Prioritizing Radiation and Targeted Systemic Therapies in Patients with Resected Brain Metastases from Lung Cancer Primaries with Targetable Mutations: A Report from a Multi-Site Single Institution
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
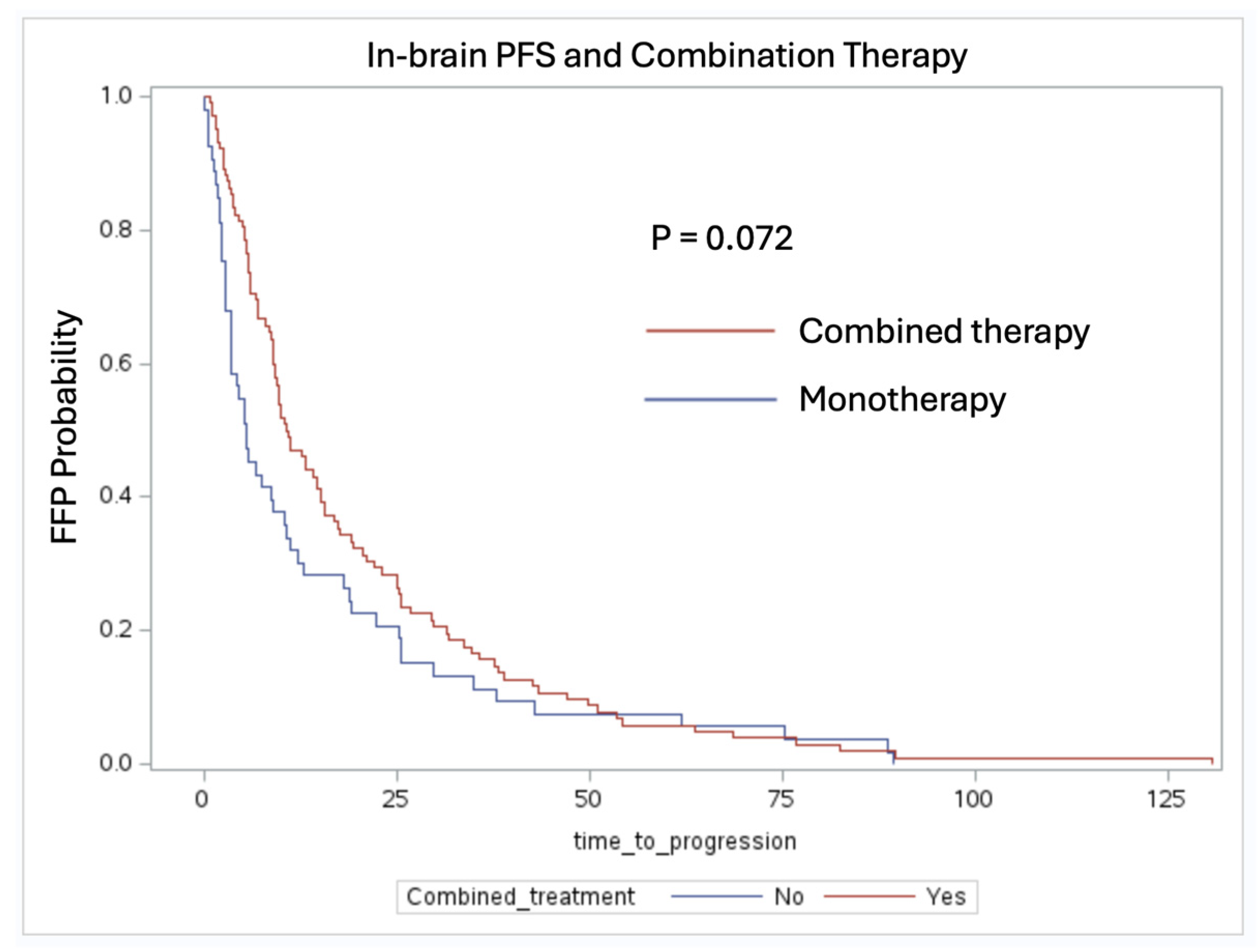
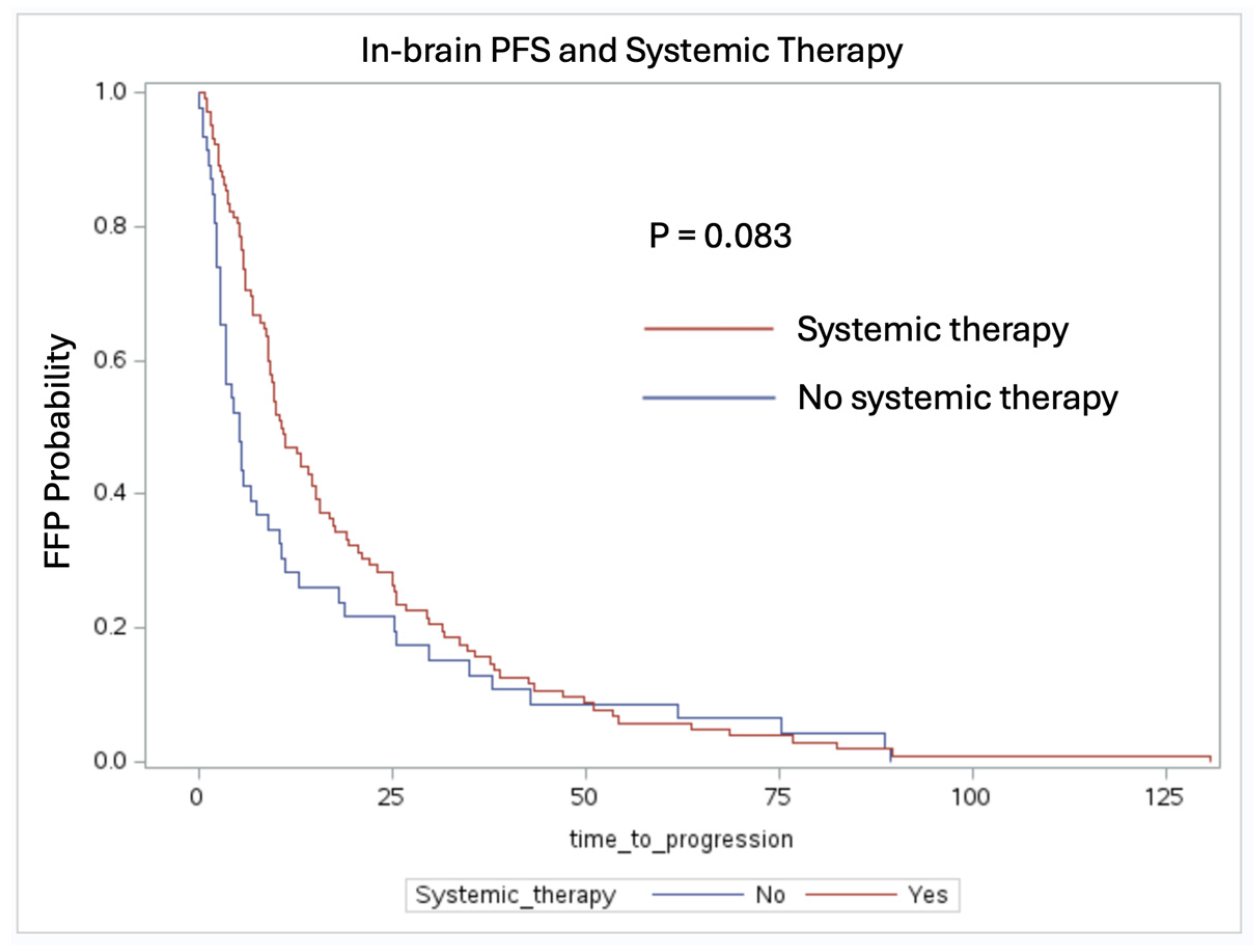
3.2. In-Brain Freedom from Progression Analysis
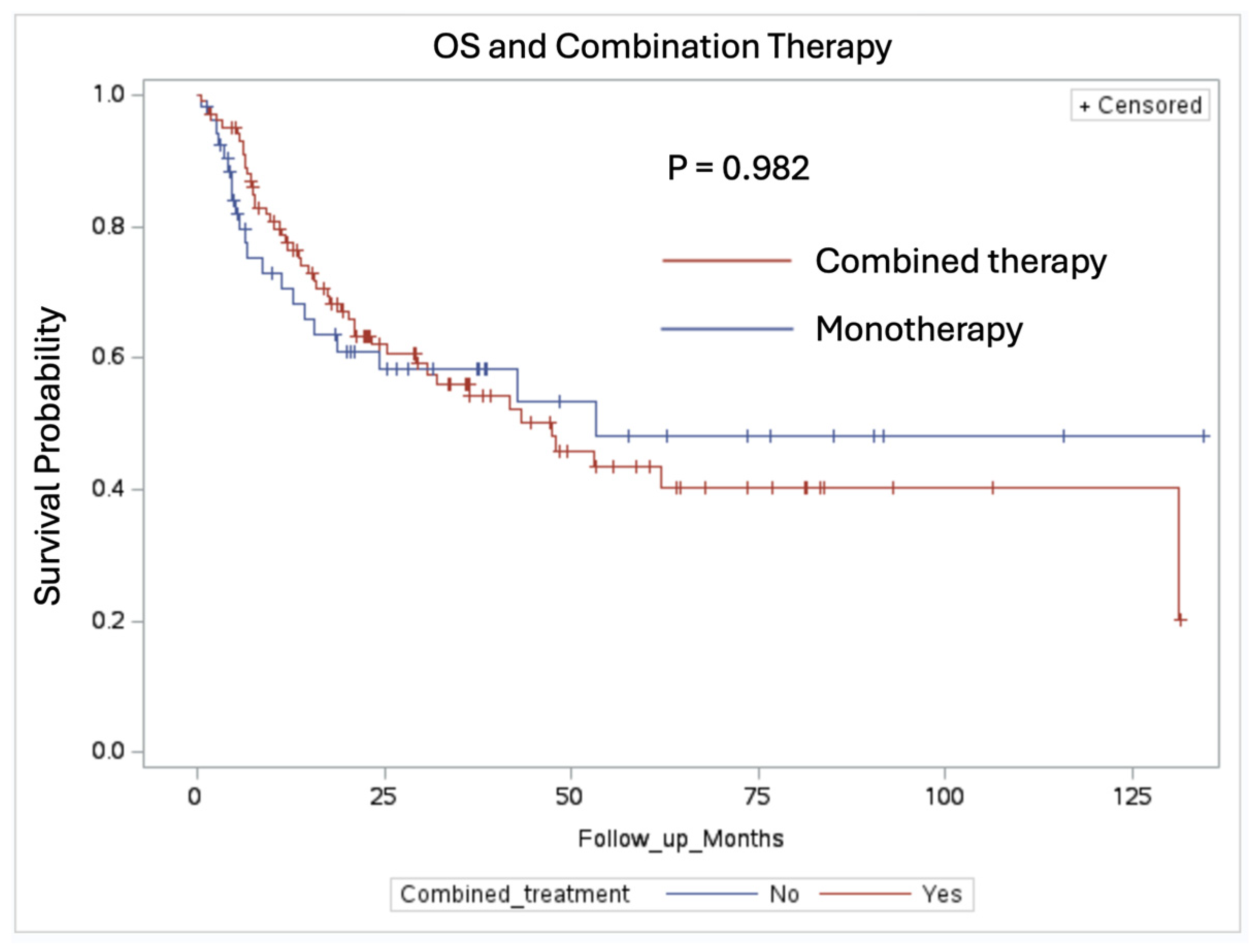
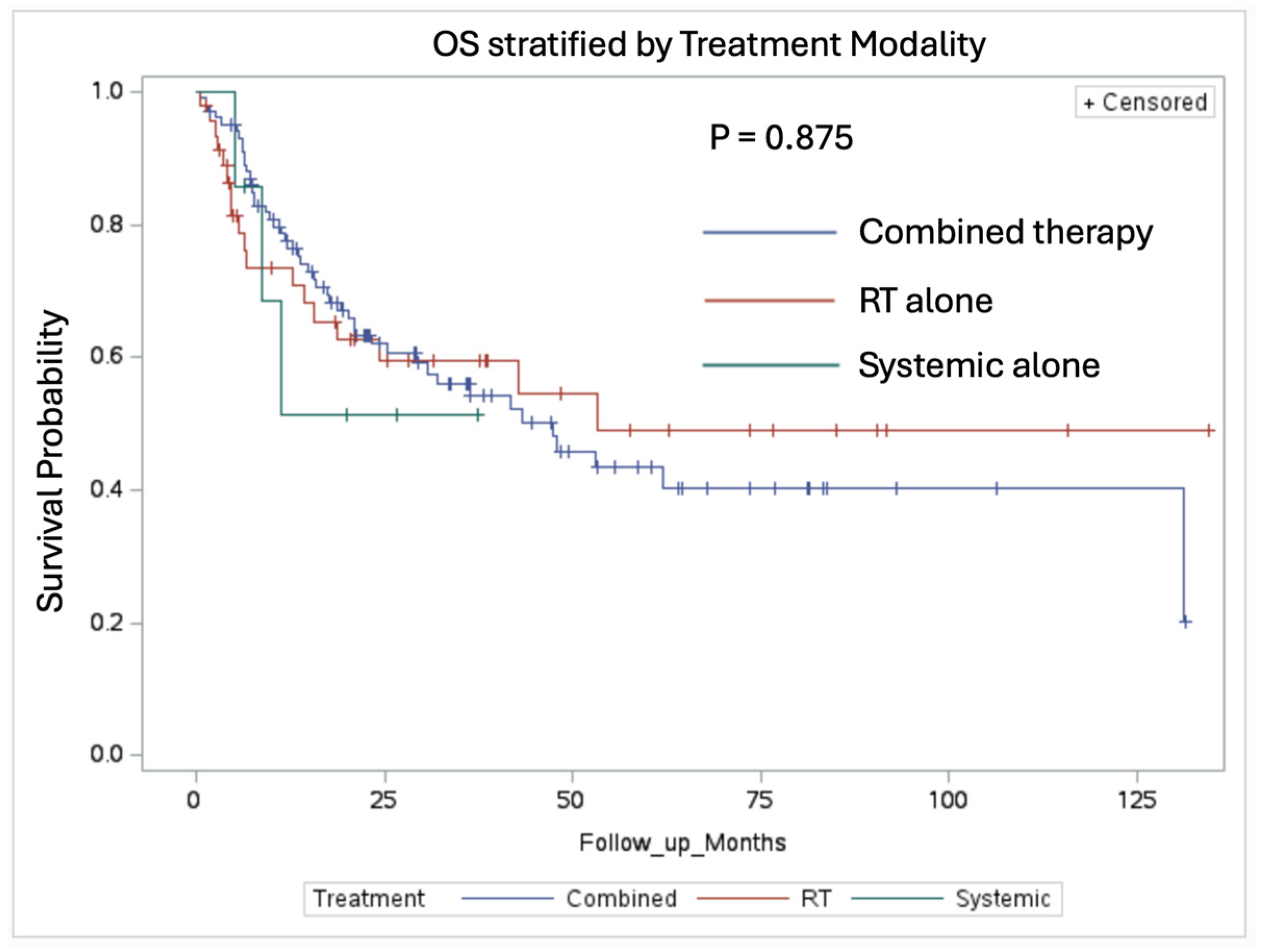
3.3. Overall Survival and Index Lesion Recurrence Analysis
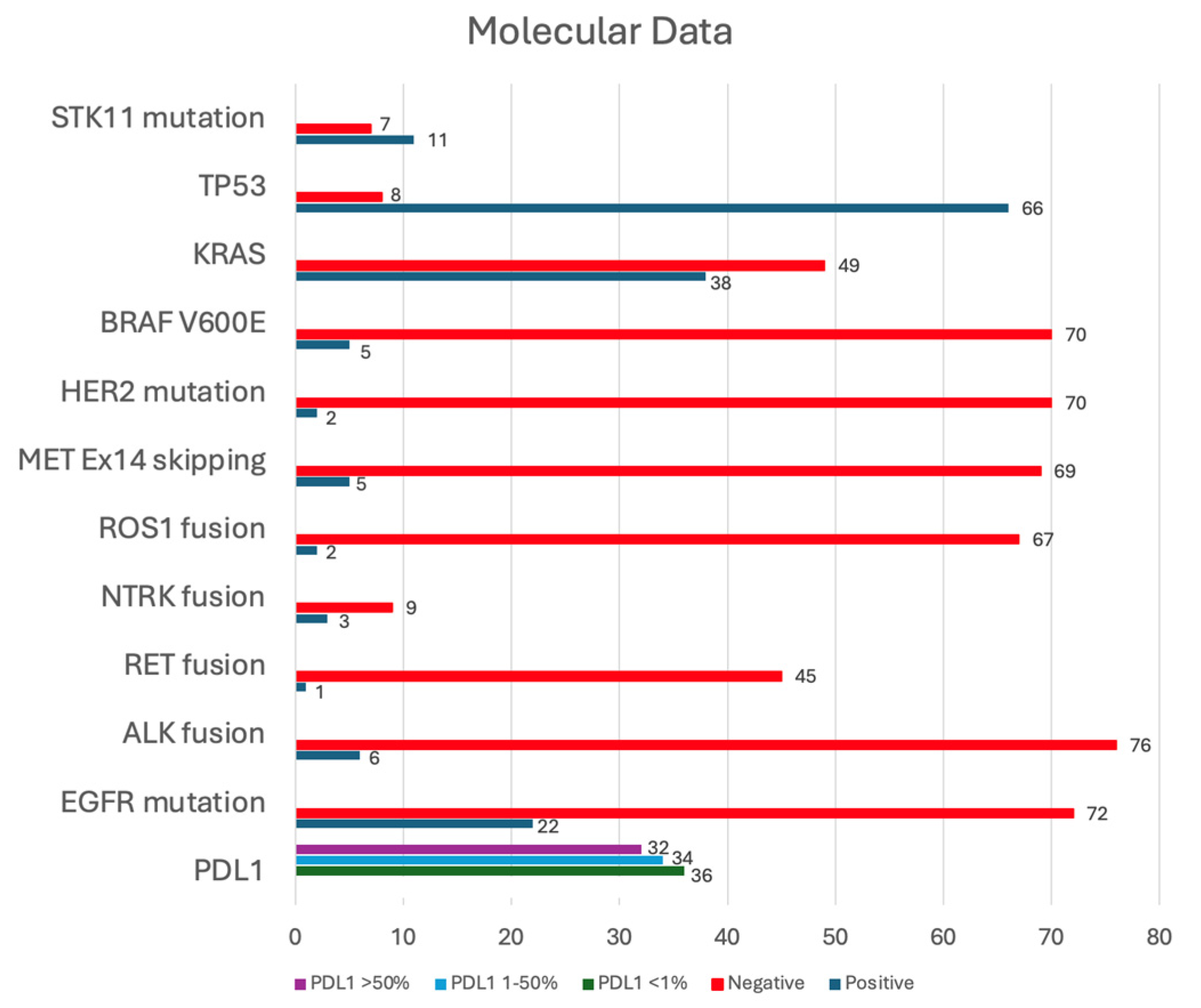
3.4. Molecular Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Ernani, V.; Stinchcombe, T.E. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J. Oncol. Pract. 2019, 15, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; Winkler, F.; Valiente, M.; Manegold, C.; Moyal, E.; Widhalm, G.; Tonn, J.C.; Zielinski, C. Recent advances in the biology and treatment of brain metastases of non-small cell lung cancer: Summary of a multidisciplinary roundtable discussion. ESMO Open 2018, 3, e000262. [Google Scholar] [CrossRef]
- Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Han, J.Y.; Katakami, N.; Kim, H.R.; Hodge, R.; Kaur, P.; Brown, A.P.; Ghiorghiu, D.; et al. CNS Efficacy of Osimertinib in Patients with T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data from a Randomized Phase III Trial (AURA3). J. Clin. Oncol. 2018, 36, 2702–2709. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- He, Z.Y.; Li, M.F.; Lin, J.H.; Lin, D.; Lin, R.J. Comparing the efficacy of concurrent EGFR-TKI and whole-brain radiotherapy vs EGFR-TKI alone as a first-line therapy for advanced EGFR-mutated non-small-cell lung cancer with brain metastases: A retrospective cohort study. Cancer Manag. Res. 2019, 11, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G.Y.; Chiang, C.L.; Yang, H.C.; Shen, C.I.; Wu, H.M.; Chen, Y.W.; Chen, C.J.; Luo, Y.H.; Hu, Y.S.; Lin, C.J.; et al. Combined stereotactic radiosurgery and tyrosine kinase inhibitor therapy versus tyrosine kinase inhibitor therapy alone for the treatment of non-small cell lung cancer patients with brain metastases. J. Neurosurg. 2021, 137, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.J.; Myall, N.J.; Sun, F.; Patil, T.; Mushtaq, R.; Yu, C.; Sinha, S.; Pollom, E.L.; Nagpal, S.; Camidge, D.R.; et al. Brain Metastases in EGFR- and ALK-Positive NSCLC: Outcomes of Central Nervous System-Penetrant Tyrosine Kinase Inhibitors Alone Versus in Combination with Radiation. J. Thorac. Oncol. 2022, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, J.A.; Zadeh, G.; Anders, C.K.; Shultz, D.B.; Brastianos, P.K. Special issue: Advances in the multimodality management of brain metastases and ongoing approaches to further improve their treatment. Neuro-Oncol. Adv. 2021, 3 (Suppl. S5), v1–v3. [Google Scholar]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Singh, K.; Saxena, S.; Khosla, A.A.; McDermott, M.W.; Kotecha, R.R.; Ahluwalia, M.S. Update on the Management of Brain Metastasis. Neurotherapeutics 2022, 19, 1772–1781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, A.; Kumar, S.; Potter, A.L.; Raman, V.; Kozono, D.E.; Lanuti, M.; Yang, C.F.J. Surgical management of non-small cell lung cancer with limited metastatic disease involving only the brain. J. Thorac. Cardiovasc. Surg. 2024, 167, 466–477.e2. [Google Scholar] [CrossRef]
- Piffko, A.; Asey, B.; Dührsen, L.; Ristow, I.; Salamon, J.; Wikman, H.; Maire, C.L.; Lamszus, K.; Westphal, M.; Sauvigny, T.; et al. Clinical determinants impacting overall survival of patients with operable brain metastases from non-small cell lung cancer. Front. Oncol. 2022, 12, 951805. [Google Scholar] [CrossRef] [PubMed]
- Myall, N.J.; Yu, H.; Soltys, S.G.; Wakelee, H.A.; Pollom, E. Management of brain metastases in lung cancer: Evolving roles for radiation and systemic treatment in the era of targeted and immune therapies. Neuro-Oncol. Adv. 2021, 3, v52–v62. [Google Scholar] [CrossRef]
- Kahraman, S.; Karakaya, S.; Kaplan, M.A.; Goksu, S.S.; Ozturk, A.; Isleyen, Z.S.; Hamdard, J.; Yildirim, S.; Dogan, T.; Isik, S.; et al. Treatment outcomes and prognostic factors in patients with driver mutant non-small cell lung cancer and de novo brain metastases. Sci. Rep. 2024, 14, 5820. [Google Scholar] [CrossRef]
- Rodrigues, A.; Li, G.; Bhambhvani, H.; Hayden-Gephart, M. Socioeconomic Disparities in Brain Metastasis Survival and Treatment: A Population-Based Study. World Neurosurg. 2022, 158, e636–e644. [Google Scholar] [CrossRef]
- Dee, E.C.; Swami, N.; Kazzi, B.; Lapen, K.; Franco, I.; Jain, B.; Patel, T.A.; Mahal, B.A.; Rimner, A.; Wu, A.; et al. Disparities in Stage at Presentation Among Hispanic and Latinx Patients with Non-Small-Cell Lung Cancer in the United States. JCO Oncol. Pract. 2024, 20, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, L.E.; Cervantes, L.; Havranek, E. Barriers in Healthcare for Latinx Patients with Limited English Proficiency-a Narrative Review. J. Gen. Intern. Med. 2023, 38, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lin, S.; Chen, J.; Dang, J. First-line treatment with TKI plus brain radiotherapy versus TKI alone in EGFR-mutated non-small cell Lung cancer with brain metastases: A systematic review and meta-analysis. BMC Cancer 2023, 23, 1043. [Google Scholar] [CrossRef]
- Kuan, A.S.; Chiang, C.L.; Wu, H.M.; Yang, H.C.; Chen, C.J.; Lin, C.J.; Guo, W.Y.; Pan, D.H.; Chung, W.Y.; Lee, C.C. Improved survival and intracranial tumor control of EGFR-mutated NSCLC patients with newly developed brain metastases following stereotactic radiosurgery and EGFR-TKI: A large retrospective cohort study and meta-analyses. J. Neuro-Oncol. 2023, 164, 729–739. [Google Scholar] [CrossRef]
- Eguren-Santamaria, I.; Sanmamed, M.F.; Goldberg, S.B.; Kluger, H.M.; Idoate, M.A.; Lu, B.Y.; Corral, J.; Schalper, K.A.; Herbst, R.S.; Gil-Bazo, I. PD-1/PD-L1 Blockers in NSCLC Brain Metastases: Challenging Paradigms and Clinical Practice. Clin. Cancer Res. 2020, 26, 4186–4197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, J.; Cai, J.; Liu, A. Combination therapy of brain radiotherapy and EGFR-TKIs is more effective than TKIs alone for EGFR-mutant lung adenocarcinoma patients with asymptomatic brain metastasis. BMC Cancer 2019, 19, 793. [Google Scholar] [CrossRef]
- Pangal, D.J.; Yarovinsky, B.; Cardinal, T.; Cote, D.J.; Ruzevick, J.; Attenello, F.J.; Chang, E.L.; Ye, J.; Neman, J.; Chow, F.; et al. The abscopal effect: Systematic review in patients with brain and spine metastases. Neuro-Oncol. Adv. 2022, 4, vdac132. [Google Scholar] [CrossRef]
- Amouzegar, A.; Haig, S.; Kahn, A.M.; Tawbi, H.A.; Jones, J.A.; Goldberg, S.B. Navigating the Complexities of Brain Metastases Management. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e433694. [Google Scholar] [CrossRef]
- Andrews, L.J.; Thornton, Z.A.; Saleh, R.; Dawson, S.; Short, S.C.; Daly, R.; Higgins, J.P.T.; Davies, P.; Kurian, K.M. Genomic landscape and actionable mutations of brain metastases derived from non-small cell lung cancer: A systematic review. Neuro-Oncol. Adv. 2023, 5, vdad145. [Google Scholar] [CrossRef]
- Gu, W.; Liu, P.; Tang, J.; Lai, J.; Wang, S.; Zhang, J.; Xu, J.; Deng, J.; Yu, F.; Shi, C.; et al. The prognosis of TP53 and EGFR co-mutation in patients with advanced lung adenocarcinoma and intracranial metastasis treated with EGFR-TKIs. Front. Oncol. 2024, 13, 1288468. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Duan, X.T.; Qiao, S.M.; Zhu, X.X. Radiotherapy combined with PD-1/PD-L1 inhibitors in NSCLC brain metastases treatment: The mechanisms, advances, opportunities, and challenges. Cancer Med. 2023, 12, 995–1006. [Google Scholar] [CrossRef]
- Frampton, J.E. Entrectinib: A Review in NTRK+ Solid Tumours and ROS1+ NSCLC. Drugs 2021, 81, 697–708. [Google Scholar] [CrossRef]
- Almquist, D.; Ernani, V. The Road Less Traveled: A Guide to Metastatic ROS1-Rearranged Non-Small-Cell Lung Cancer. JCO Oncol. Pract. 2021, 17, 7–14. [Google Scholar] [CrossRef]
- Jänne, P.A.; Planchard, D.; Kobayashi, K.; Cheng, Y.; Lee, C.K.; Valdiviezo, N.; Laktionov, K.; Yang, T.Y.; Yu, Y.; Kato, T.; et al. CNS Efficacy of Osimertinib with or without Chemotherapy in Epidermal Growth Factor Receptor-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.R.G.; Miao, E.; Boe, L.A.; Patil, T.; Imber, B.S.; Myall, N.J.; Pollom, E.L.; Hui, C.; Qu, V.; Langston, J.; et al. Tyrosine Kinase Inhibitors with and without Up-Front Stereotactic Radiosurgery for Brain Metastases from EGFR and ALK Oncogene-Driven Non-Small Cell Lung Cancer (TURBO-NSCLC). J. Clin. Oncol. 2024, JCO2302668. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Patients (n = 155) |
|---|---|
| Age at brain metastasis diagnosis, years Median (range) | 65 (33–83) |
| Gender, n (%) | |
| Male Female | 63 (40.7) 92 (59.3) |
| Race, n (%) | |
| Asian | 13 (8.4) |
| Black | 25 (16.1) |
| White/Hispanic | 16 (10.3) |
| White/non-Hispanic | 101 (65.2) |
| Smoking status, n (%) | |
| Never | 27 (17.4) |
| Former | 85 (54.8) |
| Current | 43 (27.8) |
| NSCLC histology, n (%) | |
| Adenocarcinoma | 126 (81.3) |
| Squamous cell carcinoma | 21 (13.6) |
| NSCLC NOS/poorly differentiated | 8 (5.1) |
| Number of brain metastases | |
| 1–4 | 136 (87.8) |
| 5–10 | 16 (10.3) |
| >10 | 3 (1.9) |
| Leptomeningeal disease | 4 (2.6) |
| Adjuvant therapy, n (%) | |
| Systemic therapy only | 7 (4.5) |
| Radiation therapy only | 46 (29.7) |
| Combined modality therapy | 102 (65.8) |
| Systemic therapy, n (%) | |
| Chemotherapy alone | 59 (38.1) |
| Immunotherapy alone | 73 (47.1) |
| Chemo and immuno | 30 (19.4) |
| Toxicity, n (%) | |
| Radiation necrosis | 12 (7.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wuu, Y.-R.; Kokabee, M.; Gui, B.; Lee, S.; Stone, J.; Karten, J.; D’Amico, R.S.; Vojnic, M.; Wernicke, A.G. Prioritizing Radiation and Targeted Systemic Therapies in Patients with Resected Brain Metastases from Lung Cancer Primaries with Targetable Mutations: A Report from a Multi-Site Single Institution. Cancers 2024, 16, 3270. https://doi.org/10.3390/cancers16193270
Wuu Y-R, Kokabee M, Gui B, Lee S, Stone J, Karten J, D’Amico RS, Vojnic M, Wernicke AG. Prioritizing Radiation and Targeted Systemic Therapies in Patients with Resected Brain Metastases from Lung Cancer Primaries with Targetable Mutations: A Report from a Multi-Site Single Institution. Cancers. 2024; 16(19):3270. https://doi.org/10.3390/cancers16193270
Chicago/Turabian StyleWuu, Yen-Ruh, Mostafa Kokabee, Bin Gui, Simon Lee, Jacob Stone, Jessie Karten, Randy S. D’Amico, Morana Vojnic, and A. Gabriella Wernicke. 2024. "Prioritizing Radiation and Targeted Systemic Therapies in Patients with Resected Brain Metastases from Lung Cancer Primaries with Targetable Mutations: A Report from a Multi-Site Single Institution" Cancers 16, no. 19: 3270. https://doi.org/10.3390/cancers16193270
APA StyleWuu, Y.-R., Kokabee, M., Gui, B., Lee, S., Stone, J., Karten, J., D’Amico, R. S., Vojnic, M., & Wernicke, A. G. (2024). Prioritizing Radiation and Targeted Systemic Therapies in Patients with Resected Brain Metastases from Lung Cancer Primaries with Targetable Mutations: A Report from a Multi-Site Single Institution. Cancers, 16(19), 3270. https://doi.org/10.3390/cancers16193270








