Novel Treatment Strategies for Low-Risk Metastatic Castration-Sensitive Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Collection of Clinical Data
2.3. Statistical Analyses
2.4. Ethical Considerations
3. Results
3.1. Patient Characteristics
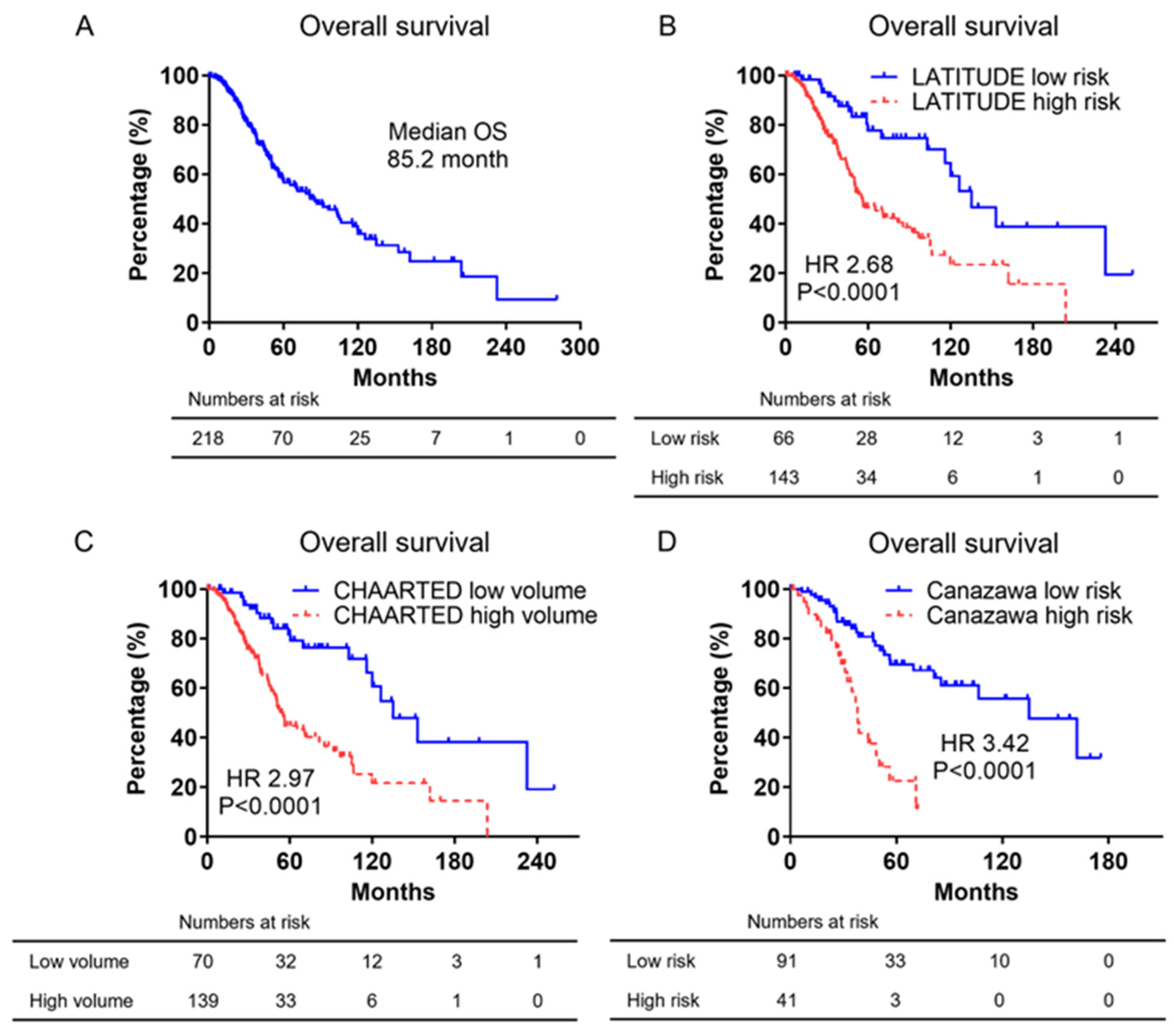
3.2. Kaplan–Meier Curves of Overall Survival
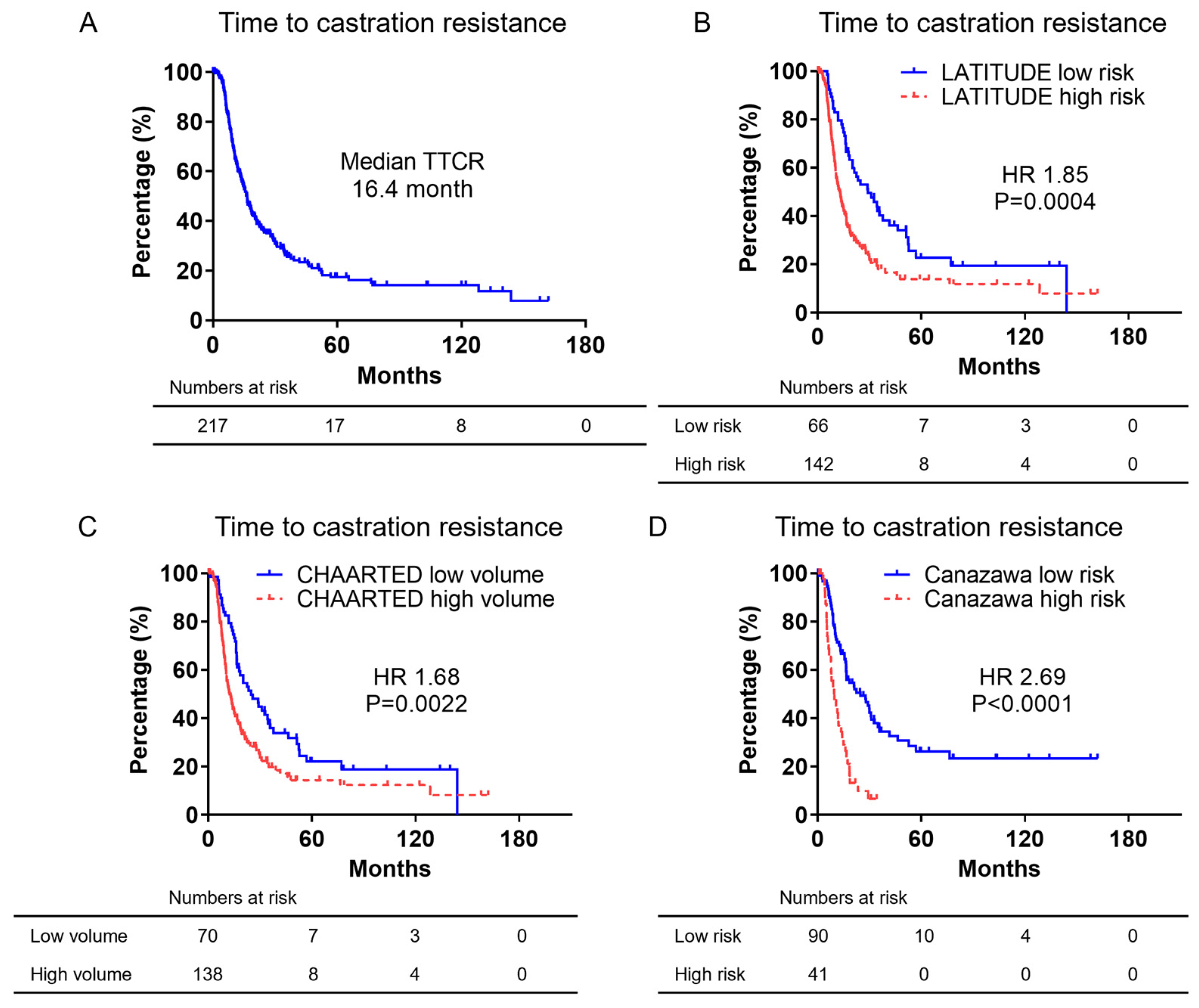
3.3. Kaplan–Meier Curves of Time to Castration Resistance
3.4. Identification of Prognostic Factors in Time to Castration Resistance in Low-Risk or Low-Volume mCSPC
3.5. Kaplan–Meier Curve of Time to Castration Resistance in Low-Risk or Low-Volume mCSPC by PSA Reduction Rate after 12 Weeks of ADT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Izumi, K.; Shimada, T.; Kano, H.; Kadomoto, S.; Makino, T.; Naito, R.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Androgen receptor signaling-targeted therapy and taxane chemotherapy induce visceral metastasis in castration-resistant prostate cancer. Prostate 2021, 81, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Schroder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Prostate-cancer mortality at 11 years of follow-up. N. Engl. J. Med. 2012, 366, 981–990. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J. Urol. 2002, 167, 948–951, discussion 952. [Google Scholar] [CrossRef]
- Akaza, H.; Hinotsu, S.; Usami, M.; Arai, Y.; Kanetake, H.; Naito, S.; Hirao, Y. Combined androgen blockade with bicalutamide for advanced prostate cancer: Long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 2009, 115, 3437–3445. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Hinotsu, S.; Namiki, M.; Carroll, P.R.; Akaza, H. Trans-Pacific variation in outcomes for men treated with primary androgen-deprivation therapy (ADT) for prostate cancer. BJU Int. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- Fukagai, T.; Namiki, T.S.; Carlile, R.G.; Yoshida, H.; Namiki, M. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006, 97, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley Sons Ltd.: Oxford, UK, 2017; pp. 191–192. [Google Scholar]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Nakagawa, R.; Iwamoto, H.; Makino, T.; Naito, R.; Kadomoto, S.; Akatani, N.; Yaegashi, H.; Kawaguchi, S.; Nohara, T.; Shigehara, K.; et al. Development of a Prognostic Model of Overall Survival for Metastatic Hormone-Naïve Prostate Cancer in Japanese Men. Cancers 2022, 14, 4822. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Terada, N.; Kitamura, H.; Kojima, T.; Saito, T.; Yokomizo, A.; Kohei, N.; Goto, T.; Kawamura, S.; Hashimoto, Y.; et al. Novel metastatic burden-stratified risk model in de novo metastatic hormone-sensitive prostate cancer. Cancer Sci. 2021, 112, 3616–3626. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, S.; Kubota, M.; Uozumi, R.; Narita, S.; Takahashi, M.; Mitsuzuka, K.; Hatakeyama, S.; Sakurai, T.; Kawamura, S.; Ishidoya, S.; et al. Development and Validation of a Novel Prognostic Model for Predicting Overall Survival in Treatment-naïve Castration-sensitive Metastatic Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 320–328. [Google Scholar] [CrossRef]
- Nakajima, K.; Edenbrandt, L.; Mizokami, A. Bone scan index: A new biomarker of bone metastasis in patients with prostate cancer. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2017, 24, 668–673. [Google Scholar] [CrossRef]
- Imbriaco, M.; Larson, S.M.; Yeung, H.W.; Mawlawi, O.R.; Erdi, Y.; Venkatraman, E.S.; Scher, H.I. A new parameter for measuring metastatic bone involvement by prostate cancer: The Bone Scan Index. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1998, 4, 1765–1772. [Google Scholar]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Cheung, L.; Chi, K.N.; Chowdhury, S.; Frydenberg, M.; Horvath, L.G.; Joshua, A.M.; et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 323–334. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- Naiki, T.; Takahara, K.; Ito, T.; Nakane, K.; Sugiyama, Y.; Koie, T.; Shiroki, R.; Miyake, H.; Yasui, T. Comparison of clinical outcomes between androgen deprivation therapy with up-front abiraterone and bicalutamide for Japanese patients with LATITUDE high-risk prostate cancer in a real-world retrospective analysis. Int. J. Clin. Oncol. 2022, 27, 592–601. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Kimura, T.; Mori, K.; Suzuki, H.; Sano, T.; Otsuka, T.; Iwamoto, Y.; Fukuokaya, W.; Miyajima, K.; Enei, Y.; et al. Abiraterone acetate versus nonsteroidal antiandrogen with androgen deprivation therapy for high-risk metastatic hormone-sensitive prostate cancer. Prostate 2022, 82, 3–12. [Google Scholar] [CrossRef]
- Watanabe, H.; Nakane, K.; Takahara, K.; Naiki, T.; Yasui, T.; Shiroki, R.; Koie, T.; Miyake, H. Prognostic outcomes in Japanese patients with metastatic castration-sensitive prostate cancer: Comparative assessments between conventional androgen deprivation therapy (ADT) and ADT with novel androgen receptor signal inhibitor. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2024, 31, 986–993. [Google Scholar] [CrossRef]
- Osawa, T.; Sasaki, K.; Machida, R.; Matsumoto, T.; Matsui, Y.; Kitamura, H.; Nishiyama, H. Real-world treatment trends for patients with advanced prostate cancer and renal cell carcinoma and their cost-a survey in Japan. Jpn. J. Clin. Oncol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Fukuokaya, W.; Yanagisawa, T.; Mori, K.; Urabe, F.; Rajwa, P.; Briganti, A.; Shariat, S.F.; Kimura, T. Radiographic Progression Without Corresponding Prostate-specific Antigen Progression in Patients with Metastatic Castration-sensitive Prostate Cancer Receiving Apalutamide: Secondary Analysis of the TITAN Trial. Eur. Urol. Oncol. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Iwamoto, H.; Nakagawa, R.; Makino, T.; Kadomoto, S.; Yaegashi, H.; Nohara, T.; Shigehara, K.; Izumi, K.; Kadono, Y.; Mizokami, A. Treatment Outcomes in Neuroendocrine Prostate Cancer. Anticancer. Res. 2022, 42, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ueno, S.; Izumi, K.; Kadono, Y.; Mizokami, A.; Hinotsu, S.; Akaza, H.; Namiki, M. Clinical outcomes and nadir prostate-specific antigen (PSA) according to initial PSA levels in primary androgen deprivation therapy for metastatic prostate cancer. World J. Urol. 2016, 34, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Teoh, J.Y.; Tsu, J.H.; Yuen, S.K.; Chan, S.Y.; Chiu, P.K.; Wong, K.W.; Ho, K.L.; Hou, S.S.; Ng, C.F.; Yiu, M.K. Survival outcomes of Chinese metastatic prostate cancer patients following primary androgen deprivation therapy in relation to prostate-specific antigen nadir level. Asia Pac. J. Clin. Oncol. 2017, 13, e65–e71. [Google Scholar] [CrossRef]
- Hu, M.; Mao, Y.; Guan, C.; Tang, Z.; Bao, Z.; Li, Y.; Liang, G. Dynamic changes in PSA levels predict prognostic outcomes in prostate cancer patients undergoing androgen -deprivation therapy: A multicenter retrospective analysis. Front. Oncol. 2023, 13, 1047388. [Google Scholar] [CrossRef]


| LATITUDE | CHAARTED | Canazawa | All | ||||
|---|---|---|---|---|---|---|---|
| Characteristics | Low-Risk | High-Risk | Low-Volume | High- Volume | Low-Risk | High-Risk | |
| Patients, n | 66 | 143 | 70 | 139 | 91 | 41 | 218 |
| Median age, yr | 71.5 | 71.0 | 70.0 | 71.0 | 71 | 73 | 71.5 |
| Median PSA, ng/mL | 103.7 | 331.8 | 117.0 | 274.5 | 200.5 | 851.8 | 239 |
| Median LDH, IU/L | 178.5 | 194.5 | 180 | 191 | 186 | 212 | 189 |
| Median ALP, IU/L | 232 | 408 | 262.5 | 403.5 | 271 | 623 | 353 |
| Histology, n (%) | |||||||
| GS ≤ 7 | 19 (28.8) | 10 (7.0) | 15 (21.4) | 14 (10.1) | 16 (17.6) | 0 | 32 (14.7) |
| GS ≥ 8 | 45 (68.2) | 132 (92.3) | 53 (75.7) | 124 (89.2) | 75 (82.4) | 41 (100) | 183 (83.9) |
| Unknown | 2 (3.0) | 1 (0.7) | 2 (2.9) | 1 (0.7) | 0 | 0 | 3 (1.4) |
| T stage, n (%) | |||||||
| T1–2 | 8 (12.1) | 10 (7.0) | 6 (8.6) | 12 (8.6) | 10 (11.0) | 4 (9.8) | 21 (9.6) |
| T3 | 20 (30.3) | 68 (47.6) | 23 (32.9) | 65 (46.8) | 47 (51.6) | 16 (39.0) | 90 (41.3) |
| T4 | 31 (47.0) | 51 (35.7) | 32 (45.7) | 50 (36.0) | 29 (31.9) | 18 (43.9) | 85 (39.0) |
| Tx | 7 (10.6) | 14 (9.8) | 9 (12.9) | 12 (8.6) | 5 (5.5) | 3 (7.3) | 22 (10.1) |
| N stage, n (%) | |||||||
| N0 | 31 (47.0) | 51 (35.7) | 31 (44.3) | 51 (36.7) | 33 (36.3) | 16 (39.0) | 86 (39.4) |
| N1 | 35 (53.0) | 88 (61.5) | 38 (54.3) | 85 (61.2) | 57 (62.6) | 25 (61.0) | 128 (58.7) |
| Nx | 0 | 4 (2.8) | 1 (1.4) | 3 (2.2) | 1 (1.1) | 0 | 4 (1.8) |
| M stage, n (%) | |||||||
| M1a | 6 (9.1) | 1 (0.7) | 6 (8.6) | 1 (0.7) | 2 (2.2) | 0 | 7 (3.2) |
| M1b | 59 (89.4) | 118 (82.5) | 64 (91.4) | 113 (81.3) | 78 (85.7) | 37 (90.2) | 186 (85.3) |
| M1c | 1 (1.5) | 24 (16.8) | 0 | 25 (18.0) | 11 (12.1) | 4 (9.8) | 25 (11.5) |
| Initial treatment, n (%) | |||||||
| CAB | 66 (100) | 142 (99.3) | 70 (100) | 138 (99.3) | 91 (100) | 41 (100) | 215 (98.6) |
| Surgical castration | 0 | 1 (0.7) | 0 | 1 (0.7) | 0 | 0 | 1 (0.5) |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.9) |
| CRPC progression, n (%) | 45 (68.2) | 110 (76.9) | 49 (70) | 106 (76.3) | 59 (64.8) | 35 (85.4) | 160 (73.4) |
| All-cause death, n (%) | 21 (31.8) | 74 (51.7) | 21 (30.0) | 74 (53.2) | 30 (33.0) | 26 (63.4) | 98 (45.0) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| n | HR (95% CI) | p | HR (95% CI) | p | ||
| PSA reduction rate, ng/mL | ≥95% | 33 | 2.44 (1.09–5.47) | 0.03 | 4.70 (1.39–15.88) | 0.013 |
| <95% | 9 | |||||
| LDH at 12 W after ADT, IU/L | <250 or reduction rate ≥ 30% | 26 | 0.90 (0.26–3.15) | 0.87 | 4.20 (0.996–17.69) | 0.051 |
| ≥250 and reduction rate < 30% | 3 | |||||
| ALP at 12 W after ADT, IU/L | <350 or reduction rate ≥ 30% | 24 | 2.98 (0.83–10.72) | 0.1 | 1.37 (0.33–5.65) | 0.66 |
| ≥350 and reduction rate < 30% | 5 | |||||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| n | HR (95% CI) | p | HR (95% CI) | p | ||
| PSA reduction rate, ng/mL | ≥95% | 32 | 2.21 (1.02–4.78) | 0.04 | 3.57 (1.17–10.95) | 0.026 |
| <95% | 10 | |||||
| LDH at 12 W after ADT, IU/L | <250 or reduction rate ≥ 30% | 25 | 2.73 (0.77–9.74) | 0.12 | 3.46 (0.86–14.02) | 0.082 |
| ≥250 and reduction rate < 30% | 3 | |||||
| ALP at 12 W after ADT, IU/L | <350 or reduction rate ≥ 30% | 22 | 1.01 (0.33–3.09) | 0.98 | 1.18 (0.36–3.86) | 0.79 |
| ≥350 and reduction rate < 30% | 6 | |||||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| n | HR (95% CI) | p | HR (95% CI) | p | ||
| PSA reduction rate, ng/mL | ≥95% | 58 | 2.17 (1.16–4.05) | 0.016 | 2.66 (1.29–5.50) | 0.008 |
| <95% | 15 | |||||
| LDH at 12 W after ADT, IU/L | <250 or reduction rate ≥ 30% | 61 | 1.91 (0.75–4.86) | 0.18 | 2.28 (0.81–6.43) | 0.12 |
| ≥250 and reduction rate < 30% | 6 | |||||
| ALP at 12 W after ADT, IU/L | <350 or reduction rate ≥ 30% | 60 | 1.13 (0.48–2.69) | 0.77 | 1.11 (0.42–2.94) | 0.84 |
| ≥350 and reduction rate < 30% | 8 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, H.; Hori, T.; Nakagawa, R.; Kano, H.; Makino, T.; Naito, R.; Yaegashi, H.; Kawaguchi, S.; Nohara, T.; Shigehara, K.; et al. Novel Treatment Strategies for Low-Risk Metastatic Castration-Sensitive Prostate Cancer. Cancers 2024, 16, 3198. https://doi.org/10.3390/cancers16183198
Iwamoto H, Hori T, Nakagawa R, Kano H, Makino T, Naito R, Yaegashi H, Kawaguchi S, Nohara T, Shigehara K, et al. Novel Treatment Strategies for Low-Risk Metastatic Castration-Sensitive Prostate Cancer. Cancers. 2024; 16(18):3198. https://doi.org/10.3390/cancers16183198
Chicago/Turabian StyleIwamoto, Hiroaki, Tomohiro Hori, Ryunosuke Nakagawa, Hiroshi Kano, Tomoyuki Makino, Renato Naito, Hiroshi Yaegashi, Shohei Kawaguchi, Takahiro Nohara, Kazuyoshi Shigehara, and et al. 2024. "Novel Treatment Strategies for Low-Risk Metastatic Castration-Sensitive Prostate Cancer" Cancers 16, no. 18: 3198. https://doi.org/10.3390/cancers16183198
APA StyleIwamoto, H., Hori, T., Nakagawa, R., Kano, H., Makino, T., Naito, R., Yaegashi, H., Kawaguchi, S., Nohara, T., Shigehara, K., Izumi, K., & Mizokami, A. (2024). Novel Treatment Strategies for Low-Risk Metastatic Castration-Sensitive Prostate Cancer. Cancers, 16(18), 3198. https://doi.org/10.3390/cancers16183198








