Repeat Faecal Immunochemical Testing for Colorectal Cancer Detection in Symptomatic and Screening Patients: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Review Questions
- Is the test positivity rate higher with multiple FITs compared with single FITs within the study populations?
- Is the rate of missed CRC and ACRN decreased with the addition of second and/or third FIT tests compared with single FITs?
- In asymptomatic, mixed, and symptomatic populations, what is the sensitivity for CRC and ACRN detection when a double-FIT strategy is used at key thresholds?
2.2. Data Search Strategy
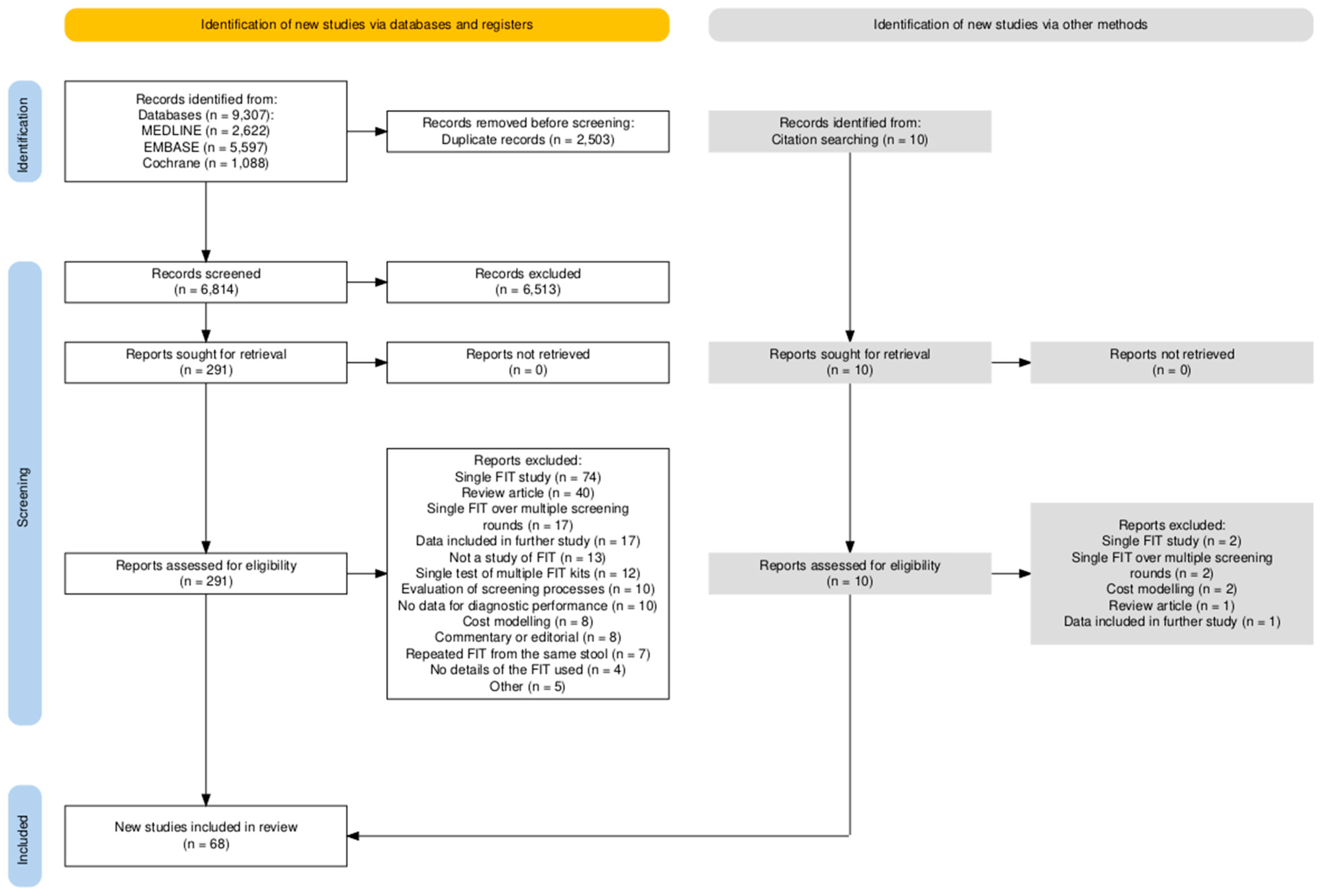
2.3. Study Selection
2.4. Data Extraction and Assessment
2.5. Outcome Measures
3. Results
3.1. Positivity Rates
3.2. Use of Additional FITs Leads to a Reduction in Missed CRC and ACRN
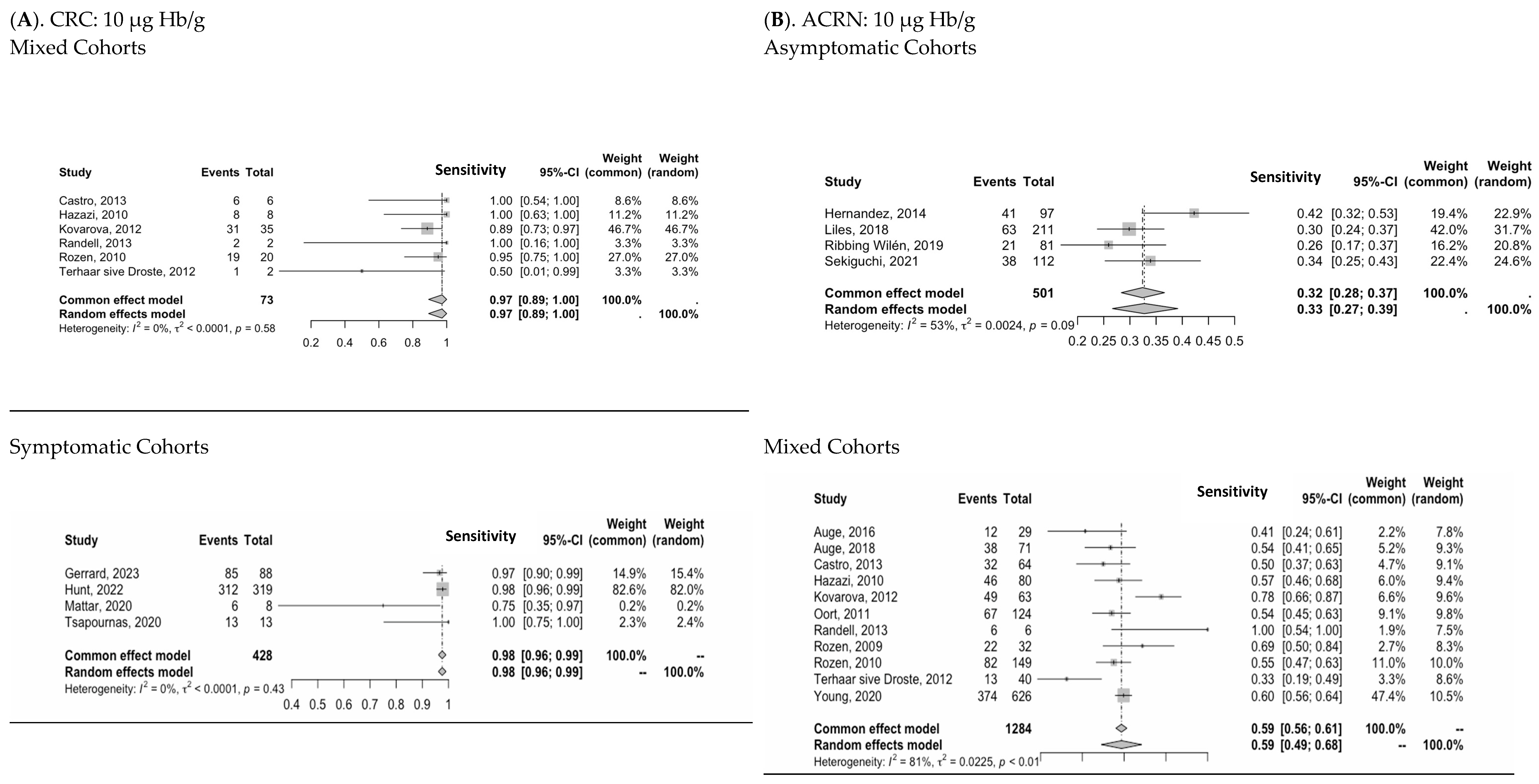
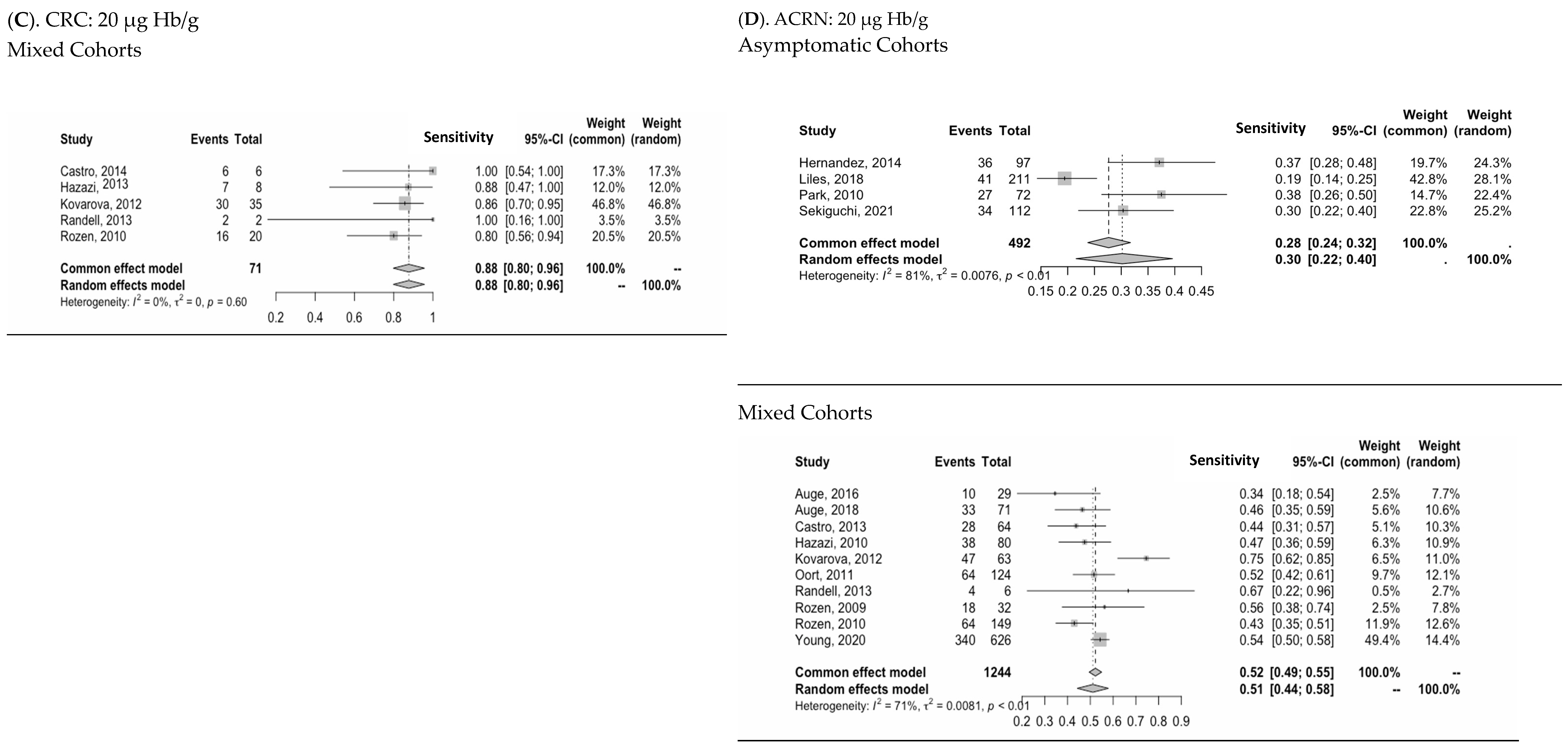
3.3. Diagnostic Performance of Two-FIT Strategy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shaukat, A.; Levin, T.R. Current and future colorectal cancer screening strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.J.; Davies, M.M.; Abulafi, M.; Banerjea, A.; Nicholson, B.D.; Arasaradnam, R.; Barker, N.; Benton, S.; Booth, R.; Burling, D.; et al. Faecal immunochemical testing (FIT) in patients with signs or symptoms of suspected colorectal cancer (CRC): A joint guideline from the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the British Society of Gastroenterology (BSG). Gut 2022, 71, 1939–1962. [Google Scholar] [CrossRef] [PubMed]
- Stonestreet, J.; Chandrapalan, S.; Woolley, D.; Uthman, U.; Arasaradnam, R.P. Systematic review and meta-analysis: Diagnostic accuracy of faecal immunochemical testing for haemoglobin (FIT) in detecting colorectal cancer for both symptomatic and screening population. Acta Gastroenterol. Belg. 2019, 82, 291–299. [Google Scholar] [PubMed]
- Saw, K.S.; Liu, C.; Xu, W.; Varghese, C.; Parry, S.; Bissett, I. Faecal immunochemical test to triage patients with possible colorectal cancer symptoms: Meta-analysis. Br. J. Surg. 2021, 109, znab411. [Google Scholar] [CrossRef]
- Pin-Vieito, N.; Tejido-Sandoval, C.; de Vicente-Bielza, N.; Sánchez-Gómez, C.; Cubiella, J. Faecal immunochemical tests safely enhance rational use of resources during the assessment of suspected symptomatic colorectal cancer in primary care: Systematic review and meta-analysis. Gut 2022, 71, 950–960. [Google Scholar] [CrossRef]
- Farkas, N.G.; Fraser, C.G.; Maclean, W.; Jourdan, I.; Rockall, T.; Benton, S.C. Replicate and repeat faecal immunochemical tests in symptomatic patients: A systematic review. Ann. Clin. Biochem. 2022, 60, 27–36. [Google Scholar] [CrossRef]
- Gerrard, A.D.; Maeda, Y.; Miller, J.; Gunn, F.; Theodoratou, E.; Noble, C.; Porteous, L.; Glancy, S.; MacLean, P.; Pattenden, R.; et al. Double faecal immunochemical testing in patients with symptoms suspicious of colorectal cancer. Br. J. Surg. 2023, 110, 471–480. [Google Scholar] [CrossRef]
- Hunt, N.; Rao, C.; Logan, R.; Chandrabalan, V.; Oakey, J.; Ainsworth, C.; Smith, N.; Banerjee, S.; Myers, M. A cohort study of duplicate faecal immunochemical testing in patients at risk of colorectal cancer from North-West England. BMJ Open 2022, 12, e059940. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Robertson, D.J.; Lee, J.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Rex, D.K. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1217–1237.e3. [Google Scholar] [CrossRef]
- Lin, J.S.; Piper, M.A.; Perdue, L.A.; Rutter, C.; Webber, E.M.; O’Connor, E.; Smith, N.; Whitlock, E.P.U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. In Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2016. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- NICE. Diagnostics Guidance [DG56] Quantitative Faecal Immunochemical Testing to Guide Colorectal Cancer Pathway Referral in Primary Care. 2023. Available online: https://www.nice.org.uk/guidance/dg56 (accessed on 10 July 2024).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Abd Jalal, N.; Ismail, N.; Kamaruddin, M.A.; Abd Mutalib, N.S.; Alias, M.R.; Mazlan, L.; Sagap, I.; Jamal, R. Colorectal screening using the immunochemical faecal occult blood test kit among the Malaysian cohort participants. Cancer Epidemiol. 2020, 65, 101656. [Google Scholar] [CrossRef]
- Cai, S.R.; Zhu, H.H.; Huang, Y.Q.; Li, Q.L.; Ma, X.Y.; Zhang, S.Z.; Zheng, S. Cost-Effectiveness between Double and Single Fecal Immunochemical Test(s) in a Mass Colorectal Cancer Screening. Biomed. Res. Int. 2016, 2016, 6830713. [Google Scholar] [CrossRef]
- Chubak, J.; Bogart, A.; Fuller, S.; Laing, S.S.; Green, B.B. Uptake and positive predictive value of fecal occult blood tests: A randomized controlled trial. Prev. Med. 2013, 57, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.R.; Young, G.P.; Esterman, A.; Cadd, B.; Morcom, J. A Randomised Trial of the Impact of New Faecal Haemoglobin Test Technologies on Population Participation in Screening for Colorectal Cancer. J. Med. Screen. 2003, 10, 117–122. [Google Scholar] [CrossRef]
- Dancourt, V.; Lejeune, C.; Lepage, C.; Gailliard, M.C.; Meny, B.; Faivre, J. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. Eur. J. Cancer 2008, 44, 2254–2258. [Google Scholar] [CrossRef]
- Faivre, J.; Dancourt, V.; Manfredi, S.; Denis, B.; Durand, G.; Gendre, I.; Bidan, J.M.; Jard, C.; Levillain, R.; Jung, S.; et al. Positivity rates and performances of immunochemical faecal occult blood tests at different cut-off levels within a colorectal cancer screening programme. Dig. Liver Dis. 2012, 44, 700–704. [Google Scholar] [CrossRef]
- Fu, W.P.; Kam, M.H.; Ling, W.M.; Ong, S.F.; Suzannah, N.; Eu, K.-W. Screening for colorectal cancer using a quantitative immunochemical faecal occult blood test: A feasibility study in an Asian population. Tech. Coloproctol. 2009, 13, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Grazzini, G.; Visioli, C.B.; Zorzi, M.; Ciatto, S.; Banovich, F.; Bonanomi, A.G.; Bortoli, A.; Castiglione, G.; Cazzola, L.; Confortini, M.; et al. Immunochemical faecal occult blood test: Number of samples and positivity cutoff. What is the best strategy for colorectal cancer screening? Br. J. Cancer 2009, 100, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Guittet, L.; Bouvier, V.; Mariotte, N.; Vallee, J.-P.; Levillain, R.; Tichet, J.; Launoy, G. Performance of immunochemical faecal occult blood test in colorectal cancer screening in average-risk population according to positivity threshold and number of samples. Int. J. Cancer 2009, 125, 1127–1133. [Google Scholar] [CrossRef]
- Hernandez, V.; Cubiella, J.; Gonzalez-Mao, M.C.; Iglesias, F.; Rivera, C.; Iglesias, M.B.; Cid, L.; Castro, I.; de Castro, L.; Vega, P.; et al. Fecal immunochemical test accuracy in average-risk colorectal cancer screening. World J. Gastroenterol. 2014, 20, 1038–1047. [Google Scholar] [CrossRef]
- Kapidzic, A.; Van Roon, A.H.C.; Van Leerdam, M.E.; Van Vuuren, A.J.; Van Ballegooijen, M.; Lansdorp-Vogelaar, I.; Spijker, W.; Izelaar, K.; Hol, L.; Kuipers, E.J. Attendance and diagnostic yield of repeated two-sample faecal immunochemical test screening for colorectal cancer. Gut 2017, 66, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.; Swan, N.; Hughes, D.J. An analysis of the duplicate testing strategy of an Irish immunochemical FOBT colorectal cancer screening programme. Color. Dis. 2013, 15, e512–e521. [Google Scholar] [CrossRef]
- Launoy, G.D.; Bertrand, H.J.; Berchi, C.; Talbourdet, V.Y.; Guizard, A.V.N.; Bouvier, V.M.; Caces, E.R. Evaluation of an immunochemical fecal occult blood test with automated reading in screening for colorectal cancer in a general average-risk population. Int. J. Cancer 2005, 115, 493–496. [Google Scholar] [CrossRef]
- Levi, Z.; Birkenfeld, S.; Vilkin, A.; Bar-Chana, M.; Lifshitz, I.; Chared, M.; Maoz, E.; Niv, Y. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int. J. Cancer 2011, 128, 2415–2424. [Google Scholar] [CrossRef]
- Liles, E.G.; Perrin, N.; Rosales, A.G.; Smith, D.H.; Feldstein, A.C.; Mosen, D.M.; Levin, T.R. Performance of a quantitative fecal immunochemical test for detecting advanced colorectal neoplasia: A prospective cohort study. BMC Cancer 2018, 18, 509. [Google Scholar] [CrossRef]
- Moosavi, S.; Enns, R.; Gentile, L.; Gondara, L.; McGahan, C.; Telford, J. Comparison of One versus Two Fecal Immunochemical Tests in the Detection of Colorectal Neoplasia in a Population-Based Colorectal Cancer Screening Program. Can. J. Gastroenterol. Hepatol. 2016, 2016, 5914048. [Google Scholar] [CrossRef]
- Nakama, H.; Zhang, B.; Fattah, A.S.M.A. A cost-effective analysis of the optimum number of stool specimens collected for immunochemical occult blood screening for colorectal cancer. Eur. J. Cancer 2000, 36, 647–650. [Google Scholar] [CrossRef]
- Nakama, H.; Zhang, B.; Fattah, A.S.M.A.; Zhang, X. Colorectal cancer in iron deficiency anemia with a positive result on immunochemical fecal occult blood. Int. J. Color. Dis. 2000, 15, 271–274. [Google Scholar] [CrossRef]
- Nakama, H.; Fukazawa, K. Colorectal cancer risk in first-degree relatives of patients with colorectal adenomatous polyp. Hepatogastroenterology 2002, 49, 157–159. [Google Scholar] [PubMed]
- Nakazato, M.; Yamano, H.O.; Matsushita, H.O.; Sato, K.; Fujita, K.; Yamanaka, Y.; Imai, Y. Immunologic fecal occult blood test for colorectal cancer screening. Jpn. Med. Assoc. J. 2006, 49, 203–207. [Google Scholar]
- Okada, T.; Odagaki, T.; López-Köstner, F.; Zárate, A.J.; Ponce, A.; Kronberg, U.; Karelovic, S.; Flores, S.; Estela, R.; Ito, T.; et al. Colorectal cancer risk factors in asymptomatic Chilean population: A survey of international collaboration between Japan and Chile. Eur. J. Cancer Prev. 2020, 29, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Park, D.I.; Ryu, S.; Kim, Y.H.; Lee, S.H.; Lee, C.K.; Eun, C.S.; Han, D.S. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am. J. Gastroenterol. 2010, 105, 2017–2025. [Google Scholar] [CrossRef]
- Raginel, T.; Puvinel, J.; Ferrand, O.; Bouvier, V.; Levillain, R.; Ruiz, A.; Lantieri, O.; Launoy, G.; Guittet, L. A Population-based Comparison of Immunochemical Fecal Occult Blood Tests for Colorectal Cancer Screening. Gastroenterology 2013, 144, 918–925. [Google Scholar] [CrossRef]
- Ribbing Wilén, H.; Blom, J.; Höijer, J.; Andersson, G.; Löwbeer, C.; Hultcrantz, R. Fecal immunochemical test in cancer screening—Colonoscopy outcome in FIT positives and negatives. Scand. J. Gastroenterol. 2019, 54, 303–310. [Google Scholar] [CrossRef]
- Rutka, M.; Bor, R.; Molnár, T.; Farkas, K.; Pigniczki, D.; Fábián, A.; Győrffy, M.; Bálint, A.; Milassin, Á.; Szűcs, M.; et al. Efficacy of the population-based pilot colorectal cancer screening, Csongrád county, Hungary, 2015. Turk. J. Med. Sci. 2020, 50, 756–763. [Google Scholar] [CrossRef]
- Sakata, N.; Sakata, Y.; Shimoda, R.; Sakata, H.; Iwakiri, R.; Fujimoto, K.; Mizuguchi, M.; Irie, H.; Shimoda, Y.; Noshiro, H. Repeated screening with fecal immunochemical tests reduced the incidence of colorectal cancers in Saga, Japan. Hepatogastroenterology 2014, 61, 1224–1228. [Google Scholar]
- Schreuders, E.H.; Grobbee, E.J.; Nieuwenburg, S.A.V.; Kapidzic, A.; van Roon, A.H.C.; van Vuuren, A.J.; Lansdorp-Vogelaar, I.; Spijker, W.W.J.; Izelaar, K.; Bruno, M.J.; et al. Multiple rounds of one sample versus two sample faecal immunochemical test-based colorectal cancer screening: A population-based study. Lancet Gastroenterol. Hepatol. 2019, 4, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, M.; Kakugawa, Y.; Ikematsu, H.; Hotta, K.; Konda, K.; Tanaka, Y.; Takamaru, H.; Yamada, M.; Sakamoto, T.; Saito, Y.; et al. Risk Stratification Score Improves Sensitivity for Advanced Colorectal Neoplasia in Colorectal Cancer Screening: The Oshima Study Workgroup. Clin. Transl. Gastroenterol. 2021, 12, e00319. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.A.; Bobo, J.K.; Church, T.R.; Rex, D.K.; Chovnick, G.; Thompson, T.D.; Zauber, A.G.; Lieberman, D.; Levin, T.R.; Joseph, D.A.; et al. A Comparison of Fecal Immunochemical and High-Sensitivity Guaiac Tests for Colorectal Cancer Screening. Am. J. Gastroenterol. 2017, 112, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Shuhaibar, M.; Walsh, C.; Lindsay, F.; Lee, N.; Walsh, P.; O’Gorman, P.; Boran, G.; McLoughlin, R.; Qasim, A.; Breslin, N.; et al. A comparative study of faecal occult blood kits in a colorectal cancer screening program in a cohort of healthy construction workers. Ir. J. Med. Sci. 2011, 180, 103–108. [Google Scholar] [CrossRef]
- Tan, W.S.; Tang, C.L.; Koo, W.H. Opportunistic screening for colorectal neoplasia in Singapore using faecal immunochemical occult blood test. Singap. Med. J. 2013, 54, 220–223. [Google Scholar] [CrossRef]
- Telford, J.; Gentile, L.; Gondara, L.; McGahan, C.; Coldman, A. Performance of a quantitative fecal immunochemical test in a colorectal cancer screening pilot program: A prospective cohort study. CMAJ Open 2016, 4, E668–E673. [Google Scholar] [CrossRef]
- Tepeš, B.; Stabuc, B.; Stefanovič, M.; Bračko, M.; Frkovič Grazio, S.; Novak Mlakar, D.; Maučec Zakotnik, J. Faecal immunochemical test-based colorectal cancer screening programme SVIT in Slovenia: Pilot phase. Eur. J. Cancer Prev. 2014, 23, 235–239. [Google Scholar] [CrossRef]
- Tepes, B.; Stefanovic, M.; Stabuc, B.; Mlakar, D.N.; Grazio, S.F.; Zakotnik, J.M. Quality Control in the Slovenian National Colorectal Cancer Screening Program. Dig. Dis. 2022, 40, 187–197. [Google Scholar] [CrossRef]
- Tourne-Garcia, C.; Perez-Riquelme, F.; Monteagudo-Piqueras, O.; Fraser, C.G.; Yepes-Garcia, P. One or two faecal immunochemical tests in an organised population-based colorectal cancer screening programme in Murcia (Spain). J. Med. Screen. 2022, 29, 231–240. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Zhu, Y.; Lu, M.; Wang, Y.; Chen, X.; Ma, W.; Du, L.; Chen, W. One-sample quantitative and two-sample qualitative faecal immunochemical tests for colorectal cancer screening: A cross-sectional study in China. BMJ Open 2022, 12, e059754. [Google Scholar] [CrossRef]
- Wong, M.C.; Ching, J.Y.; Chan, V.C.; Lam, T.Y.; Shum, J.P.; Luk, A.K.; Wong, S.S.; Ng, S.C.; Ng, S.S.; Wu, J.C.; et al. Diagnostic Accuracy of a Qualitative Fecal Immunochemical Test Varies With Location of Neoplasia But Not Number of Specimens. Clin. Gastroenterol. Hepatol. 2015, 13, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, L.; Naik, I.; Braunstein, Z.; Zhong, J.; Ren, B. Transcription Factor C/EBP Homologous Protein in Health and Diseases. Front. Immunol. 2017, 8, 1612. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Huang, Q.; Li, Q.; Jiang, X.; Mamat, M.; Tang, M.; Wang, J.; Chen, K. Comparative Evaluation of Preliminary Screening Methods for Colorectal Cancer in a Mass Program. Dig. Dis. Sci. 2017, 62, 2532–2541. [Google Scholar] [CrossRef] [PubMed]
- Auge, J.M.; Rodriguez, C.; Pellise, M.; Bernal, A.; Grau, J.; Castells, A.; Filella, X.; Molina, R. Analytical and clinical performance of Kroma iT, a compact fully-automated immunochemistry analyzer for fecal occult hemoglobin. Anticancer. Res. 2013, 33, 5633–5637. [Google Scholar] [PubMed]
- Auge, J.M.; Fraser, C.G.; Rodriguez, C.; Roset, A.; Lopez-Ceron, M.; Grau, J.; Castells, A.; Jimenez, W. Clinical utility of one versus two faecal immunochemical test samples in the detection of advanced colorectal neoplasia in symptomatic patients. Clin. Chem. Lab. Med. 2016, 54, 125–132. [Google Scholar] [CrossRef]
- Auge, J.M.; Rodriguez, C.; Espanyol, O.; Rivero, L.; Sandalinas, S.; Grau, J.; Jimenez, W.; Castells, A. An evaluation of the SENTiFIT 270 analyser for quantitation of faecal haemoglobin in the investigation of patients with suspected colorectal cancer. Clin. Chem. Lab. Med. 2018, 56, 625–633. [Google Scholar] [CrossRef]
- Castro, I.; Cubiella, J.; Rivera, C.; González-Mao, C.; Vega, P.; Soto, S.; Hernandez, V.; Iglesias, F.; Teresa Alves, M.; Bujanda, L.; et al. Fecal immunochemical test accuracy in familial risk colorectal cancer screening. Int. J. Cancer 2014, 134, 367–375. [Google Scholar] [CrossRef]
- Chew, M.H.; Suzanah, N.; Ho, K.S.; Lim, J.F.; Ooi, B.S.; Tang, C.L.; Eu, K.W. Colorectal cancer mass screening event utilising quantitative faecal occult blood test. Singap. Med. J. 2009, 50, 348–353. [Google Scholar]
- Cruz-Correa, M.; Schultz, K.; Jagannath, S.; Harris, M.; Kantsevoy, S.; Bedine, M.; Kalloo, A.N. Performance Characteristics and Comparison of Two Fecal Occult Blood Tests in Patients Undergoing Colonoscopy. Dig. Dis. Sci. 2007, 52, 1009–1013. [Google Scholar] [CrossRef]
- Guimarães, D.P.; Fregnani, J.H.; Reis, R.M.; Taveira, L.N.; Scapulatempo-Neto, C.; Matsushita, M.; Silva, S.R.M.; Oliveira, C.Z.; Longatto-Filho, A.; Eklund, C.; et al. Comparison of a New-generation Fecal Immunochemical Test (FIT) With Guaiac Fecal Occult Blood Test (gFOBT) in Detecting Colorectal Neoplasia Among Colonoscopy-referral Patients. Anticancer Res. 2019, 39, 261–269. [Google Scholar] [CrossRef]
- Hazazi, R.; Rozen, P.; Leshno, M.; Levi, Z.; Samuel, Z.; Waked, A.; Vilkin, A.; Maoz, E.; Birkenfeld, S.; Niv, Y. Can patients at high risk for significant colorectal neoplasms and having normal quantitative faecal occult blood test postpone elective colonoscopy? Aliment. Pharmacol. Ther. 2010, 31, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kovarova, J.T.; Zavoral, M.; Zima, T.; Zak, A.; Kocna, P.; Kohout, P.; Granatova, J.; Vanickova, Z.; Vranova, J.; Suchanek, S.; et al. Improvements in colorectal cancer screening programmes—Quantitative immunochemical faecal occult blood testing—How to set the cut-off for a particular population. Biomed. Pap. 2012, 156, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Hu, J.; Li, N.; Liu, Y.; Wu, Z.; Zheng, Y.; Wang, H.; Wu, K.; Ye, H.; et al. New immunochemical fecal occult blood test with two-consecutive stool sample testing is a cost-effective approach for colon cancer screening: Results of a prospective multicenter study in Chinese patients. Int. J. Cancer 2006, 118, 3078–3083. [Google Scholar] [CrossRef]
- Oort, F.A.; Van Turenhout, S.T.; Coupé, V.M.; Van Der Hulst, R.W.; Wesdorp, E.I.; Terhaar Sive Droste, J.S.; Larbi, I.B.; Kanis, S.L.; Van Hengel, E.; Bouman, A.A.; et al. Double sampling of a faecal immunochemical test is not superior to single sampling for detection of colorectal neoplasia: A colonoscopy controlled prospective cohort study. BMC Cancer 2011, 11, 434. [Google Scholar] [CrossRef]
- Randell, E.; Kennell, M.; Taher, A.; Antle, S.; Bursey, F.; Tavenor, T.; Hammond, M.; Stone, S.; Mahar, D.; Smith, S.; et al. Evaluation of Hemo Techt NS-Plus system for use in a province-wide colorectal cancer screening program. Clin. Biochem. 2013, 46, 365–368. [Google Scholar] [CrossRef]
- Redwood, D.; Provost, E.; Asay, E.; Roberts, D.; Haverkamp, D.; Perdue, D.; Bruce, M.G.; Sacco, F.; Espey, D. Comparison of fecal occult blood tests for colorectal cancer screening in an Alaska Native population with high prevalence of Helicobacter pylori infection, 2008–2012. Prev. Chronic Dis. 2014, 11, E56. [Google Scholar] [CrossRef] [PubMed]
- Rozen, P.; Levi, Z.; Hazazi, R.; Waked, A.; Vilkin, A.; Maoz, E.; Birkenfeld, S.; Niv, Y. Quantitative colonoscopic evaluation of relative efficiencies of an immunochemical faecal occult blood test and a sensitive guaiac test for detecting significant colorectal neoplasms. Aliment. Pharmacol. Ther. 2009, 29, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Rozen, P.; Comaneshter, D.; Levi, Z.; Hazazi, R.; Vilkin, A.; Maoz, E.; Birkenfeld, S.; Niv, Y. Cumulative evaluation of a quantitative immunochemical fecal occult blood test to determine its optimal clinical use. Cancer 2010, 116, 2115–2125. [Google Scholar] [CrossRef]
- Terhaar Sive Droste, J.S.; Van Turenhout, S.T.; Oort, F.A.; Van Der Hulst, R.W.; Steeman, V.A.; Coblijn, U.; Van Der Eem, L.; Duijkers, R.; Bouman, A.A.; Meijer, G.A.; et al. Faecal immunochemical test accuracy in patients referred for surveillance colonoscopy: A multi-centre cohort study. BMC Gastroenterol. 2012, 12, 94. [Google Scholar] [CrossRef]
- Vasilyev, S.; Smirnova, E.; Popov, D.; Semenov, A.; Eklund, C.; Hendolin, P.; Paloheimo, L.; Syrjänen, K. A New-Generation Fecal Immunochemical Test (FIT) Is Superior to Quaiac-based Test in Detecting Colorectal Neoplasia Among Colonoscopy Referral Patients. Anticancer. Res. 2015, 35, 2873–2880. [Google Scholar]
- Wong, W.M.; Lam, S.K.; Cheung, K.L.; Tong, T.S.M.; Rozen, P.; Young, G.P.; Chu, K.W.; Ho, J.; Law, W.L.; Tung, H.M.; et al. Evaluation of an automated immunochemical fecal occult blood test for colorectal neoplasia detection in a Chinese population. Cancer 2003, 97, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.C.Y.; Wong, W.M.; Cheung, K.L.; Tong, T.S.M.; Rozen, P.; Young, G.P.; Chu, K.W.; Ho, J.; Law, W.L.; Tung, H.M.; et al. A sensitive guaiac faecal occult blood test is less useful than an immunochemical test for colorectal cancer screening in a Chinese population. Aliment. Pharmacol. Ther. 2003, 18, 941–946. [Google Scholar] [CrossRef]
- Wu, D.; Luo, H.-Q.; Zhou, W.-X.; Qian, J.-M.; Li, J.-N. The Performance of Three-Sample Qualitative Immunochemical Fecal Test to Detect Colorectal Adenoma and Cancer in Gastrointestinal Outpatients: An Observational Study. PLoS ONE 2014, 9, e106648. [Google Scholar] [CrossRef] [PubMed]
- Young, G.P.; Woodman, R.J.; Ang, F.L.I.; Symonds, E.L. Both Sample Number and Test Positivity Threshold Determine Colonoscopy Efficiency in Detection of Colorectal Cancer with Quantitative Fecal Immunochemical Tests. Gastroenterology 2020, 159, 1561–1563.e1563. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; Clèries, R.; Boadas, J.; Ribes, J.; Oliva, J.C.; Alsius, A.; Sanz, X.; Martínez-Bauer, E.; Galter, S.; Pujals, M.; et al. Prediction of advanced colonic neoplasm in symptomatic patients: A scoring system to prioritize colonoscopy (COLONOFIT study). BMC Cancer 2019, 19, 734. [Google Scholar] [CrossRef]
- Högberg, C.; Gunnarsson, U.; Jansson, S.; Thulesius, H.; Cronberg, O.; Lilja, M. Diagnosing colorectal cancer in primary care: Cohort study in Sweden of qualitative faecal immunochemical tests, haemoglobin levels, and platelet counts. Br. J. Gen. Pract. 2020, 70, e843–e851. [Google Scholar] [CrossRef] [PubMed]
- Mattar, R.; Marques, S.B.; Minata, M.K.; Silva-Etto, J.M.K.D.; Sakai, P.; De Moura, E.G.H. Diagnostic accuracy of one sample or two samples quantitative fecal immunochemical tests for intestinal neoplasia detection. Arq. Gastroenterol. 2020, 57, 316–322. [Google Scholar] [CrossRef]
- Oono, Y.; Iriguchi, Y.; Doi, Y.; Tomino, Y.; Kishi, D.; Oda, J.; Takayanagi, S.; Mizutani, M.; Fujisaki, T.; Yamamura, A.; et al. A retrospective study of immunochemical fecal occult blood testing for colorectal cancer detection. Clin. Chim. Acta 2010, 411, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Tsapournas, G.; Hellström, P.M.; Cao, Y.; Olsson, L.I. Diagnostic accuracy of a quantitative faecal immunochemical test vs. symptoms suspected for colorectal cancer in patients referred for colonoscopy. Scand. J. Gastroenterol. 2020, 55, 184–192. [Google Scholar] [CrossRef]
- Smith, A.; Young, G.P.; Cole, S.R.; Bampton, P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 2006, 107, 2152–2159. [Google Scholar] [CrossRef]
- Guittet, L.; Bouvier, V.; Mariotte, N.; Vallee, J.P.; Levillain, R.; Tichet, J.; Launoy, G. Comparison of a guaiac and an immunochemical faecal occult blood test for the detection of colonic lesions according to lesion type and location. Br. J. Cancer 2009, 100, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ge, Z.; Dai, J.; Li, X.; Gao, Y. Effectiveness of the Immunofecal Occult Blood Test for Colorectal Cancer Screening in a Large Population. Dig. Dis. Sci. 2011, 56, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, M.S.; Macleod, C.; Digby, J.; Al-Azzawi, Y.; Pang, G.; Watson, A.J.M.; Strachan, J.; Mowat, C.; McSorley, S.T. Prevalence of repeat faecal immunochemical testing in symptomatic patients attending primary care. Color. Dis. 2022, 24, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.J.; Banerjea, A.; Humes, D.J.; Allen, J.; Oliver, S.; Ford, A.; Hardy, K.; Djedovic, N.; Logan, R.F.; Morling, J.R. Choice of faecal immunochemical test matters: Comparison of OC-Sensor and HM-JACKarc, in the assessment of patients at high risk of colorectal cancer. Clin. Chem. Lab. Med. 2021, 59, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.E.; Fraser, C.G.; Halloran, S.P.; Young, G.P. Comparing Fecal Immunochemical Tests: Improved Standardization Is Needed. Gastroenterology 2012, 142, 422–424. [Google Scholar] [CrossRef]
- Murphy, C.C.; Sen, A.; Watson, B.; Gupta, S.; Mayo, H.; Singal, A.G. A Systematic Review of Repeat Fecal Occult Blood Tests for Colorectal Cancer Screening. Cancer Epidemiol. Biomark. Prev. 2020, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Georgiou Delisle, T.; D’Souza, N.; Davies, B.; Benton, S.; Chen, M.; Ward, H.; Abulafi, M. Faecal immunochemical test for suspected colorectal cancer symptoms: Patient survey of usability and acceptability. BJGP Open 2021, 6, BJGPO.2021.0102. [Google Scholar] [CrossRef]

| Author | Population |
|---|---|
| Asymptomatic | |
| Abdullah, 2020 [17] | Asymptomatic, Age 35–65 |
| Cai, 2016 [18] | Asymptomatic, Age 40–74 |
| Chubak, 2013 [19] | Asymptomatic, Age 50–74 |
| Cole, 2003 [20] | Asymptomatic, Age 50–69 |
| Dancourt, 2008 [21] | Asymptomatic, Age 50–74 |
| Faivre, 2012 [22] | Asymptomatic, Age 50–74 |
| Fu, 2009 [23] | Asymptomatic, Age 40 and Over |
| Grazzini, 2009 [24] | Asymptomatic, Age 50–69 |
| Guittet, 2009 [25] | Asymptomatic, Age 50–74 |
| Hernandez, 2014 [26] | Asymptomatic, Age 50–69 |
| Kapidzic, 2017 [27] | Asymptomatic, Age 50–74 |
| Kelley, 2013 [28] | Asymptomatic, Age 50–75 |
| Launoy, 2005 [29] | Asymptomatic, Age 50–74 |
| Levi, 2011 [30] | Asymptomatic, Age 50–75 |
| Liles, 2018 [31] | Asymptomatic, Age 49–75 |
| Moosavi, 2016 [32] | Asymptomatic, Age 50–75 |
| Nakama (A), 2000 [33] | Asymptomatic, Age 40 and Over |
| Nakama (B), 2000 [34] | Asymptomatic, Age 40–60 |
| Nakama, 2002 [35] | Asymptomatic, Adult Population |
| Nakazato, 2006 [36] | Asymptomatic, Adult Population |
| Okada, 2020 [37] | Asymptomatic, Age 50–75 |
| Park, 2010 [38] | Asymptomatic, Age 50–74 |
| Raginel, 2013 [39] | Asymptomatic, Age 50–74 |
| Ribbing Wilén, 2019 [40] | Asymptomatic, Age 60 |
| Rutka, 2020 [41] | Asymptomatic, Age 50–70 |
| Sakata, 2014 [42] | Asymptomatic, Age 40 and Over |
| Schreuders, 2019 [43] | Asymptomatic, Age 50–74 |
| Sekiguchi, 2021 [44] | Asymptomatic, Age 49–79 |
| Shapiro, 2017 [45] | Asymptomatic, Age 50–74 |
| Shuhaibar, 2011 [46] | Asymptomatic, Age 50 and Over |
| Smith, 2006 [82] | Asymptomatic, Age 50–75 |
| Tan, 2013 [47] | Asymptomatic, Age 50 and Over |
| Telford, 2016 [48] | Asymptomatic, Age 50–74 |
| Tepeš, 2014 [49] | Asymptomatic, Age 64–68 |
| Tepeš, 2022 [50] | Asymptomatic, Age 50–74 |
| Tourne-Garcia, 2022 [51] | Asymptomatic, Age 50–69 |
| Wang, 2022 [52] | Asymptomatic, Age 40–74 |
| Wong M.C.S, 2015 [53] | Asymptomatic, Age 50–74 |
| Yang, 2011 [54] | Asymptomatic, Mean Age 55.18 ± 15.67 |
| Ye, 2017 [55] | Asymptomatic, Age 40–74 |
| Mixed | |
| Auge, 2013 [56] | Surveillance or Symptomatic, Adult Population |
| Auge, 2016 [57] | Surveillance or Symptomatic, Adult Population |
| Auge, 2018 [58] | Surveillance or Symptomatic, Adult Population |
| Castro, 2013 [59] | High Risk Screening of FDR with CRC, Adult Population |
| Chew, 2009 [60] | Screening, Surveillance or Symptomatic, Adult Population |
| Cruz-Correa, 2007 [61] | Screening, Surveillance, Previous CRC or Significant Family History, Adult Population |
| Guimarães, 2019 [62] | Screening, Surveillance or Symptomatic, Adult Population |
| Hazazi, 2010 [63] | High Risk Screening or Surveillance, Adult Population |
| Kovarova, 2012 [64] | Screening, Surveillance or Symptomatic, Adult Population |
| Li, 2006 [65] | Screening, Surveillance or Symptomatic, Adult Population |
| Oort, 2011 [66] | Screening, Surveillance or Symptomatic, Adult Population |
| Randell, 2013 [67] | High Risk Screening or Symptomatic, Adult Population |
| Redwood, 2014 [68] | Asymptomatic or Surveillance, Adult Population |
| Rozen, 2009 [69] | High Risk Screening, Surveillance or Symptomatic, Adult Population |
| Rozen, 2010 [70] | High Risk Screening, Surveillance or Symptomatic, Adult Population |
| Terhaar sive Droste, 2012 [71] | High Risk Screening or Surveillance, Adult Population |
| Vasilyev, 2015 [72] | Screening, Surveillance or Symptomatic, Adult Population |
| Wong B.C, 2003 [73] | Surveillance or Symptomatic, Adult Population |
| Wong W.M, 2003 [74] | Surveillance or Symptomatic, Adult Population |
| Wu, 2014 [75] | Screening, Surveillance or Symptomatic, Adult Population |
| Young, 2020 [76] | High Risk Screening or Surveillance, Adult Population |
| Symptomatic | |
| Fernández-Bañares, 2019 [77] | Symptomatic, Adult Population |
| Gerrard, 2023 [7] | Symptomatic, Adult Population |
| Högberg, 2020 [78] | Symptomatic, Adult Population |
| Hunt N., 2022 [8] | Symptomatic, Adult Population |
| Mattar, 2020 [79] | Symptomatic, Adult Population |
| Oono, 2010 [80] | Symptomatic, Adult Population |
| Smith, 2006 [82] | Symptomatic, Adult Population |
| Tsapournas, 2020 [81] | Symptomatic, Adult Population |
| Population | Author | CRCs | 1T FN | 2T FN | 3T FN | Relative Reduction in Missed CRC by 2T | Relative Reduction in Missed CRC by 3T |
|---|---|---|---|---|---|---|---|
| A. 10 µg Hb/g: CRC | |||||||
| Asymptomatic | Hernandez, 2014 [26] | 5 | 0 | 0 | - | n/a | - |
| Sekiguchi, 2021 [44] | 10 | 2 | 0 | - | 100% | - | |
| Mixed | Castro, 2014 [59] | 6 | 0 | 0 | - | n/a | - |
| Hazazi, 2010 [63] | 8 | 0 | 0 | 0 | n/a | n/a | |
| Kovarova, 2012 [64] | 35 | 4 | 4 | - | 0.0% | - | |
| Randell, 2013 [67] | 2 | 0 | 0 | - | n/a | - | |
| Rozen, 2010 [70] | 20 | 5 | 1 | 0 | 80.0% | 100% | |
| Symptomatic | Gerrard, 2023 [7] | 88 | 6 | 3 | - | 50.0% | - |
| Tsapournas, 2020 [81] | 13 | 1 | 0 | - | 100% | - | |
| B. 20 µg Hb/g: CRC | |||||||
| Asymptomatic | Hernandez, 2014 [26] | 5 | 0 | 0 | - | n/a | - |
| Park, 2010 [38] | 13 | 4 | 2 | 2 | 50.0% | 50.0% | |
| Sekiguchi, 2021 [44] | 10 | 2 | 0 | - | 100% | - | |
| Mixed | Castro, 2014 [59] | 6 | 0 | 0 | - | n/a | - |
| Hazazi, 2010 [63] | 8 | 1 | 1 | 0 | 0.0% | 100% | |
| Kovarova, 2012 [64] | 35 | 5 | 5 | - | 0.0% | - | |
| Randell, 2013 [67] | 2 | 0 | 0 | - | n/a | - | |
| Rozen, 2010 [70] | 20 | 7 | 4 | 5 | 42.9% | 71.4% | |
| Symptomatic | Gerrard, 2023 [7] | 88 | 12 | 9 | - | 25.0% | - |
| Tsapournas, 2020 [81] | 13 | 2 | 1 | - | 50.0% | - | |
| Population | Author | ACRNs | 1T FN | 2T FN | 3T FN | Relative Reduction in Missed ACRN by 2T | Relative Reduction in Missed ACRN by 3T |
| C. 10 µg Hb/g: ACRN | |||||||
| Asymptomatic | Hernandez, 2014 [26] | 97 | 63 | 56 | - | 11.1% | - |
| Liles, 2018 [31] | 211 | 163 | 148 | - | 9.2% | - | |
| Ribbing Wilén, 2019 [40] | 81 | 65 | 60 | - | 7.7% | - | |
| Sekiguchi, 2021 [44] | 112 | 85 | 74 | - | 12.9% | - | |
| Mixed | Auge, 2016 [57] | 29 | 19 | 17 | 10.5% | - | |
| Auge, 2018 [58] | 71 | 39 | 33 | - | 13.3% | ||
| Castro, 2014 [59] | 64 | 34 | 32 | - | 5.9% | - | |
| Hazazi, 2010 [63] | 80 | 44 | 34 | 28 | 22.5% | 37.6% | |
| Kovarova, 2012 [64] | 63 | 15 | 14 | - | 6.7% | - | |
| Oort, 2011 [66] | 124 | 65 | 57 | - | 12.3% | - | |
| Randell, 2013 [67] | 6 | 1 | 0 | - | 100% | - | |
| Rozen, 2009 [69] | 32 | 15 | 10 | 8 | 33.5% | 46.7% | |
| Rozen, 2010 [70] | 149 | 87 | 67 | 58 | 22.9% | 33.4% | |
| Young, 2020 [76] | 626 | 350 | 252 | - | 28.0% | - | |
| Symptomatic | Gerrard, 2023 [7] | 185 | 48 | 34 | - | 29.2% | - |
| Tsapournas, 2020 [81] | 28 | 8 | 7 | - | 12.5% | - | |
| D. 20 µg Hb/g: ACRN | |||||||
| Asymptomatic | Hernandez, 2014 [26] | 97 | 66 | 61 | - | 7.6% | - |
| Liles, 2018 [31] | 211 | 181 | 170 | - | 5.9% | - | |
| Park, 2010 [38] | 72 | 49 | 45 | 41 | 9.0% | 17.3% | |
| Sekiguchi, 2021 [44] | 112 | 87 | 78 | - | 10.4% | ||
| Mixed | Auge, 2016 [57] | 29 | 20 | 19 | - | 5.1% | - |
| Auge, 2018 [58] | 71 | 45 | 38 | - | 15.9% | - | |
| Castro, 2014 [59] | 64 | 38 | 36 | - | 5.3% | - | |
| Hazazi, 2010 [63] | 80 | 52 | 42 | 32 | 19.1% | 38.3% | |
| Kovarova, 2012 [64] | 63 | 18 | 16 | - | 11.2% | - | |
| Oort, 2011 [66] | 124 | 68 | 60 | - | 11.8% | - | |
| Randell, 2013 [67] | 6 | 2 | 2 | - | 0.0% | - | |
| Rozen, 2009 [69] | 32 | 17 | 14 | 10 | 17.7% | 41.2% | |
| Rozen, 2010 [70] | 149 | 102 | 85 | 73 | 16.8% | 28.5% | |
| Young, 2020 [76] | 626 | 413 | 286 | - | 30.8% | - | |
| Symptomatic | Gerrard, 2023 [7] | 185 | 64 | 46 | - | 28.1% | - |
| Tsapournas, 2020 [81] | 28 | 11 | 9 | - | 18.2% | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerrard, A.D.; Garau, R.; Xu, W.; Maeda, Y.; Dunlop, M.G.; Theodoratou, E.; Din, F.V.N. Repeat Faecal Immunochemical Testing for Colorectal Cancer Detection in Symptomatic and Screening Patients: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 3199. https://doi.org/10.3390/cancers16183199
Gerrard AD, Garau R, Xu W, Maeda Y, Dunlop MG, Theodoratou E, Din FVN. Repeat Faecal Immunochemical Testing for Colorectal Cancer Detection in Symptomatic and Screening Patients: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(18):3199. https://doi.org/10.3390/cancers16183199
Chicago/Turabian StyleGerrard, Adam D., Roberta Garau, Wei Xu, Yasuko Maeda, Malcolm G. Dunlop, Evropi Theodoratou, and Farhat V. N. Din. 2024. "Repeat Faecal Immunochemical Testing for Colorectal Cancer Detection in Symptomatic and Screening Patients: A Systematic Review and Meta-Analysis" Cancers 16, no. 18: 3199. https://doi.org/10.3390/cancers16183199
APA StyleGerrard, A. D., Garau, R., Xu, W., Maeda, Y., Dunlop, M. G., Theodoratou, E., & Din, F. V. N. (2024). Repeat Faecal Immunochemical Testing for Colorectal Cancer Detection in Symptomatic and Screening Patients: A Systematic Review and Meta-Analysis. Cancers, 16(18), 3199. https://doi.org/10.3390/cancers16183199





