Response Rates and Transplantation Impact in Patients with Relapsed Acute Promyelocytic Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
3.2. Patient Characteristics and Distribution by Salvage Therapy and Transplant Consolidation
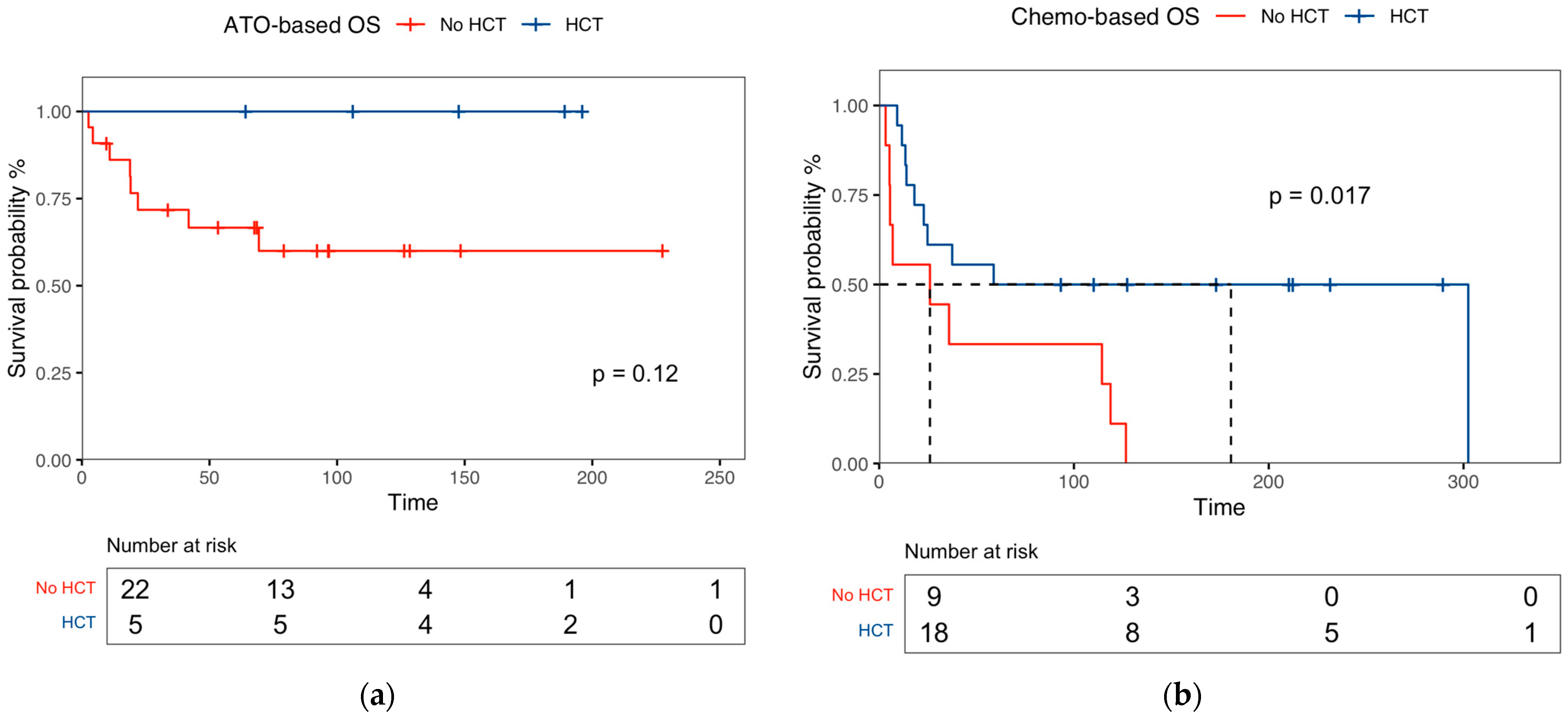
3.3. Response Rates of Salvage Therapies, Transplant Outcomes, and Predictive Factors for Survival
3.4. Second Relapse Rates and Predictive Factors for Relapse
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo-Coco, F.; Hasan, S.K. Understanding the molecular pathogenesis of acute promyelocytic leukemia. Best Pract. Res. Clin. Haematol. 2014, 27, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lo-Coco, F.; Ammatuna, E. The Biology of Acute Promyelocytic Leukemia and Its Impact on Diagnosis and Treatment. Hematology 2006, 2006, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Breccia, M.; Gallo, E.; Rambaldi, A.; Paoloni, F.; Fioritoni, G.; Ferrara, F.; Specchia, G.; et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: Results of the AIDA-2000 trial of the GIMEMA Group. Blood 2010, 116, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Bowen, D.; Kell, J.; Knapper, S.; Morgan, Y.G.; Lok, J.; Grech, A.; Jones, G.; et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): Results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef]
- Sanz, M.A.; Montesinos, P.; Rayón, C.; Holowiecka, A.; de la Serna, J.; Milone, G.; de Lisa, E.; Brunet, S.; Rubio, V.; Ribera, J.M.; et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: Further improvements in treatment outcome. Blood 2010, 115, 5137–5146. [Google Scholar] [CrossRef]
- Iland, H.J.; Bradstock, K.; Supple, S.G.; Catalano, A.; Collins, M.; Hertzberg, M.; Browett, P.; Grigg, A.; Firkin, F.; Hugman, A.; et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 2012, 120, 1570–1580. [Google Scholar] [CrossRef]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.J.; et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef]
- Raffoux, E.; Rousselot, P.; Poupon, J.; Daniel, M.T.; Cassinat, B.; Delarue, R.; Taksin, A.L.; Réa, D.; Buzyn, A.; Tibi, A.; et al. Combined Treatment with Arsenic Trioxide and All-Trans-Retinoic Acid in Patients with Relapsed Acute Promyelocytic Leukemia. J. Clin. Oncol. 2016, 21, 2326–2334. [Google Scholar] [CrossRef]
- Cicconi, L.; Breccia, M.; Franceschini, L.; Latagliata, R.; Molica, M.; Divona, M.; Diverio, D.; Rizzo, M.; Ottone, T.; Iaccarino, L.; et al. Prolonged treatment with arsenic trioxide (ATO) and all-trans-retinoic acid (ATRA) for relapsed acute promyelocytic leukemia previously treated with ATRA and chemotherapy. Ann. Hematol. 2018, 97, 1797–1802. [Google Scholar] [CrossRef]
- Russell, N.; Burnett, A.; Hills, R.; Betteridge, S.; Dennis, M.; Jovanovic, J.; Dillon, R.; Grimwade, D.; NCRI AML Working Group. Attenuated arsenic trioxide plus ATRA therapy for newly diagnosed and relapsed APL: Long-term follow-up of the AML17 trial. Blood 2018, 132, 1452–1454. [Google Scholar] [CrossRef]
- Fouzia, N.A.; Sharma, V.; Ganesan, S.; Palani, H.K.; Balasundaram, N.; David, S.; Kulkarni, U.P.; Korula, A.; Devasia, A.J.; Nair, S.C.; et al. Management of relapse in acute promyelocytic leukaemia treated with up-front arsenic trioxide-based regimens. Br. J. Haematol. 2021, 192, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Yanada, M.; Yano, S.; Kanamori, H.; Gotoh, M.; Emi, N.; Watakabe, K.; Kurokawa, M.; Nishikawa, A.; Mori, T.; Tomita, N.; et al. Autologous hematopoietic cell transplantation for acute promyelocytic leukemia in second complete remission: Outcomes before and after the introduction of arsenic trioxide. Leuk. Lymphoma 2017, 58, 1061–1067. [Google Scholar] [CrossRef]
- Sasaki, K.; Ravandi, F.; Kadia, T.; DiNardo, C.D.; Yilmaz, M.; Short, N.; Jabbour, E.; Patel, K.P.; Loghavi, S.; Pierce, S.; et al. Outcome of Patients with Relapsed Acute Promyelocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2024, 24, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Avvisati, G.; Lo-Coco, F.; Paoloni, F.P.; Petti, M.C.; Diverio, D.; Vignetti, M.; Latagliata, R.; Specchia, G.; Baccarani, M.; Di Bona, E.; et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: Very long-term results and role of maintenance. Blood 2011, 117, 4716–4725. [Google Scholar] [CrossRef] [PubMed]
- Douer, D.; Zickl, L.N.; Schiffer, C.A.; Appelbaum, F.R.; Feusner, J.H.; Shepherd, L.; Willman, C.L.; Bloomfield, C.D.; Paietta, E.; Gallagher, R.E.; et al. Late Relapses Following All-Trans Retinoic Acid for Acute Promyelocytic Leukemia Are Uncommon, Respond Well to Salvage Therapy and Occur Independently of Prognostic Factors At Diagnosis: Long-Term Follow-up of North American Intergroup Study I0129. Blood 2011, 118, 83. [Google Scholar] [CrossRef]
- Yanada, M.; Tsuzuki, M.; Fujita, H.; Fujimaki, K.; Fujisawa, S.; Sunami, K.; Taniwaki, M.; Ohwada, A.; Tsuboi, K.; Maeda, A.; et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood 2013, 121, 3095–3102. [Google Scholar] [CrossRef]
- Shigeno, K.; Naito, K.; Sahara, N.; Kobayashi, M.; Nakamura, S.; Fujisawa, S.; Shinjo, K.; Takeshita, A.; Ohno, R.; Ohnishi, K. Arsenic trioxide therapy in relapsed or refractory Japanese patients with acute promyelocytic leukemia: Updated outcomes of the phase II study and postremission therapies. Int. J. Hematol. 2005, 82, 224–229. [Google Scholar] [CrossRef]
- Sanz, J.; Labopin, M.; Sanz, M.A.; Aljurf, M.; Sousa, A.B.; Craddock, C.; Zuckerman, T.; Labussière-Wallet, H.; Campos, A.; Grillo, G.; et al. Hematopoietic stem cell transplantation for adults with relapsed acute promyelocytic leukemia in second complete remission. Bone Marrow Transplant. 2020, 56, 1272–1280. [Google Scholar] [CrossRef]
- Ganzel, C.; Mathews, V.; Alimoghaddam, K.; Ghavamzadeh, A.; Kuk, D.; Devlin, S.; Wang, H.; Zhang, M.J.; Weisdorf, D.; Douer, D.; et al. Autologous transplant remains the preferred therapy for relapsed APL in CR2. Bone Marrow Transplant. 2016, 51, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Mohty, R.; Reljic, T.; Yassine, F.; Kettaneh, C.; Al-Husni, D.; Keller, K.; Badar, T.; Murthy, H.; Foran, J.; Kumar, A.; et al. Efficacy of Autologous and Allogeneic Hematopoietic Cell Transplantation in Adults with Acute Promyelocytic Leukemia: Results of a Systematic Review and Meta-Analysis. Transplant. Cell. Ther. 2024, 30, 599.e1–599.e10. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Grimwade, D.; Tallman, M.S.; Lowenberg, B.; Fenaux, P.; Estey, E.H.; Naoe, T.; Lengfelder, E.; Büchner, T.; Döhner, H.; et al. Management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2009, 113, 1875–1891. [Google Scholar] [CrossRef] [PubMed]


| Recommendations | Class/Level |
|---|---|
| For patients with confirmed molecular relapse (in independent labs in two consecutive positive PCR), immediate preemptive therapy is crucial to prevent overt relapse. | IIa/B |
| Salvage therapy for molecular persistence, molecular relapse, or hematologic relapse should be based on prior first-line treatment and the timing of the first relapse. | IV/C |
| Patients who relapse after ATRA + CHT should be managed with ATRA + ATO; patients who relapse after ATRA + ATO should be managed with ATRA + CHT.† | IV/C |
| Patients achieving CR2 should ideally undergo intensification with either HCT or CHT, if feasible. | IV/C |
| Allogeneic HCT is recommended for patients who do not achieve a second molecular remission. | IV/C |
| Autologous HCT is the preferred option for patients with no detectable MRD in the marrow and an adequate PCR harvest. | IIa/B |
| For patients ineligible for HCT, options include repeated ATO cycles with or without ATRA and/or CHT. | IV/C |
| For CNS relapse, induction involves weekly triple ITT with MTX, hydrocortisone, and Ara-C until cerebrospinal fluid blast clearance, followed by 6–10 consolidation ITT treatments. Systemic treatment should adhere to the previously mentioned recommendations. | IV/C |
| Variables | |
|---|---|
| At baseline | |
| Males, n (%) | 36 (53.7%) |
| Median age at diagnosis, years (IQR) | 45.8 (32.2–58.7) |
| Sanz risk score, n (%) | |
| Standard risk | 45 (67.2) |
| High risk | 22 (32.8) |
| Morphology, hypergranular/microgranular; n (%) | 50/17 (74.6/25.4) |
| Leukocyte count at diagnosis, ×109/L (IQR) | 2.6 (1.3–15.7) |
| Platelet count at diagnosis, ×109/L (IQR) | 27.5 (16.7–49.0) |
| First-line regimen, n (%) | |
| AIDA0493 | 23 (34.3) |
| AIDA2000 | 38 (56.7) |
| APL0406 | 3 (4.5) |
| AIDA + GO | 1 (1.5) |
| IDA + Ara-C | 1 (1.5) |
| DNR | 1 (1.5) |
| Maintenance therapy, n (%) | 50 (74.6) |
| At relapse | |
| Median age at relapse, years (IQR) | 48.2 (34.9–71.3) |
| Relapsed during maintenance therapy, n (%) | 29 (58.0) |
| Type of relapse, n (%) | |
| Molecular relapse | 25 (37.3) |
| Morphological relapse | 42 (62.7) |
| Extramedullary relapse, n (%) | 11 (16.4) |
| Site of extramedullary relapse | |
| Skin | 1 (9.1) |
| Central nervous system | 9 (81.8) |
| Paravertebral mass | 1 (9.1) |
| Time to relapse from diagnosis, median months (IQR) | 17.3 (12.2–35.3) |
| Molecular relapse | 20.9 (12.9–39.9) |
| Morphological relapse | 31.4 (15.5–48.8) |
| Extramedullary relapse | 27.6 (15.0–50.3) |
| Variables | ATO ± ATRA | Chemo-Based | ATRA ± GO | p |
|---|---|---|---|---|
| Total cohort, n (%) | 27 (40.3) | 27 (40.3) | 7 (10.4) | |
| Median age at relapse, years (IQR) | 50.1 (33.0–60.6) | 41.7 (33.0–52.3) | 65.9 (54.2–74.9) | 0.013 * |
| Female gender, n (%) | 11 (40.7) | 14 (51.8) | 2 (28.5) | 0.481 |
| Morphology, hypergranular/microgranular; n (%) | 20/7 (74.0/26.0) | 21/6 (77.7/22.3) | 4/3 (57.1/42.9) | 0.541 |
| Year of relapse, range | 2000–2023 | 1984–2007 | 1996–2018 | <0.001 * |
| Sanz risk score at diagnosis, n (%) | 0.010 * | |||
| Standard risk | 17 (63.0) | 16 (59.3) | 7 (100) | |
| High risk | 10 (37.0) | 11 (40.7) | 0 (0) | |
| First-line regimen, n (%) | <0.001 * | |||
| AIDA0493 | 1 (3.7) | 17 (62.9) | 2 (28.6) | |
| AIDA2000 | 25 (92.6) | 8 (29.7) | 3 (42.8) | |
| APL0406 | 1 (3.7) | 0 | 1 (14.3) | |
| AIDA + GO | 0 | 1 (3.7) | 0 | |
| IDA + Ara-C | 0 | 1 (3.7) | 0 | |
| DNR | 0 | 0 | 1 (14.3) | |
| Response to salvage therapy, n (%) | 0.112 | |||
| Morphological CR2 | 6 (27.2) | 8 (29.7) | 1 (14.3) | |
| Molecular CR2 | 16 (72.7) | 19 (70.3) | 5 (71.4) | |
| No response | 0 | 0 | 1 (14.3) | |
| Consolidation at CR2, n (%) | ||||
| Auto-HCT, n (%) | 2 (7.4) | 14 (51.8) | 1 (14.3) | 0.565 |
| Allo-HCT, n (%) | 3 (11.1) | 4 (14.8) | 2 (28.6) | |
| Second relapse, n (%) | 9 (33.3) | 13 (48.1) | 4 (57.1) | 0.539 |
| Median time from diagnosis of relapse to 2nd relapse, months (IQR) | 11.6 (5.3–22.4) | 9.09 (6.9–21.3) | 13.5 (3.6–29.0) | 0.236 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Female gender | 0.685 | 0.3–1.2 | 0.273 | 1.230 | 0.9–1.0 | 0.270 |
| Age at relapse | 1.020 | 1.0–1.041 | 0.054 | 0.430 | 0.1–1.2 | 0.453 |
| Sanz risk score at diagnosis | 0.374 | 0.6–1.4 | 0.393 | 0.73 | 0.3–1.5 | 0.390 |
| Time of relapse from diagnosis | 0.968 | 0.94–0.99 | 0.016 * | 0.992 | 0.9–1.0 | 0.623 |
| Relapse during maintenance | 1.959 | 1.0–3.8 | 0.045 * | 2.012 | 1.1–3.8 | 0.049 * |
| Chemotherapy | 0.674 | 0.8–3.4 | 0.169 | 1.553 | 0.7–2.9 | 0.250 |
| ATO-therapy | 0.397 | 0.1–0.8 | 0.025 | 0.423 | 0.1–0.89 | 0.026 * |
| HCT consolidation | 0.516 | 0.2–1.1 | 0.087 | 0.52 | 0.24–1.1 | 0.087 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Female gender | 1.183 | 0.5–2.5 | 0.669 | 1.348 | 0.5–3.2 | 0.509 |
| Age at relapse | 0.993 | 0.9–1.0 | 0.538 | 0.999 | 0.9–1.03 | 0.946 |
| ATO-therapy | 0.637 | 0.2–1.4 | 0.274 | 0.852 | 0.3–2.3 | 0.759 |
| HCT consolidation | 1.325 | 0.6–2.9 | 0.481 | 1.207 | 0.3–3.8 | 0.752 |
| Molecular CR | 0.355 | 0.1–0.9 | 0.035 * | 0.338 | 0.1–0.9 | 0.036 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Gurnari, C.; Scalzulli, E.; Cicconi, L.; Guarnera, L.; Carmosino, I.; Cerretti, R.; Bisegna, M.L.; Capria, S.; Minotti, C.; et al. Response Rates and Transplantation Impact in Patients with Relapsed Acute Promyelocytic Leukemia. Cancers 2024, 16, 3214. https://doi.org/10.3390/cancers16183214
Costa A, Gurnari C, Scalzulli E, Cicconi L, Guarnera L, Carmosino I, Cerretti R, Bisegna ML, Capria S, Minotti C, et al. Response Rates and Transplantation Impact in Patients with Relapsed Acute Promyelocytic Leukemia. Cancers. 2024; 16(18):3214. https://doi.org/10.3390/cancers16183214
Chicago/Turabian StyleCosta, Alessandro, Carmelo Gurnari, Emilia Scalzulli, Laura Cicconi, Luca Guarnera, Ida Carmosino, Raffaella Cerretti, Maria Laura Bisegna, Saveria Capria, Clara Minotti, and et al. 2024. "Response Rates and Transplantation Impact in Patients with Relapsed Acute Promyelocytic Leukemia" Cancers 16, no. 18: 3214. https://doi.org/10.3390/cancers16183214
APA StyleCosta, A., Gurnari, C., Scalzulli, E., Cicconi, L., Guarnera, L., Carmosino, I., Cerretti, R., Bisegna, M. L., Capria, S., Minotti, C., Iori, A. P., Torrieri, L., Venditti, A., Pulsoni, A., Martelli, M., Voso, M. T., & Breccia, M. (2024). Response Rates and Transplantation Impact in Patients with Relapsed Acute Promyelocytic Leukemia. Cancers, 16(18), 3214. https://doi.org/10.3390/cancers16183214









