shozo Mori, Akimasa Sakamoto, Miku Iwata, Mikiya Shine, Chihiro Ito, Mio Uraoka, Yoshitomo Ueno, Kei Tamura, Yuzo Umeda, and et al. 2024. "C-Reactive Protein-to-Albumin Ratio as a Predictive Indicator for Evaluating Tolerability in S-1 Adjuvant Chemotherapy after Curative Surgery for Pancreatic Cancer: An External Validation Cohort Study" Cancers 16, no. 19: 3372. https://doi.org/10.3390/cancers16193372
APA StyleFunamizu, N., Mori, S., Sakamoto, A., Iwata, M., Shine, M., Ito, C., Uraoka, M., Ueno, Y., Tamura, K., Umeda, Y., Aoki, T., & Takada, Y. (2024). C-Reactive Protein-to-Albumin Ratio as a Predictive Indicator for Evaluating Tolerability in S-1 Adjuvant Chemotherapy after Curative Surgery for Pancreatic Cancer: An External Validation Cohort Study. Cancers, 16(19), 3372. https://doi.org/10.3390/cancers16193372







