Are Dual-Phase 18F-Fluorodeoxyglucose PET-mpMRI Diagnostic Performances to Distinguish Brain Tumour Radionecrosis/Recurrence after Cranial Radiotherapy Usable in Routine?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. 18F-FDG PET-mpMRI Protocol
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of Brain Metastases and Leptomeningeal Disease. Neuro-Oncology 2021, 23, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Bodensohn, R.; Kaempfel, A.-L.; Boulesteix, A.-L.; Orzelek, A.M.; Corradini, S.; Fleischmann, D.F.; Forbrig, R.; Garny, S.; Hadi, I.; Hofmaier, J.; et al. Stereotactic Radiosurgery versus Whole-Brain Radiotherapy in Patients with 4–10 Brain Metastases: A Nonrandomized Controlled Trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2023, 186, 109744. [Google Scholar] [CrossRef]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Tonse, R.; Tom, M.C.; Mehta, M.P.; Ahluwalia, M.S.; Kotecha, R. Integration of Systemic Therapy and Stereotactic Radiosurgery for Brain Metastases. Cancers 2021, 13, 3682. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 Cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-Analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef]
- Kohutek, Z.A.; Yamada, Y.; Chan, T.A.; Brennan, C.W.; Tabar, V.; Gutin, P.H.; Yang, T.J.; Rosenblum, M.K.; Ballangrud, Å.; Young, R.J.; et al. Long-Term Risk of Radionecrosis and Imaging Changes after Stereotactic Radiosurgery for Brain Metastases. J. Neurooncol. 2015, 125, 149–156. [Google Scholar] [CrossRef]
- Leyrat, B.; Khalill, T.; Lemaire, J.-J.; Casile, M.; Molnar, I.; Dedieu, V.; Chassin, V.; Dupic, G.; Bellière, A.; Durando, X.; et al. Local Control and Radionecrosis of Brain Metastases from Non-Small-Cell Lung Cancer Treated by Hypofractionated Stereotactic Radiotherapy: Evaluation of Predictive Factors. Clin. Transl. Radiat. Oncol. 2022, 36, 1–8. [Google Scholar] [CrossRef]
- Walker, A.J.; Ruzevick, J.; Malayeri, A.A.; Rigamonti, D.; Lim, M.; Redmond, K.J.; Kleinberg, L. Postradiation Imaging Changes in the CNS: How Can We Differentiate between Treatment Effect and Disease Progression? Future Oncol. 2014, 10, 1277–1297. [Google Scholar] [CrossRef]
- Stockham, A.L.; Tievsky, A.L.; Koyfman, S.A.; Reddy, C.A.; Suh, J.H.; Vogelbaum, M.A.; Barnett, G.H.; Chao, S.T. Conventional MRI Does Not Reliably Distinguish Radiation Necrosis from Tumor Recurrence after Stereotactic Radiosurgery. J. Neurooncol. 2012, 109, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gramling, R.; Stanek, S.; Han, P.K.J.; Duberstein, P.; Quill, T.E.; Temel, J.S.; Alexander, S.C.; Anderson, W.G.; Ladwig, S.; Norton, S.A. Distress Due to Prognostic Uncertainty in Palliative Care: Frequency, Distribution, and Outcomes among Hospitalized Patients with Advanced Cancer. J. Palliat. Med. 2018, 21, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, W.H.T.; Govaerts, C.W.; Kramer, M.C.A.; Labrecque, J.A.; Smits, M.; Dirven, L.; van der Hoorn, A. Diagnostic Accuracy of MRI Techniques for Treatment Response Evaluation in Patients with Brain Metastasis: A Systematic Review and Meta-Analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2022, 177, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, K.; Nakasu, Y.; Horiguchi, S.; Harada, H.; Nishimura, T.; Bando, E.; Okawa, H.; Furukawa, Y.; Hirai, T.; Endo, M. Perfusion Weighted Magnetic Resonance Imaging to Distinguish the Recurrence of Metastatic Brain Tumors from Radiation Necrosis after Stereotactic Radiosurgery. J. Neurooncol. 2010, 99, 81–88. [Google Scholar] [CrossRef]
- Muto, M.; Frauenfelder, G.; Senese, R.; Zeccolini, F.; Schena, E.; Giurazza, F.; Jäger, H.R. Dynamic Susceptibility Contrast (DSC) Perfusion MRI in Differential Diagnosis between Radionecrosis and Neoangiogenesis in Cerebral Metastases Using rCBV, rCBF and K2. Radiol. Med. 2018, 123, 545–552. [Google Scholar] [CrossRef]
- Barajas, R.F.; Chang, J.S.; Sneed, P.K.; Segal, M.R.; McDermott, M.W.; Cha, S. Distinguishing Recurrent Intra-Axial Metastatic Tumor from Radiation Necrosis Following Gamma Knife Radiosurgery Using Dynamic Susceptibility-Weighted Contrast-Enhanced Perfusion MR Imaging. AJNR Am. J. Neuroradiol. 2009, 30, 367–372. [Google Scholar] [CrossRef]
- Chao, S.T.; Suh, J.H.; Raja, S.; Lee, S.Y.; Barnett, G. The Sensitivity and Specificity of FDG PET in Distinguishing Recurrent Brain Tumor from Radionecrosis in Patients Treated with Stereotactic Radiosurgery. Int. J. Cancer 2001, 96, 191–197. [Google Scholar] [CrossRef]
- Horky, L.L.; Hsiao, E.M.; Weiss, S.E.; Drappatz, J.; Gerbaudo, V.H. Dual Phase FDG-PET Imaging of Brain Metastases Provides Superior Assessment of Recurrence versus Post-Treatment Necrosis. J. Neurooncol. 2011, 103, 137–146. [Google Scholar] [CrossRef]
- Pyatigorskaya, N.; Sgard, B.; Bertaux, M.; Yahia-Cherif, L.; Kas, A. Can FDG-PET/MR Help to Overcome Limitations of Sequential MRI and PET-FDG for Differential Diagnosis between Recurrence/Progression and Radionecrosis of High-Grade Gliomas? J. Neuroradiol. J. Neuroradiol. 2021, 48, 189–194. [Google Scholar] [CrossRef]
- Otman, H.; Farce, J.; Meneret, P.; Palard-Novello, X.; Le Reste, P.-J.; Lecouillard, I.; Vauleon, E.; Chanchou, M.; Carsin Nicol, B.; Bertaux, M.; et al. Delayed [ 18 F]-FDG PET Imaging Increases Diagnostic Performance and Reproducibility to Differentiate Recurrence of Brain Metastases From Radionecrosis. Clin. Nucl. Med. 2022, 47, 800–806. [Google Scholar] [CrossRef]
- Matuszak, J.; Waissi, W.; Clavier, J.B.; Noël, G.; Namer, I.J. Métastases cérébrales: Apport de l’acquisition tardive en TEP/TDM au 18F-FDG pour le diagnostic différentiel entre récurrence tumorale et radionécrose. Médecine Nucl. 2016, 40, 196. [Google Scholar] [CrossRef]
- Li, H.; Deng, L.; Bai, H.X.; Sun, J.; Cao, Y.; Tao, Y.; States, L.J.; Farwell, M.D.; Zhang, P.; Xiao, B.; et al. Diagnostic Accuracy of Amino Acid and FDG-PET in Differentiating Brain Metastasis Recurrence from Radionecrosis after Radiotherapy: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2018, 39, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Maurizi Enrici, R.; Scopinaro, F. Accuracy of F-DOPA PET and Perfusion-MRI for Differentiating Radionecrotic from Progressive Brain Metastases after Radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 103–111. [Google Scholar] [CrossRef]
- Le Rhun, E.; Guckenberger, M.; Smits, M.; Dummer, R.; Bachelot, T.; Sahm, F.; Galldiks, N.; de Azambuja, E.; Berghoff, A.S.; Metellus, P.; et al. EANO-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up of Patients with Brain Metastasis from Solid Tumours. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Kocher, M.; Ceccon, G.; Werner, J.-M.; Brunn, A.; Deckert, M.; Pope, W.B.; Soffietti, R.; Le Rhun, E.; Weller, M.; et al. Imaging Challenges of Immunotherapy and Targeted Therapy in Patients with Brain Metastases: Response, Progression, and Pseudoprogression. Neuro-Oncology 2020, 22, 17–30. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET Imaging in Patients with Brain Metastasis-Report of the RANO/PET Group. Neuro-Oncology 2019, 21, 585–595. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, X.-W.; Deng, L.-J.; She, H.-L. Differentiation between Glioma Recurrence and Treatment Effects Using Amide Proton Transfer Imaging: A Mini-Bayesian Bivariate Meta-Analysis. Front. Oncol. 2022, 12, 852076. [Google Scholar] [CrossRef]
- Ma, B.; Blakeley, J.O.; Hong, X.; Zhang, H.; Jiang, S.; Blair, L.; Zhang, Y.; Heo, H.-Y.; Zhang, M.; van Zijl, P.C.M.; et al. Applying Amide Proton Transfer-Weighted MRI to Distinguish Pseudoprogression from True Progression in Malignant Gliomas. J. Magn. Reson. Imaging JMRI 2016, 44, 456–462. [Google Scholar] [CrossRef]
- Lindner, T.; Bolar, D.S.; Achten, E.; Barkhof, F.; Bastos-Leite, A.J.; Detre, J.A.; Golay, X.; Günther, M.; Wang, D.J.J.; Haller, S.; et al. Current State and Guidance on Arterial Spin Labeling Perfusion MRI in Clinical Neuroimaging. Magn. Reson. Med. 2023, 89, 2024–2047. [Google Scholar] [CrossRef]
- Nichelli, L.; Casagranda, S. Current Emerging MRI Tools for Radionecrosis and Pseudoprogression Diagnosis. Curr. Opin. Oncol. 2021, 33, 597–607. [Google Scholar] [CrossRef]
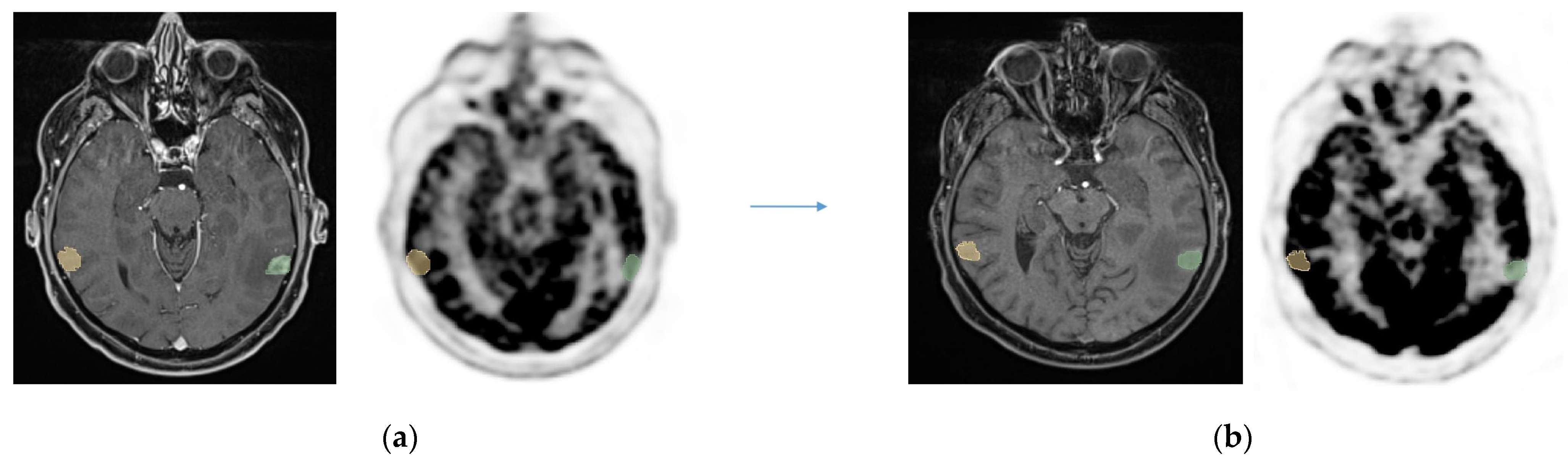
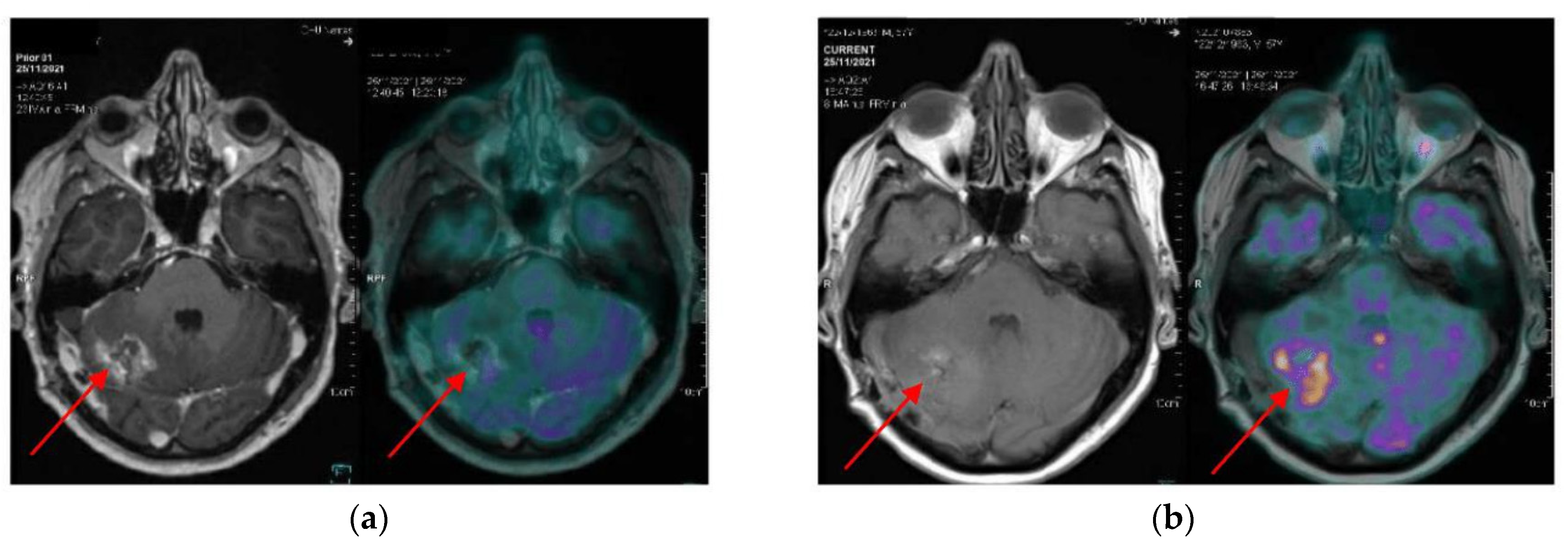
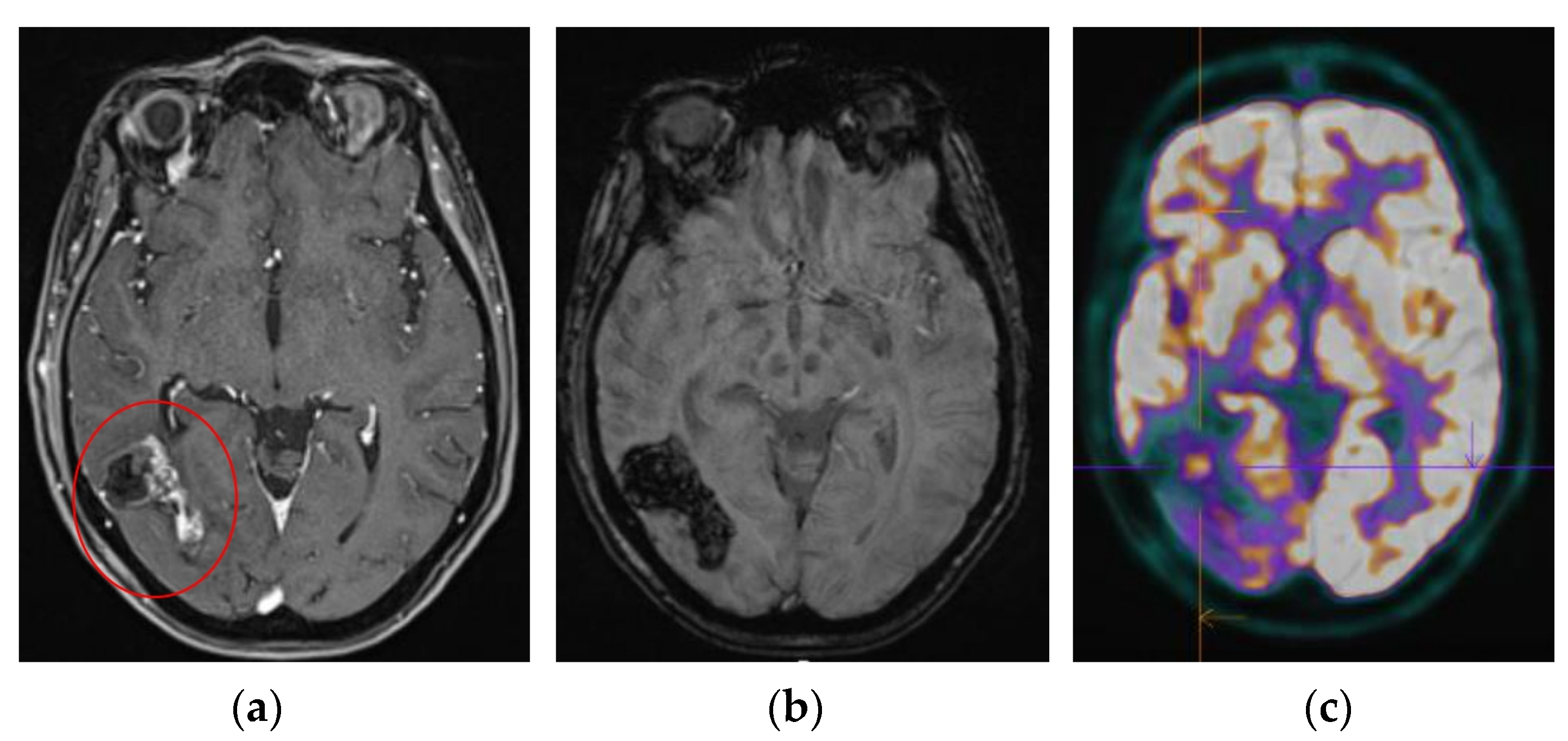
| N = 23 | |
|---|---|
| Sex | |
| Male | 10 |
| Female | 13 |
| Median age (years, range) | 60.5 (29–74) |
| Pathological analyses | N = 24 |
| Glioblastoma | 9 |
| Oligodendroglioma | 1 |
| Lung adenocarcinoma | 8 |
| Small cell lung cancer | 2 |
| Breast cancer | 1 |
| Melanoma | 3 |
| Treatment | N = 24 |
| Post-surgery | 10 |
| RT alone | 14 |
| Lesions’ location | N = 24 |
| Sus-tentorial | 19 |
| Sub-tentorial | 3 |
| Meningeal contact | 10 |
| Concomitant therapy, n (%) | N = 14 (60.9) |
| Immunotherapy | 7 |
| Alectinib | 1 |
| Temozolomide | 6 |
| Re-irradiation, n (%) | N = 8 (34.8) |
| Whole brain | 2 |
| IMRT (partial brain) | 3 |
| Stereotactic RT | 3 |
| Patient | Histology | Diagnosis Evocated at Initial mpMRI | 18F-FDG PET-mpMRI | Gold Standard | ||
|---|---|---|---|---|---|---|
| Qualitative | nIR Contralateral | nIR Frontal | ||||
| 1 | Glioblastoma | Relapse | RN | RN | RN | RN |
| 2 | NSCL mets | RN | RN | RN | RN | RN |
| 3 | Glioblastoma | Equivocal | RN | RN | RN | Relapse |
| 4 | Oligodendroglioma | Equivocal | Relapse | Relapse | Relapse | Relapse |
| 5 | NSCL mets | Equivocal | Relapse | Relapse | Relapse | Relapse |
| 6 | Glioblastoma | RN | Relapse | Relapse | RN | RN |
| 7 | NSCL mets | Equivocal | Relapse | Relapse | Relapse | Relapse |
| 8 | Melanoma mets | RN | RN | RN | RN | RN |
| 9 | Melanoma mets | Equivocal | RN | RN | RN | RN |
| 10 | Breast mets | Equivocal | RN | RN | Relapse | RN |
| 11 | Glioblastoma | Equivocal | RN | RN | RN | RN |
| 12 | Glioblastoma | RN | Relapse | RN | Relapse | Relapse |
| 13 | NSCL mets | RN | RN | RN | RN | RN |
| 14 | NSCL mets | Equivocal | RN | RN | RN | RN |
| 15 | Glioblastoma | RN | RN | RN | RN | Relapse |
| 16 | Glioblastoma | Equivocal | RN | RN | RN | RN |
| 17 | Glioblastoma | Relapse | Relapse | RN | RN | Relapse |
| 18 | Melanoma mets | Equivocal | Relapse | Relapse | Relapse | Relapse |
| 19 | SCLC mets | Equivocal | RN | RN | RN | RN |
| 20 | NSCL mets | Relapse | Relapse | RN | RN | RN |
| 21 | NSCL mets | Relapse | RN | RN | RN | RN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cailleteau, A.; Ferrer, L.; Geffroy, D.; Fleury, V.; Lalire, P.; Doré, M.; Rousseau, C. Are Dual-Phase 18F-Fluorodeoxyglucose PET-mpMRI Diagnostic Performances to Distinguish Brain Tumour Radionecrosis/Recurrence after Cranial Radiotherapy Usable in Routine? Cancers 2024, 16, 3216. https://doi.org/10.3390/cancers16183216
Cailleteau A, Ferrer L, Geffroy D, Fleury V, Lalire P, Doré M, Rousseau C. Are Dual-Phase 18F-Fluorodeoxyglucose PET-mpMRI Diagnostic Performances to Distinguish Brain Tumour Radionecrosis/Recurrence after Cranial Radiotherapy Usable in Routine? Cancers. 2024; 16(18):3216. https://doi.org/10.3390/cancers16183216
Chicago/Turabian StyleCailleteau, Axel, Ludovic Ferrer, Delphine Geffroy, Vincent Fleury, Paul Lalire, Mélanie Doré, and Caroline Rousseau. 2024. "Are Dual-Phase 18F-Fluorodeoxyglucose PET-mpMRI Diagnostic Performances to Distinguish Brain Tumour Radionecrosis/Recurrence after Cranial Radiotherapy Usable in Routine?" Cancers 16, no. 18: 3216. https://doi.org/10.3390/cancers16183216
APA StyleCailleteau, A., Ferrer, L., Geffroy, D., Fleury, V., Lalire, P., Doré, M., & Rousseau, C. (2024). Are Dual-Phase 18F-Fluorodeoxyglucose PET-mpMRI Diagnostic Performances to Distinguish Brain Tumour Radionecrosis/Recurrence after Cranial Radiotherapy Usable in Routine? Cancers, 16(18), 3216. https://doi.org/10.3390/cancers16183216





