The Adenosinergic Pathway in Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
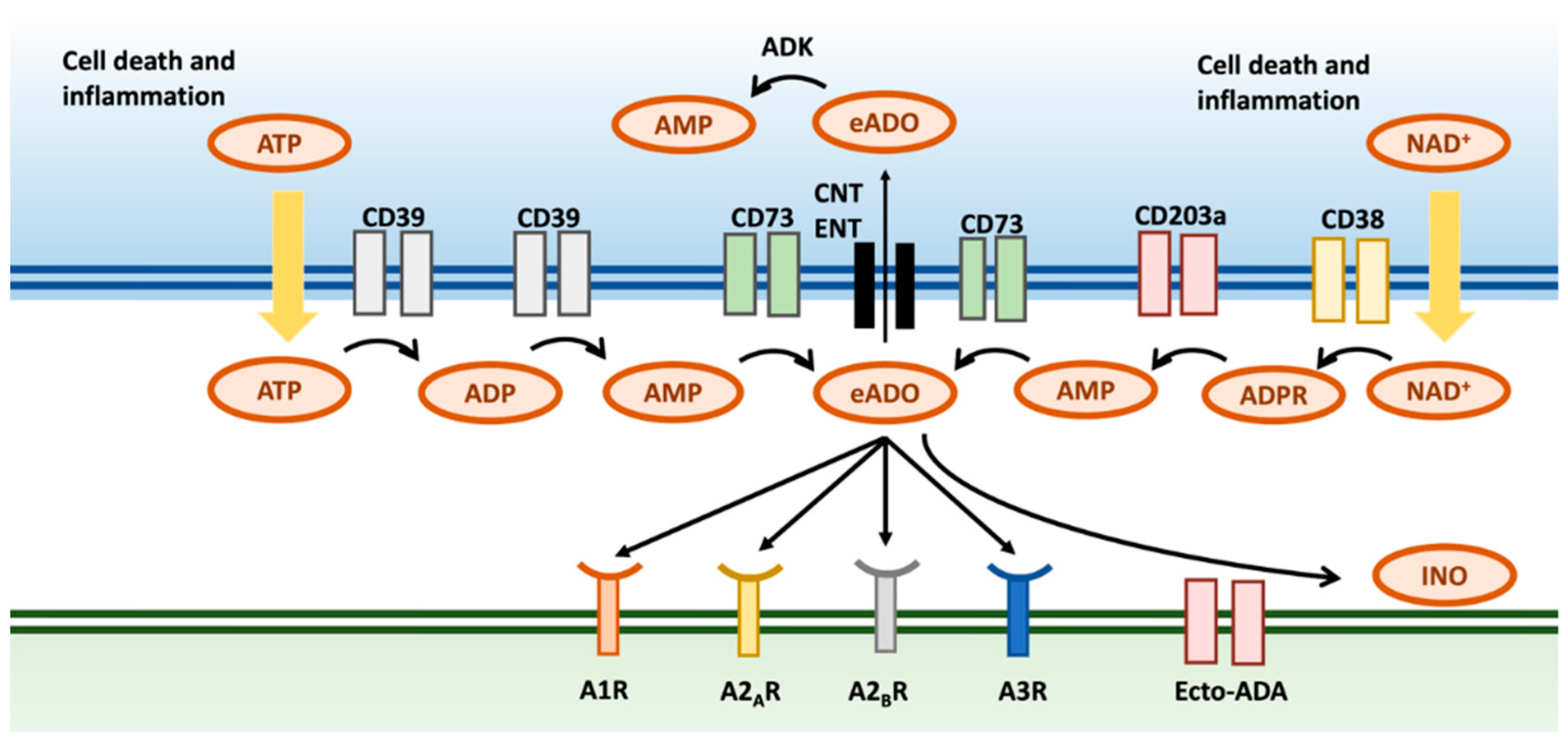
2. Generation and Metabolism of Adenosine
2.1. Adenosine Is Formed by a Canonical and a Non-Canonical Pathway
2.2. Hypoxia Induces the Formation of eADO
2.3. The Formation of eADO Is Upregulated within the TME
2.4. eADO Is Either Degraded in the Extracellular Space or Transported Intracellularly
2.5. Overview of the Four Adenosine Receptors
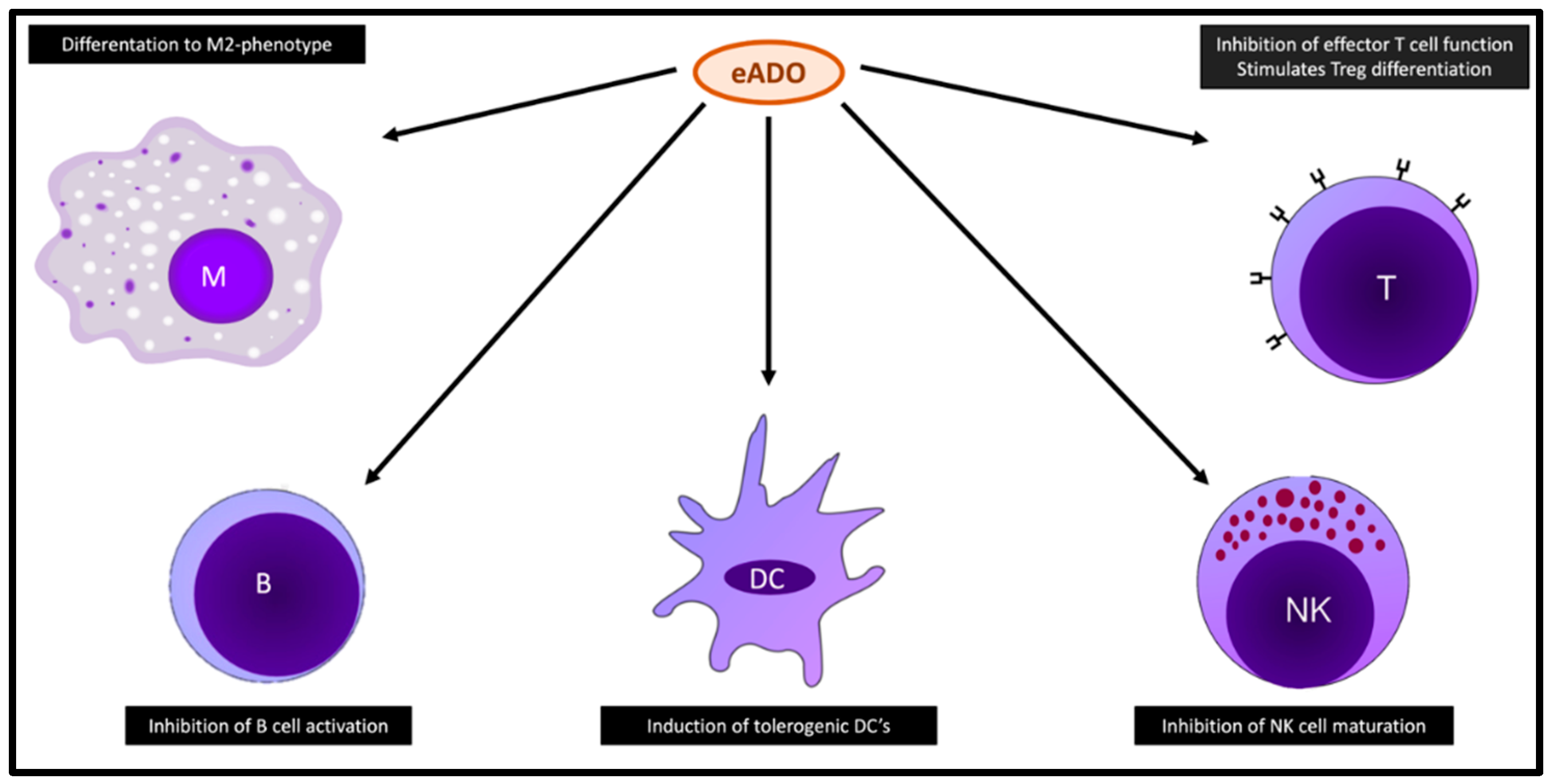
3. Functions of eADO
3.1. eADO and Immune Cells
3.2. eADO and Tumor Cells
3.3. eADO and Other Cells within the TME
4. Preclinical Data on Adenosine Pathway Inhibition
4.1. CD73 Inhibitors
4.2. CD39 Inhibitors
4.3. A2A Receptor Antagonists
4.4. A2B and Dual A2/A3 Receptor Antagonists
5. Clinical Data on Adenosine Pathway Inhibition
5.1. CD73 Inhibition
5.1.1. Oleclumab/MEDI9447
5.1.2. Mupadolimab/CPI-006
5.1.3. BMS-986179
5.1.4. Uliledlimab/TJD5
5.1.5. NZV930
5.2. A2A Receptor Inhibition
5.2.1. Taminadenant/PBF509/NIR178
5.2.2. Imaradenant/AZD4635
5.2.3. Ciforadenant/CPI-444
5.2.4. Inupadenant/EOS850
5.3. A2B Receptor Inhibition
PBF-1129
5.4. Dual A2A and A2B Receptor Inhibition
Etrumadenant/AB928
| Molecule | Target | Phase | Trial Setup | Target Population | NSCLC /Total | Primary Endpoints | Secondary Endpoints | Reference |
|---|---|---|---|---|---|---|---|---|
| MEDI9447 Oleclumab | CD73 | I | Monotherapy, dose finding | Advanced malignancies, refractory to SOC | 0/6 | Safety: 1 grade 3 AE, no dose reductions, no deaths | ORR and DCR at week 8: ORR 0%; DCR 0% | Kondo et al. [108] |
| MEDI9447 Oleclumab | CD73 | I | Monotherapy (dose escalation) or oleclumab + durvalumab | Advanced malignancies, at least 1 prior line of therapy; NSCLC had to be EGFRm | 42/192 (only oleclumab + durvalumab arm) | Safety and optimal dosing: grade 3/4 AEs in 14.5% of all patients; 1 treatment-related death in the colorectal group; fatigue and rash most common in the NSCLC group | ORR in the NSCLC group: 9.5% (4 PR); 6-month PFS rate in the NSCLC group: 16% | Bendell et al. [109] |
| MEDI9447 Oleclumab | CD73 | II | Durvalumab mono vs. durvalumab + oleclumab vs. durvalumab + monalizumab | Stage III unresectable NSCLC, no progression after cCRT | 189/189 | ORR: 30% (D + O); 35.5% (D + M); 17.9% (D mono) | DCR at week 16: 81.7% (D + O), 77.4% (D + M), 58.3 (D mono); Median PFS: NR (D + O), 15.1 m (D + M), 6.3 m (D mono); Safety: similar toxicity in all 3 arms, 4 deaths due to study drug | Herbst et al. [87] |
| MEDI9447 Oleclumab | CD73 | II | Neoadjuvant therapy. Durvalumab mono vs. durvalumab + oleclumab vs. durvalumab + monalizumab vs. durvalumab + danvatirsen | Stage IA3—IIIA resectable NSCLC | 84/84 | MPR: 11.1% (D mono), 19% (D + O), 30% (D + M), 31.3% (D + D) | Safety: no added toxicity of the combination groups compared to monotherapy | Cascone et al. [90] |
| MEDI9447 Oleclumab | CD73 | Ib/II | Oleclumab + osimertinib, dose finding | Advanced EGFRm and tissue T790M-negative NSCLC, progression on a 1/2 gen TKI | 26/26 | Safety: 1 treatment discontinuation due to pneumonitis, no grade 4/5 TRAEs; ORR: PR in 6 patients, higher ORR and DCR in patients negative for T790M on both tissue and ctDNA | / | Kim et al. [86] |
| MEDI9447 Oleclumab | CD73 | II | NACT + SABR combined with durvalumab or durvalumab + oleclumab | Operable high-risk luminal B breast cancer | 0/136 | Residual cancer burden on surgical specimen, results ongoing | ORR primary tumor, ORR pathologic lymph nodes; Safety: results ongoing | De Caluwé et al. [110] |
| CPI-006 Mupadolimab | CD73 | I | Dose escalation: mupadolimab monotherapy and in combination with ciforadenant | Relapsed advanced cancers | ?/17 | Optimal dosing and safety: no DLTs | Tumor reduction seen in 1 patient with prostate cancer; favorable effect on peripheral lymphocytes | Luke et al. [91] |
| TJD5 Uliledlimab | CD73 | Ib/II | 2 different doses of uliledlimab in combination with toripalimab | Treatment naïve NSCLC without driver mutations | 66/66 | Safety: grade 3 AEs in 15.2% of patients; 1 treatment discontinuation | ORR: 31.3% and 50% in CD73 high cohort; DCR: 79.2% | Zhou et al. [93] |
| NZV930 * | CD73 | I | Dose escalation: NZV930 monotherapy vs. combination with spartalizumab vs. combo with taminadenant vs. combo with S + T | Advanced cancers, progression on standard therapy | 8/105 | Safety: grade 3 AEs in 14% of patients, DLTs in 6.7% of patients | ORR: 0%; DCR: 11% | Fu et al. [94] |
| BMS-986179 | CD73 | I/IIa | BMS-986179 + Nivolumab, after 2-week monotherapy lead-in | Advanced cancers | ?/59 | Safety: grade 3 AEs in 15% of patients; 1 treatment discontinuation; no grade 4/5 AEs reported | ORR: 11.8%; DCR: 28.8% | Siu et al. [92] |
| TTX-030 | CD39 | TTX-030 + budigalimab + FOLFOX | Locally advanced or metastatic gastric/gastro-esophageal junction carcinoma | 0/44 | Safety: grade 3/4 AEs in 11% of patients; no grade 5 toxicity | ORR: 61% | Wainberg et al. [111] | |
| CPI-444 Ciforadenant | A2A receptor | Ib/II | Ciforadenant + atezolizumab vs. docetaxel | Advanced NSCLC, progression on platinum-doublet and PD-(L)1 inhibition | 29/29 | ORR: 6.7% (C + A), 21.4% (D); Safety: no grade 5 AEs or AEs leading to treatment discontinuation in the experimental arm | Median PFS: 2.3 m (C + A); 3.2 m (D) | Felip et al. [99] |
| CPI-444 Ciforadenant | A2A receptor | I/Ib | Ciforadenant mono and ciforadenant + atezolizumab | Advanced cancers (including NSCLC), at least 1 and no more than 5 prior therapies | 26/34 | Safety: 1 grade 3 AE, no dose reductions, no deaths; DCR at week 8: 36% (C mono), 71% (C + A) | / | Fong et al. [98] |
| CPI-444 Ciforadenant | A2A receptor | I/Ib | Ciforadenant mono and ciforadenant + atezolizumab | Advanced RCC, progression on at 1 least 1 prior therapy | 0/68 | Safety: 9 grade 3/4 AEs, no dose reductions, no deaths; DCR at month 6: 17% (C mono), 39% (C + A) | / | Fong et al. [100] |
| CPI-444 Ciforadenant | A2A receptor | I/Ib | Ciforadenant mono and ciforadenant + atezolizumab | Advanced mCRPC, progression on at least 1 prior therapy | 0/33 | Safety: 2 grade 3/4 AEs, no dose reductions, no deaths; ORR: 0% (C mono), 1 PR in the combination arm | / | Harshman et al. [101] |
| PBF509/NIR178 Taminadenant | A2A receptor | I/Ib | Taminadenant mono and taminadenant + spartalizumab | Advanced NSCLC, at least 1 prior line of therapy | 50/50 | Determination of DLTs and MTD | Safety: 13 grade 3/4 AEs among both arms, 3 SAEs among both arms leading to treatment discontinuation; DCR at data cutoff: 42.9% (T mono), 66.7% (T + S) | Chiappori et al. [95] |
| PBF509/NIR178 Taminadenant | A2A receptor | II | Taminadenant continuous vs. taminadenant intermittent 2 weeks vs. taminadenant intermittent 1 week combined with spartalizumab | Advanced NSCLC, ICI-naïve, 1–3 prior lines of therapy | 62/62 | ORR: 9% (C); 0% (Int2); 10% (Int1) | Safety: 1 grade 3/4 AE in each treatment arm, no treatment discontinuation or deaths | Lin et al. [96] |
| AZD4635 Imaradenant | A2A receptor | Ia/Ib | Imaradenant and Imaradenant + durvalumab | Dose expansion phase: 1 cohort NSCLC post-ICI | 30/250 | Safety: 3 grade 3 AEs among both arms, 1 event of sudden death in colorectal cohort | ORR: 0% in both arms; DCR at 22 weeks: 6.7% (I), 20% (I + D) | Lim et al. [97] |
| AZD4635 Imaradenant | A2A receptor | I | Imaradenant monotherapy | Advanced malignancies, at least 1 prior line of therapy | 0/10 | Safety: no grade 3 AEs, no dose reduction, 2 AEs leading to a temporary dose interruption | ORR: 0%; DCR at week 15: 0% | Matsubara et al. [112] |
| EOS-850 Inupadenant | A2A receptor | I | Inupadenant monotherapy | Advanced malignancies, dose expansion trial | ?/42 | Optimal dosing; Safety: 7 SAEs leading to treatment discontinuation, no dose reductions | ORR: 4.8%; DCR: 33.3% | Buisseret et al. [102] |
| EOS-850 Inupadenant | A2A receptor | II | Part 1: carboplatin + pemetrexed + inupadenant dose finding—Part 2: C + P + inupadenant vs. C + P + placebo | NSQ metastatic NSCLC, chemo-naïve and progressive on ICI | 40 + 150/190 | Part 1: RP2D, results pending; Part 2: PFS, results ongoing | ORR, OS, and AEs: results ongoing | O’Brien et al. [103] |
| PBF-1129 | A2B receptor | I | Dose escalation trial of PBF-1129 monotherapy | Advanced NSCLC, progression on chemotherapy and immunotherapy | 21/21 | Safety: no DLTs, 3 grade 3 AEs | ORR: 0%—DCR: 14.2%—PFS: 1.5 months—mOS: 4.6 months | Evans et al. [81] |
| AB928 Etrumadenant | Dual A2A and A2B receptor | I/Ib | Dose finding: etrumadenant + carbo-pem-pembro; dose expansion: etrumadenant + carbo-pem-zimberelimab | Ph 1: NSCLC with genetic alteration and chemo-ICI naïve; Ph Ib: EGFRm | 11/11 | Safety: 2 SAEs were noted | PR was achieved in 4 patients in total | Spira et al. (ARC-4) [104] |
| AB928 Etrumadenant | Dual A2A and A2B receptor | II | Zimberelimab vs. domvanalimab + zimberelimab vs. domvanalimab + zimberelimab + etrumadenant | Treatment naïve NSCLC without driver mutations | 149/149 (133 patients analyzed) | ORR: 12% (Z), 18% (D + Z), 18% (D + E + Z); mPFS: 5.4 m (Z), 12 m (D + Z), 10.9 m (D + E + Z) | Safety: grade 3 AEs in 58% (Z), 47% (D + Z), and 52% (D + E + Z) of patients | Johnson et al. (ARC-7) [107] |
| AB928 Etrumadenant | Dual A2A and A2B receptor | II | Domvanalimab + zimberelimab + sacituzumab govitecan vs. domvanalimab + zimberelimab + etrumadenant | Treatment naïve NSCLC without driver mutations | 69 to 289 patients to be enrolled | ORR: results ongoing | PFS, OS, and safety: results ongoing | Spira et al. (VELOCITY-lung) [106] |
| CF-102 Namodenoson | A3 receptor | II | Namodenoson vs. placebo | Hepatocellular carcinoma in Child B cirrhosis | 0/78 | mOS: 4.1 m (N) vs. 4.3 m (P) | ORR: 9% (N) vs. 0% (P); Safety: no treatment discontinuations or deaths | Stemmer et al. [113] |
6. Combination Strategies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. WHO Fact Sheet on Lung Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer#:~:text=GLOBOCAN%202020%20estimates%20of%20cancer,deaths%20(18%25)%20in%202020 (accessed on 13 July 2023).
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab plus Ipilimumab Combined with Two Cycles of Chemotherapy in Patients with Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Passaro, A.; Brahmer, J.; Antonia, S.; Mok, T.; Peters, S. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J. Clin. Oncol. 2022, 40, 598–610. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front. Pharmacol. 2021, 12, 688625. [Google Scholar] [CrossRef] [PubMed]
- Genova, C.; Dellepiane, C.; Carrega, P.; Sommariva, S.; Ferlazzo, G.; Pronzato, P.; Gangemi, R.; Filaci, G.; Coco, S.; Croce, M. Therapeutic Implications of Tumor Microenvironment in Lung Cancer: Focus on Immune Checkpoint Blockade. Front. Immunol. 2022, 12, 799455. [Google Scholar] [CrossRef]
- Brambilla, E.; Le Teuff, G.; Marguet, S.; Lantuejoul, S.; Dunant, A.; Graziano, S.; Pirker, R.; Douillard, J.Y.; Le Chevalier, T.; Filipits, M.; et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The Immune Contexture in Cancer Prognosis and Treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ramis-Cabrer, D.; Curull, V.; Wang, X.; Mateu-Jiménez, M.; Pijuan, L.; Duran, X.; Qin, L.; Rodríguez-Fuster, A.; Aguiló, R.; et al. B Cells and Tertiary Lymphoid Structures Influence Survival in Lung Cancer Patients with Resectable Tumors. Cancers 2020, 12, 2644. [Google Scholar] [CrossRef] [PubMed]
- Vanhersecke, L.; Brunet, M.; Guégan, J.P.; Rey, C.; Bougouin, A.; Cousin, S.; Le Moulec, S.; Besse, B.; Loriot, Y.; Larroquette, M.; et al. Mature Tertiary Lymphoid Structures Predict Immune Checkpoint Inhibitor Efficacy in Solid Tumors Independently of PD-L1 Expression. Nat. Cancer 2021, 2, 794–802. [Google Scholar] [CrossRef]
- Wolberg, G.; Zimmerman, T.P.; Hiemstra, K.; Winston, M.; Chu, L.-C. Adenosine Inhibition of Lymphocyte-Mediated Cytolysis: Possible Role of Cyclic Adenosine Monophosphate. Science 1975, 187, 957–959. [Google Scholar] [CrossRef]
- Ohta, A.; Gorelik, E.; Prasad, S.J.; Ronchese, F.; Lukashev, D.; K Wong, M.K.; Huang, X.; Caldwell, S.; Liu, K.; Smith, P.; et al. A2A Adenosine Receptor Protects Tumors from Antitumor T Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13132–13137. [Google Scholar] [CrossRef]
- Blay, J.; White, T.D.; Hoskin, D.W. The Extracellular Fluid of Solid Carcinomas Contains Immunosuppressive Concentrations of Adenosine. Cancer Res. 1997, 57, 2602–2605. [Google Scholar]
- Han, Y.; Lee, T.; He, Y.; Raman, R.; Irizarry, A.; Martin, M.L.; Giaccone, G. The Regulation of CD73 in Non-Small Cell Lung Cancer. Eur. J. Cancer 2022, 170, 91–102. [Google Scholar] [CrossRef]
- Le, X.; Negrao, M.V.; Reuben, A.; Federico, L.; Diao, L.; McGrail, D.; Nilsson, M.; Robichaux, J.; Munoz, I.G.; Patel, S.; et al. Characterization of the Immune Landscape of EGFR-Mutant NSCLC Identifies CD73/Adenosine Pathway as a Potential Therapeutic Target. J. Thorac. Oncol. 2021, 16, 583–600. [Google Scholar] [CrossRef]
- Gao, Z.W.; Liu, C.; Yang, L.; Chen, H.C.; Yang, L.F.; Zhang, H.Z.; Dong, K. CD73 Severed as a Potential Prognostic Marker and Promote Lung Cancer Cells Migration via Enhancing EMT Progression. Front. Genet. 2021, 12, 728200. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Yoshimura, K.; Kurabe, N.; Kahyo, T.; Kawase, A.; Tanahashi, M.; Ogawa, H.; Inui, N.; Funai, K.; Shinmura, K.; et al. Prognostic Impact of CD73 and A2A Adenosine Receptor Expression in Non-Small Cell Lung Cancer. Oncotarget 2017, 8, 8738–8751. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Horenstein, A.L.; Canzonetta, C.; Costa, F.; Morandi, F. Canonical and Non-Canonical Adenosinergic Pathways. Immunol. Lett. 2019, 205, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Falzoni, S.; Giuliani, A.L.; Adinolfi, E. P2 Receptors in Cancer Progression and Metastatic Spreading. Curr. Opin. Pharmacol. 2016, 29, 17–25. [Google Scholar] [CrossRef]
- Kaczmarek, E.; Koziak, K.; Sé Vigny, J.; Siegel, J.B.; Anrather, J.; Beaudoin, A.R.; Bach, F.H.; Robson, S.C. Identification and Characterization of CD39/Vascular ATP Diphosphohydrolase. J. Biol. Chem. 1996, 271, 33116–33122. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Chillemi, A.; Zaccarello, G.; Bruzzone, S.; Quarona, V.; Zito, A.; Serra, S.; Malavasi, F. A CD38/CD203A/CD73 Ectoenzymatic Pathway Independent of CD39 Drives a Novel Adenosinergic Loop in Human T Lymphocytes. Oncoimmunology 2013, 2, e26246. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Ohh, M.; Won Park, C.; Ivan, M.; Hoffman, M.A.; Kim, T.-Y.; Eric Huang, L.; Pavletich, N.; Chau, V.; Kaelin, W.G. Ubiquitination of Hypoxia-Inducible Factor Requires Direct Binding to the β-Domain of the von Hippel-Lindau Protein. Nat. Cell Biol. 2000, 2, 423–427. [Google Scholar] [CrossRef]
- Semenza, G.L. Annual Review of Medicine Regulation of Erythropoiesis by the Hypoxia-Inducible Factor Pathway: Effects of Genetic and Pharmacological Perturbations. Annu. Rev. Med. 2023, 74, 307–319. [Google Scholar] [CrossRef]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-Inducible Nuclear Factors Bind to an Enhancer Element Located 3′ to the Human Erythropoietin Gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovski, D.A.; DiFazio, L.T.; Bogdanovski, A.K.; Csóka, B.; Jordan, G.B.; Paul, E.R.; Antonioli, L.; Pilip, S.A.; Nemeth, Z.H. Hypoxia-Inducible-Factor-1 in Trauma and Critical Care. J. Crit. Care 2017, 42, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Synnestvedt, K.; Furuta, G.T.; Comerford, K.M.; Louis, N.; Karhausen, J.; Eltzschig, H.K.; Hansen, K.R.; Thompson, L.F.; Colgan, S.P. Ecto-5′-Nucleotidase (CD73) Regulation by Hypoxia-Inducible Factor-1 Mediates Permeability Changes in Intestinal Epithelia. J. Clin. Investig. 2002, 110, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ahmad, S.; Glover, L.; Miller, S.M.; Shannon, J.M.; Guo, X.; Franklin, W.A.; Bridges, J.P.; Schaack, J.B.; Colgan, S.P.; et al. Adenosine A 2A Receptor Is a Unique Angiogenic Target of HIF-2 in Pulmonary Endothelial Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 10684–10689. [Google Scholar] [CrossRef]
- Kong, T.; Westerman, K.A.; Faigle, M.; Eltzschig, H.K.; Colgan, S.P. HIF-dependent Induction of Adenosine A2B Receptor in Hypoxia. FASEB J. 2006, 20, 2242–2250. [Google Scholar] [CrossRef]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The Ectonucleotidases CD39 and CD73: Novel Checkpoint Inhibitor Targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef]
- Regateiro, F.S.; Howie, D.; Nolan, K.F.; Agorogiannis, E.I.; Greaves, D.R.; Cobbold, S.P.; Waldmann, H. Generation of Anti-Inflammatory Adenosine by leukocytes Is Regulated by TGF-β. Eur. J. Immunol. 2011, 41, 2955–2965. [Google Scholar] [CrossRef]
- Ryzhov, S.V.; Pickup, M.W.; Chytil, A.; Gorska, A.E.; Zhang, Q.; Owens, P.; Feoktistov, I.; Moses, H.L.; Novitskiy, S.V. Role of TGF-β Signaling in Generation of CD39+CD73+ Myeloid Cells in Tumors. J. Immun. 2014, 193, 3155–3164. [Google Scholar] [CrossRef]
- Yeung, K.T.; Yang, J. Epithelial–Mesenchymal Transition in Tumor Metastasis. Mol. Oncol. 2017, 11, 28–39. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Dijke, P. Ten. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Spychala, J.; Kitajewski, J. Wnt and β-Catenin Signaling Target the Expression of Ecto-5′-Nucleotidase and Increase Extracellular Adenosine Generation. Exp. Cell Res. 2004, 296, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lupia, M.; Angiolini, F.; Bertalot, G.; Freddi, S.; Sachsenmeier, K.F.; Chisci, E.; Kutryb-Zajac, B.; Confalonieri, S.; Smolenski, R.T.; Giovannoni, R.; et al. CD73 Regulates Stemness and Epithelial-Mesenchymal Transition in Ovarian Cancer-Initiating Cells. Stem. Cell Rep. 2018, 10, 1412–1425. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Quarona, V.; Toscani, D.; Costa, F.; Chillemi, A.; Pistoia, V.; Giuliani, N.; Malavasi, F. Adenosine Generated in the Bone Marrow Niche through a CD38-Mediated Pathway Correlates with Progression of Human Myeloma. Mol. Med. 2016, 22, 694–704. [Google Scholar] [CrossRef]
- Chen, L.; Diao, L.; Yang, Y.; Yi, X.; Rodriguez, B.L.; Li, Y.; Villalobos, P.A.; Cascone, T.; Liu, X.; Tan, L.; et al. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov. 2018, 8, 1156–1175. [Google Scholar] [CrossRef] [PubMed]
- Latini, S.; Pedata, F. Adenosine in the Central Nervous System: Release Mechanisms and Extracellular Concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef]
- Collis, M.G.; Hourani, S.M. Adenosine Receptor Subtypes. Trends Pharmacol. Sci. 1993, 14, 360–366. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic Regulation of the Immune System. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Fornai, M.; Pellegrini, C.; D’Antongiovanni, V.; Turiello, R.; Morello, S.; Haskó, G.; Blandizzi, C. Tumor Microenvironment; Birbrair, A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; Volume 1270. [Google Scholar] [CrossRef]
- Powell, J.D.; Delgoffe, G.M. The Mammalian Target of Rapamycin: Linking T Cell Differentiation, Function, and Metabolism. Immunity 2010, 33, 301–311. [Google Scholar] [CrossRef]
- Mastelic-Gavillet, B.; Navarro Rodrigo, B.; Décombaz, L.; Wang, H.; Ercolano, G.; Ahmed, R.; Lozano, L.E.; Ianaro, A.; Derré, L.; Valerio, M.; et al. Adenosine Mediates Functional and Metabolic Suppression of Peripheral and Tumor-Infiltrating CD8+ T Cells. J. Immunother. Cancer 2019, 7, 257. [Google Scholar] [CrossRef]
- Leone, R.D.; Sun, I.M.; Oh, M.H.; Sun, I.H.; Wen, J.; Englert, J.; Powell, J.D. Inhibition of the Adenosine A2a Receptor Modulates Expression of T Cell Coinhibitory Receptors and Improves Effector Function for Enhanced Checkpoint Blockade and ACT in Murine Cancer Models. Cancer Immunol. Immunother. 2018, 67, 1271–1284. [Google Scholar] [CrossRef]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing CGAS–STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Saigi, M.; Tani, T.; Springer, B.F.; Shibata, H.; Kitajima, S.; Mahadevan, N.R.; Campisi, M.; Kim, W.; Kobayashi, Y.; et al. MET-Induced CD73 Restrains STING-Mediated Immunogenicity of EGFR-Mutant Lung Cancer. Cancer Res. 2022, 82, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The Development and Immunosuppressive Functions of CD4+ CD25+ FoxP3+ Regulatory T Cells Are under Influence of the Adenosine-A2A Adenosine Receptor Pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ngiow, S.F.; Gao, Y.; Patch, A.M.; Barkauskas, D.S.; Messaoudene, M.; Lin, G.; Coudert, J.D.; Stannard, K.A.; Zitvogel, L.; et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018, 78, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Minguet, S.; Huber, M.; Rosenkranz, L.; Schamel, W.W.A.; Reth, M.; Brummer, T. Adenosine and CAMP Are Potent Inhibitors of the NF-κB Pathway Downstream of Immunoreceptors. Eur. J. Immunol. 2005, 35, 31–41. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Pinhal-Enfield, G.; Elson, G.; Cronstein, B.N.; Hasko, G.; Outram, S.; Leibovich, S.J. The Adenosine-Dependent Angiogenic Switch of Macrophages to an M2-like Phenotype Is Independent of Interleukin-4 Receptor Alpha (IL-4Rα) Signaling. Inflamm 2013, 36, 921–931. [Google Scholar] [CrossRef]
- Challier, J.; Bruniquel, D.; Sewell, A.K.; Laugel, B. Adenosine and CAMP Signalling Skew Human Dendritic Cell Differentiation towards a Tolerogenic Phenotype with Defective CD8+ T-Cell Priming Capacity. Immunology 2013, 138, 402–410. [Google Scholar] [CrossRef]
- Morello, S.; Miele, L. Targeting the Adenosine A2b Receptor in the Tumor Microenvironment Overcomes Local Immunosuppression by Myeloid-Derived Suppressor Cells. Oncoimmunology 2014, 3, e27989. [Google Scholar] [CrossRef]
- Iannone, R.; Miele, L.; Maiolino, P.; Pinto, A.; Morello, S. Blockade of A2b Adenosine Receptor Reduces Tumor Growth and Immune Suppression Mediated by Myeloid-Derived Suppressor Cells in a Mouse Model of Melanoma. Neoplasia 2013, 15, 1400–1409. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Tang, J.; Wang, R. CD73 (NT5E) Promotes the Proliferation and Metastasis of Lung Adenocarcinoma through the EGFR/AKT/MTOR Pathway. Biomed. Res. Int. 2022, 2022, 9944847. [Google Scholar] [CrossRef]
- Bertolini, G.; Compagno, M.; Belisario, D.C.; Bracci, C.; Genova, T.; Mussano, F.; Vitale, M.; Horenstein, A.; Malavasi, F.; Ferracini, R.; et al. CD73/Adenosine Pathway Involvement in the Interaction of Non-Small Cell Lung Cancer Stem Cells and Bone Cells in the Pre-Metastatic Niche. Int. J. Mol. Sci. 2022, 23, 5126. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wu, Z.; Miao, J.; Du, S.; Ai, S.; Xu, E.; Feng, M.; Song, J.; Guan, W. Adenosine Interaction with Adenosine Receptor A2a Promotes Gastric Cancer Metastasis by Enhancing PI3K-AKT-MTOR Signaling. Mol. Biol. Cell 2019, 30, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chu, X.; Deng, F.; Tong, L.; Tong, G.; Yi, Y.; Liu, J.; Tang, J.; Tang, Y.; Xia, Y.; et al. The Adenosine A2b Receptor Promotes Tumor Progression of Bladder Urothelial Carcinoma by Enhancing MAPK Signaling Pathway. Oncotarget 2017, 8, 48755–48768. [Google Scholar] [CrossRef]
- Sadej, R.; Skladanowski, A.C. Dual, Enzymatic and Non-Enzymatic, Function of Ecto-5′-Nucleotidase (EN, CD73) in Migration and Invasion of A375 Melanoma Cells. Acta Biochim. Pol. 2012, 59, 647–652. [Google Scholar] [CrossRef]
- Yu, M.; Guo, G.; Huang, L.; Deng, L.; Chang, C.S.; Achyut, B.R.; Canning, M.; Xu, N.; Arbab, A.S.; Bollag, R.J.; et al. CD73 on Cancer-Associated Fibroblasts Enhanced by the A2B-Mediated Feedforward Circuit Enforces an Immune Checkpoint. Nat. Commun. 2020, 11, 515. [Google Scholar] [CrossRef]
- Mediavilla-Varela, M.; Luddy, K.; Noyes, D.; Khalil, F.K.; Neuger, A.M.; Soliman, H.; Antonia, S.J. Antagonism of Adenosine A2A Receptor Expressed by Lung Adenocarcinoma Tumor Cells and Cancer Associated Fibroblasts Inhibits Their Growth. Cancer Biol. Ther. 2013, 14, 860–868. [Google Scholar] [CrossRef]
- Allard, B.; Cousineau, I.; Allard, D.; Buisseret, L.; Pommey, S.; Chrobak, P.; Stagg, J. Adenosine A2a Receptor Promotes Lymphangiogenesis and Lymph Node Metastasis. Oncoimmunology 2019, 8, 1601481. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Turcotte, M.; Spring, K.; Pommey, S.; Royal, I.; Stagg, J. Anti-CD73 Therapy Impairs Tumor Angiogenesis. Int. J. Cancer 2014, 134, 1466–1473. [Google Scholar] [CrossRef]
- Geoghegan, J.C.; Diedrich, G.; Lu, X.; Rosenthal, K.; Sachsenmeier, K.F.; Wu, H.; Dall’Acqua, W.F.; Damschroder, M.M. Inhibition of CD73 AMP Hydrolysis by a Therapeutic Antibody with a Dual, Non-Competitive Mechanism of Action. MAbs 2016, 8, 454–467. [Google Scholar] [CrossRef]
- Hay, C.M.; Sult, E.; Huang, Q.; Mulgrew, K.; Fuhrmann, S.R.; McGlinchey, K.A.; Hammond, S.A.; Rothstein, R.; Rios-Doria, J.; Poon, E.; et al. Targeting CD73 in the Tumor Microenvironment with MEDI9447. Oncoimmunology 2016, 5, e1208875. [Google Scholar] [CrossRef]
- Miller, R.A.; Luke, J.J.; Hu, S.; Mahabhashyam, S.; Jones, W.B.; Marron, T.; Merchan, J.R.; Hughes, B.G.M.; Willingham, S.B. Anti-CD73 Antibody Activates Human B Cells, Enhances Humoral Responses and Induces Redistribution of B Cells in Patients with Cancer. J. Immunother. Cancer 2022, 10, e005802. [Google Scholar] [CrossRef]
- Jia, H.; Li, J.; Pei, F.; Greenwood, L.; Pejza, L.; Long, Y.; Chen, K.; Perer, J.; Wang, M.; Zou, H. Abstract 4259: PT199, a next Generation Anti-CD73 MAb That Inhibits Both Membrane-Bound and Soluble CD73 Activity to Completion without “Hook Effect”. Cancer Res. 2022, 82 (Suppl. S12), 4259. [Google Scholar] [CrossRef]
- Spatola, B.N.; Lerner, A.G.; Wong, C.; dela Cruz, T.; Welch, M.; Fung, W.; Kovalenko, M.; Losenkova, K.; Yegutkin, G.G.; Beers, C.; et al. Fully Human Anti-CD39 Antibody Potently Inhibits ATPase Activity in Cancer Cells via Uncompetitive Allosteric Mechanism. MAbs 2020, 12, 1838036. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla-Varela, M.; Castro, J.; Chiappori, A.; Noyes, D.; Hernandez, D.C.; Allard, B.; Stagg, J.; Antonia, S.J. A Novel Antagonist of the Immune Checkpoint Protein Adenosine A2a Receptor Restores Tumor-Infiltrating Lymphocyte Activity in the Context of the Tumor Microenvironment. Neoplasia 2017, 19, 530–536. [Google Scholar] [CrossRef]
- Borodovsky, A.; Barbon, C.M.; Wang, Y.; Ye, M.; Prickett, L.; Chandra, D.; Shaw, J.; Deng, N.; Sachsenmeier, K.; Clarke, J.D.; et al. Small Molecule AZD4635 Inhibitor of A2AR Signaling Rescues Immune Cell Function Including CD103+ Dendritic Cells Enhancing Anti-Tumor Immunity. J. Immunother. Cancer 2020, 8, e000417. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Ho, P.Y.; Hotson, A.; Hill, C.; Piccione, E.C.; Hsieh, J.; Liu, L.; Buggy, J.J.; McCaffery, I.; Miller, R.A. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol. Res. 2018, 6, 1136–1149. [Google Scholar] [CrossRef]
- Houthuys, E.; Basilico, P.; Brouwer, M.; Deregnaucourt, T.; Detheux, M.; Driessens, G.; Gomes, B.; Leroy, X.; Marchante, J.; Marillier, R.; et al. Abstract 3261: EOS100850, a Non-Brain Penetrant Highly Selective A2Areceptor Antagonist, Uniquely Maintains High Potency within the Adenosine Rich Tumor Microenvironment. Cancer Res. 2019, 79 (Suppl. S13), 3261. [Google Scholar] [CrossRef]
- Pastore, D.R.E.; Mookhtiar, K.; Schwartz, B.; Kumar, S.; Nagaraj, R.; Meru, A.V. Abstract 3454: Adenosine Receptor Antagonists A2AR (TT-10) and A2BR (TT-4) Demonstrate Anti-Tumor Activity in 4T1-Induced Syngeneic Breast Cancer Mouse Model. Cancer Res. 2022, 82 (Suppl. S12), 3454. [Google Scholar] [CrossRef]
- Evans, J.V.; Suman, S.; Goruganthu, M.U.L.; Tchekneva, E.E.; Guan, S.; Arasada, R.R.; Antonucci, A.; Piao, L.; Ilgisonis, I.; Bobko, A.A.; et al. Improving Combination Therapies: Targeting A2B adenosine Receptor to Modulate Metabolic Tumor Microenvironment and Immunosuppression. J. Natl. Cancer Inst. 2023, 115, 1404–1419. [Google Scholar] [CrossRef]
- Fan, P.; Housley, F.; Haberstock, H.; Horne, K.; Liu, J.; Elzein, E.; Yao, L. Abstract 55: TT-702, a Selective and Potent A2B Receptor Antagonist for the Treatment of Cancer. Cancer Res. 2021, 81 (Suppl. S13), 55. [Google Scholar] [CrossRef]
- Seifert, M.; Benmebarek, M.R.; Briukhovetska, D.; Märkl, F.; Dörr, J.; Cadilha, B.L.; Jobst, J.; Stock, S.; Andreu-Sanz, D.; Lorenzini, T.; et al. Impact of the Selective A2AR and A2BR Dual Antagonist AB928/Etrumadenant on CAR T Cell Function. Br. J. Cancer 2022, 127, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Zaynagetdinov, R.; Schiemann, K.; Nallaparaju, K.; Belousova, N.; Matevossian, A.; Chen, Z.; Kradjian, G.; Pandya, M.; Dawra, N.; Krauel, E.-M.; et al. Abstract 3499: M1069 as Dual A2A/A2B Adenosine Receptor Antagonist Counteracts Immune-Suppressive Mechanisms of Adenosine and Reduces Tumor Growth in Vivo. Cancer Res. 2022, 82 (Suppl. S12), 3499. [Google Scholar] [CrossRef]
- Cohen, S.; Stemmer, S.M.; Zozulya, G.; Ochaion, A.; Patoka, R.; Barer, F.; Bar-Yehuda, S.; Rath-Wolfson, L.; Jacobson, K.A.; Fishman, P. CF102 an A3 Adenosine Receptor Agonist Mediates Anti-Tumor and Anti-Inflammatory Effects in the Liver. J. Cell. Physiol. 2011, 226, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, S.W.; Camidge, D.R.; Shu, C.A.; Marrone, K.A.; Le, X.; Blakely, C.M.; Park, K.; Chang, G.C.; Patel, S.P.; et al. CD73 Inhibitor Oleclumab Plus Osimertinib in Previously Treated Patients with Advanced T790M-Negative EGFR-Mutated NSCLC: A Brief Report. J. Thorac. Oncol. 2023, 18, 650–656. [Google Scholar] [CrossRef]
- Herbst, R.S.; Majem, M.; Barlesi, F.; Carcereny, E.; Chu, Q.; Monnet, I.; Sanchez-Hernandez, A.; Dakhil, S.; Ross Camidge, D.; Winzer, L.; et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination with Oleclumab or Monalizumab in Patients with Unresectable, Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 3383–3393. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Barlesi, F.; Goldberg, S.B.; Mann, H.; Gopinathan, A.; Newton, M.D.; Aggarwal, C. Phase 3 Study of Durvalumab Combined with Oleclumab or Monalizumab in Patients with Unresectable Stage III NSCLC (PACIFIC-9). J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS8610. [Google Scholar] [CrossRef]
- Cascone, T.; Kar, G.; Spicer, J.D.; García-Campelo, R.; Weder, W.; Daniel, D.B.; Spigel, D.R.; Hussein, M.; Mazieres, J.; Oliveira, J.; et al. Neoadjuvant Durvalumab Alone or Combined with Novel Immuno-Oncology Agents in Resectable Lung Cancer: The Phase II NeoCOAST Platform Trial. Cancer Discov. 2023, 13, 2394–2411. [Google Scholar] [CrossRef]
- Luke, J.J.; Powderly, J.D.; Merchan, J.R.; Barve, M.A.; Hotson, A.N.; Mobasher, M.; Kwei, L.; Luciano, G.; Buggy, J.J.; Piccione, E.; et al. Immunobiology, Preliminary Safety, and Efficacy of CPI-006, an Anti-CD73 Antibody with Immune Modulating Activity, in a Phase 1 Trial in Advanced Cancers. J. Clin. Oncol. 2019, 37 (Suppl. S15), 2505. [Google Scholar] [CrossRef]
- Siu, L.L.; Burris, H.; Le, D.T.; Hollebecque, A.; Steeghs, N.; Delord, J.-P.; Hilton, J.; Barnhart, B.; Sega, E.; Sanghavi, K.; et al. Abstract CT180: Preliminary Phase 1 Profile of BMS-986179, an Anti-CD73 Antibody, in Combination with Nivolumab in Patients with Advanced Solid Tumors. Cancer Res. 2018, 78 (Suppl. S13), CT180. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, L.; Jiang, B.; Du, Y.; Wang, B.; Hu, X.; He, Y.; Zhao, M.; Yao, Y.; Cui, J.; et al. Uliledlimab and Toripalimab Combination Therapy in Treatment Naive Advanced NSCLC: Phase 1b/2 Clinical Trial Results Using CD73 as a Potential Predictive Biomarker. J. Clin. Oncol. 2023, 41 (Suppl. S16), 2570. [Google Scholar] [CrossRef]
- Fu, S.; Banerji, U.; Bedard, P.L.; Ferrándiz, A.C.; Chiappori, A.; Desai, J.; Jamal, R.; Perez, D.R.; Yamamoto, N.; Vieira, E.; et al. Abstract CT503: A Phase I/Ib Study of the Safety and Preliminary Efficacy of NZV930 Alone and in Combination with Spartalizumab and/or Taminadenant in Patients (Pts) with Advanced Malignancies. Cancer Res. 2022, 82 (Suppl. S12), CT503. [Google Scholar] [CrossRef]
- Chiappori, A.A.; Creelan, B.; Tanvetyanon, T.; Gray, J.E.; Haura, E.B.; Thapa, R.; Barlow, M.L.; Chen, Z.; Chen, D.T.; Beg, A.A.; et al. Phase i Study of Taminadenant (PBF509/NIR178), an Adenosine 2A Receptor Antagonist, with or without Spartalizumab (PDR001), in Patients with Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 2313–2320. [Google Scholar] [CrossRef]
- Lin, C.C.; Joerger, M.; Grell, P.; Chiappori, A.A.; Leal, T.A.; Kasper, S.; Jerusalem, G.; Gonçalves, A.; Wolf, J.; De Braud, F.; et al. Continuous vs Intermittent Adenosine 2A Receptor (A2AR) Inhibition in Preclinical Colon Cancer (CC) Models and in a Phase (Ph) II Study of Taminadenant (NIR178) + Spartalizumab (PDR001) in Patients (Pts) with Non-Small Cell Lung Cancer (NSCLC). Eur. J. Cancer 2020, 138, S12–S13. [Google Scholar] [CrossRef]
- Lim, E.A.; Bendell, J.C.; Falchook, G.S.; Bauer, T.M.; Drake, C.G.; Choe, J.H.; George, D.J.; Karlix, J.L.; Ulahannan, S.; Sachsenmeier, K.F.; et al. Phase Ia/b, Open-Label, Multicenter Study of AZD4635 (an Adenosine A2A Receptor Antagonist) as Monotherapy or Combined with Durvalumab, in Patients with Solid Tumors. Clin. Cancer Res. 2022, 28, 4871–4884. [Google Scholar] [CrossRef]
- Fong, L.; Forde, P.M.; Powderly, J.D.; Goldman, J.W.; Nemunaitis, J.J.; Luke, J.J.; Hellmann, M.D.; Kummar, S.; Doebele, R.C.; Mahadevan, D.; et al. Safety and Clinical Activity of Adenosine A2a Receptor (A2aR) Antagonist, CPI-444, in Anti-PD1/PDL1 Treatment-Refractory Renal Cell (RCC) and Non-Small Cell Lung Cancer (NSCLC) Patients. J. Clin. Oncol. 2017, 35 (Suppl. S15), 3004. [Google Scholar] [CrossRef]
- Felip, E.; Lim, F.L.; Johnson, M.; O’Brien, M.; Barlesi, F.; Mazieres, J.; Solomon, B.; Moreno, V.; Boni, V.; Swalduz, A.; et al. 1315P Phase Ib/II Open-Label, Randomised Evaluation of Atezolizumab (Atezo) + CPI-444 vs Docetaxel as Second/Third-Line Therapy in MORPHEUS-NSCLC (Non-Small Cell Lung Cancer). Ann. Oncol. 2020, 31, S850. [Google Scholar] [CrossRef]
- Fong, L.; Hotson, A.; Powderly, J.D.; Sznol, M.; Heist, R.S.; Choueiri, T.K.; George, S.; Hughes, B.G.M.; Hellmann, M.D.; Shepard, D.R.; et al. Adenosine 2A Receptor Blockade as an Immunotherapy for Treatment-Refractory Renal Cell Cancer. Cancer Discov. 2020, 10, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Harshman, L.C.; Chu, M.; George, S.; Hughes, B.G.M.; Carthon, B.C.; Fong, L.; Merchan, J.R.; Kwei, L.; Hotson, A.N.; Mobasher, M.; et al. Adenosine Receptor Blockade with Ciforadenant +/− Atezolizumab in Advanced Metastatic Castration-Resistant Prostate Cancer (MCRPC). J. Clin. Oncol. 2020, 38 (Suppl. S6), 129. [Google Scholar] [CrossRef]
- Buisseret, L.; Rottey, S.; De Bono, J.S.; Migeotte, A.; Delafontaine, B.; Manickavasagar, T.; Martinoli, C.; Wald, N.; Rossetti, M.; Gangolli, E.A.; et al. Phase 1 Trial of the Adenosine A 2A Receptor Antagonist Inupadenant (EOS-850): Update on Tolerability, and Antitumor Activity Potentially Associated with the Expression of the A 2A Receptor within the Tumor. J. Clin. Oncol. 2021, 39 (Suppl. 15), 2562. [Google Scholar] [CrossRef]
- O’Brien, M.E.R.; Cheema, P.K.; Grohé, C.; Costa, E.C.; Girard, N.; Chiappori, A.; Ross, S.; Rossetti, M.; Dubois, F.; Lager, J.J.; et al. Randomized Phase 2 Study Evaluating Efficacy and Safety of Inupadenant in Combination with Chemotherapy in Adults with Metastatic Non–Small Cell Lung Cancer (MNSCLC) Who Progressed on Immunotherapy. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS9158. [Google Scholar] [CrossRef]
- Spira, A.I.; Conkling, P.R.; Johnson, M.L.; Gardner, O.; Gilbert, H.N.; Scharville, M.; Yin, F.; Krishnan, K.; Paoloni, M.C.; Chaudhry, A. ARC-4 Study: Efficacy and Safety of AB928 plus Carboplatin, Pemetrexed and a PD-1 Antibody in Participants with Metastatic Non-Small Cell Lung Cancer (MNSCLC). J. Clin. Oncol. 2020, 38 (Suppl. S15), e21659. [Google Scholar] [CrossRef]
- Powderly, J.; Spira, A.; Gutierrez, R.; DiRenzo, D.; Udyavar, A.; Karakunnel, J.J.; Rieger, A.; Colabella, J.; Lai, D.W.; de Souza, P. Phase I Evaluation of AB928, a Novel Dual Adenosine Receptor Antagonist, Combined with Chemotherapy or AB122 (Anti-PD-1) in Patients (Pts) with Advanced Malignancies. Ann. Oncol. 2019, 30, v493. [Google Scholar] [CrossRef]
- Spira, A.I.; Chiu, J.; Wang, C.C.; Zer, A.; Conibear, J.; Phuong, P.H.; Park, J.K.; Seto, A.; Zhang, J.; Cho, B.C. VELOCITY-Lung: A Phase (Ph) 2 Study Evaluating Safety and Efficacy of Domvanalimab (Dom) + Zimberelimab (Zim) + Sacituzumab Govitecan (SG), or Etrumadenant (Etruma) + Dom + Zim, or Etruma + Zim in Patients (Pts) with Treatment-Naïve Metastatic Non-Small Cell Lung Cancer (MNSCLC). J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS9155. [Google Scholar] [CrossRef]
- Johnson, M.L.; Fox, W.; Lee, Y.-G.; Lee, K.H.; Ahn, H.K.; Kim, Y.-C.; Lee, K.-Y.; Lee, J.-S.; He, X.; Park, C.; et al. ARC-7: Randomized Phase 2 Study of Domvanalimab + Zimberelimab ± Etrumadenant versus Zimberelimab in First-Line, Metastatic, PD-L1-High Non-Small Cell Lung Cancer (NSCLC). J. Clin. Oncol. 2022, 40 (Suppl. S36), 397600. [Google Scholar] [CrossRef]
- Kondo, S.; Iwasa, S.; Koyama, T.; Fujita, T.; Sugibayashi, K.; Murayama, K.; Yamamoto, N. Safety, Tolerability, Pharmacokinetics, and Antitumour Activity of Oleclumab in Japanese Patients with Advanced Solid Malignancies: A Phase I, Open-Label Study. Int. J. Clin. Oncol. 2022, 27, 1795–1804. [Google Scholar] [CrossRef]
- Bendell, J.; LoRusso, P.; Overman, M.; Noonan, A.M.; Kim, D.-W.; Strickler, J.H.; Kim, S.-W.; Clarke, S.; George, T.J.; Grimison, P.S.; et al. First-in-Human Study of Oleclumab, a Potent, Selective Anti-CD73 Monoclonal Antibody, Alone or in Combination with Durvalumab in Patients with Advanced Solid Tumors. Cancer Immunol. Immun. 2023, 72, 2443–2458. [Google Scholar] [CrossRef]
- De Caluwé, A.; Buisseret, L.; Poortmans, P.; Van Gestel, D.; Salgado, R.; Sotiriou, C.; Larsimont, D.; Paesmans, M.; Craciun, L.; Stylianos, D.; et al. Neo-CheckRay: Radiation Therapy and Adenosine Pathway Blockade to Increase Benefit of Immuno-Chemotherapy in Early Stage Luminal B Breast Cancer, a Randomized Phase II Trial. BMC Cancer 2021, 21, 899. [Google Scholar] [CrossRef]
- Wainberg, Z.; Kang, Y.-K.; Lee, K.-W.; Kim, S.T.; Chao, J.; Catenacci, D.; Oh, S.Y.; Soares, H.P.; Selfridge, J.E.; Cha, Y.; et al. Abstract CT015: Safety and Efficacy of TTX-030, an Anti-CD39 Antibody, in Combination with Chemoimmunotherapy for the First Line Treatment of Locally Advanced or Metastatic Gastric/GEJ Cancer. Cancer Res. 2022, 82 (Suppl. S12), CT015. [Google Scholar] [CrossRef]
- Matsubara, N.; Kusuhara, S.; Yamamoto, N.; Sudo, K.; Yanagita, M.; Murayama, K.; Kawasumi, H.; Russell, D.L.; Yin, D.; Shimizu, T. Safety and Pharmacokinetics of Imaradenant (AZD4635) in Japanese Patients with Advanced Solid Malignancies: A Phase I, Open-Label Study. Cancer Chemother. Pharmacol. 2024, 93, 341–352. [Google Scholar] [CrossRef]
- Stemmer, S.M.; Manojlovic, N.S.; Marinca, M.V.; Petrov, P.; Cherciu, N.; Ganea, D.; Ciuleanu, T.E.; Pusca, I.A.; Beg, M.S.; Purcell, W.T.; et al. Namodenoson in Advanced Hepatocellular Carcinoma and Child–Pugh B Cirrhosis: Randomized Placebo-Controlled Clinical Trial. Cancers 2021, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Bareche, Y.; Pommey, S.; Carneiro, M.; Buisseret, L.; Cousineau, I.; Thebault, P.; Chrobak, P.; Communal, L.; Allard, D.; Robson, S.C.; et al. High-dimensional analysis of the adenosine pathway in high-grade serous ovarian cancer. J. Immunother. Cancer 2021, 9, e001965. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ngiow, S.F.; Barkauskas, D.S.; Sult, E.; Hay, C.; Blake, S.J.; Huang, Q.; Liu, J.; Takeda, K.; Teng, M.W.; et al. Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses. Cancer Cell 2016, 30, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, W.; Wang, Z.; Wang, H.; Liu, J.; Huang, C.; Zhao, T.; Wang, X.; Gao, S.; Ma, Y.; et al. CD73 induces gemcitabine resistance in pancreatic ductal adenocarcinoma: A promising target with non-canonical mechanisms. Cancer Lett. 2021, 519, 289–303. [Google Scholar] [CrossRef]
- Wirsdörfer, F.; de Leve, S.; Cappuccini, F.; Eldh, T.; Meyer, A.V.; Gau, E.; Thompson, L.F.; Chen, N.-Y.; Karmouty-Quintana, H.; Fischer, U.; et al. Extracellular Adenosine Production by ecto-5′-Nucleotidase (CD73) Enhances Radiation-Induced Lung Fibrosis. Cancer Res. 2016, 76, 3045–3056. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Kerkhove, O.; Verfaillie, S.; Maes, B.; Cuppens, K. The Adenosinergic Pathway in Non-Small Cell Lung Cancer. Cancers 2024, 16, 3142. https://doi.org/10.3390/cancers16183142
Van Kerkhove O, Verfaillie S, Maes B, Cuppens K. The Adenosinergic Pathway in Non-Small Cell Lung Cancer. Cancers. 2024; 16(18):3142. https://doi.org/10.3390/cancers16183142
Chicago/Turabian StyleVan Kerkhove, Olivier, Saartje Verfaillie, Brigitte Maes, and Kristof Cuppens. 2024. "The Adenosinergic Pathway in Non-Small Cell Lung Cancer" Cancers 16, no. 18: 3142. https://doi.org/10.3390/cancers16183142
APA StyleVan Kerkhove, O., Verfaillie, S., Maes, B., & Cuppens, K. (2024). The Adenosinergic Pathway in Non-Small Cell Lung Cancer. Cancers, 16(18), 3142. https://doi.org/10.3390/cancers16183142





