Characterization of HER2-Low Breast Tumors among a Cohort of Colombian Women
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Immunohistochemistry
2.3. Gene Expression Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
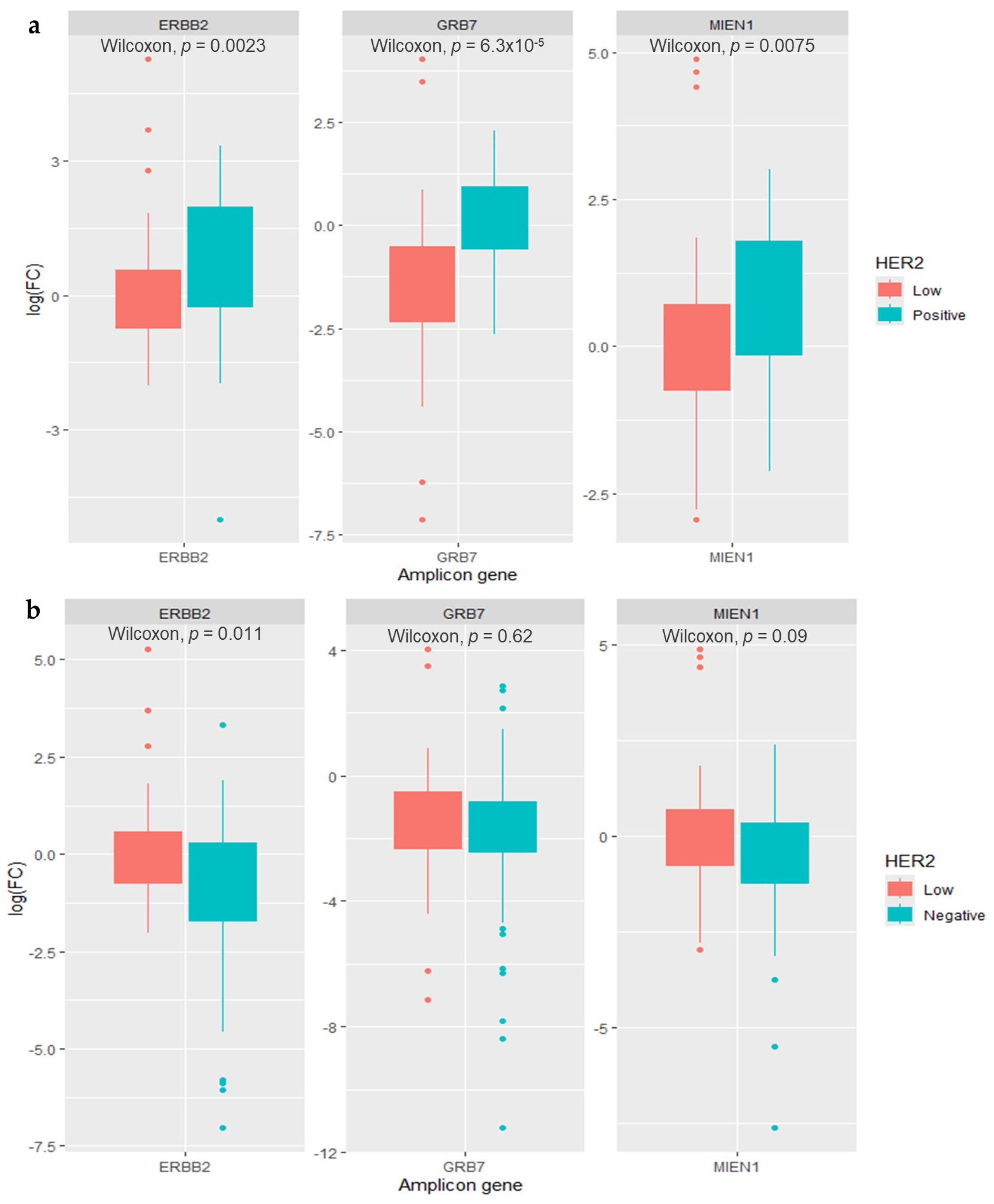
3.2. Clinicopathological Characteristics and HER2 Amplicon Gene Expression of HER2-Low Breast Cancer Patients
3.3. Survival and Risk of Mortality of HER2-Low Breast Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. Adv. Breast Cancer 2008, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef]
- Bartsch, R.; Bergen, E. SABCS 2017: Update on Chemotherapy, Targeted Therapy, and Immunotherapy. Memo 2018, 11, 204. [Google Scholar] [CrossRef]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; Mcshane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Elizabeth Hale Hammond, M.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Shu, L.; Tong, Y.; Li, Z.; Chen, X.; Shen, K. Can HER2 1+ Breast Cancer Be Considered as HER2-Low Tumor? A Comparison of Clinicopathological Features, Quantitative HER2 MRNA Levels, and Prognosis among HER2-Negative Breast Cancer. Cancers 2022, 14, 4250. [Google Scholar] [CrossRef]
- Xu, K.; Bayani, J.; Mallon, E.; Pond, G.R.; Piper, T.; Hasenburg, A.; Markopoulos, C.J.; Dirix, L.; Seynaeve, C.M.; van de Velde, C.J.H.; et al. Discordance between Immunohistochemistry and Erb-B2 Receptor Tyrosine Kinase 2 MRNA to Determine Human Epidermal Growth Factor Receptor 2 Low Status for Breast Cancer. J. Mol. Diagn. 2022, 24, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing Antibody-Drug Conjugates (ADCs) in HER2-Positive Breast Cancer: State of the Art and Future Directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, Pathological, and PAM50 Gene Expression Features of HER2-Low Breast Cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Ahn, J.; Kim, Y.; Kim, J.S.; Song, J.Y.; Kim, H.K.; Lee, J.; Park, H.K.; Kim, Y.S. Clinical Significance of HER2-Low Expression in Early Breast Cancer: A Nationwide Study from the Korean Breast Cancer Society. Breast Cancer Res. 2022, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. Her2-Low Breast Cancer: Molecular Characteristics and Prognosis. Cancers 2021, 13, 2824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tsang, J.Y.; Tam, F.; Loong, T.; Tse, G.M. Comprehensive Characterization of HER2-Low Breast Cancers: Implications in Prognosis and Treatment. EBioMedicine 2023, 91, 104571. [Google Scholar] [CrossRef]
- Sweeney, C.; Bernard, P.S.; Factor, R.E.; Kwan, M.L.; Habel, L.A.; Quesenberry, C.P.; Shakespear, K.; Weltzien, E.K.; Stijleman, I.J.; Davis, C.A.; et al. Intrinsic Subtypes from PAM50 Gene Expression Assay in a Population-Based Breast Cancer Cohort: Differences by Age, Race, and Tumor Characteristics. Cancer Epidemiol. Biomark. Prev. 2014, 23, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, C.I. Racial Disparities in Breast Cancer Diagnosis and Treatment by Hormone Receptor and HER2 Status. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1666–1672. [Google Scholar] [CrossRef]
- Kurian, A.W.; Fish, K.; Shema, S.J.; Clarke, C.A. Lifetime Risks of Specific Breast Cancer Subtypes among Women in Four Racial/Ethnic Groups. Breast Cancer Res. 2010, 12, R99. [Google Scholar] [CrossRef]
- Parise, C.A.; Bauer, K.R.; Caggiano, V. Variation in Breast Cancer Subtypes with Age and Race/Ethnicity. Crit. Rev. Oncol. Hematol. 2010, 76, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Gómez, S.J.; Sanabria-Salas, M.C.; Garay, J.; Baddoo, M.C.; Hernández-Suarez, G.; Mejía, J.C.; García, O.; Miele, L.; Fejerman, L.; Zabaleta, J. Ancestry as a Potential Modifier of Gene Expression in Breast Tumors from Colombian Women. PLoS ONE 2017, 12, e0183179. [Google Scholar] [CrossRef]
- Norris, E.T.; Wang, L.; Conley, A.B.; Rishishwar, L.; Mariño-Ramírez, L.; Valderrama-Aguirre, A.; Jordan, I.K. Genetic Ancestry, Admixture and Health Determinants in Latin America. BMC Genom. 2018, 19, 861. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and Molecular Characteristics of HER2-Low-Positive Breast Cancer: Pooled Analysis of Individual Patient Data from Four Prospective, Neoadjuvant Clinical Trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Abudureheiyimu, N.; Mo, H.; Guan, X.; Lin, S.; Wang, Z.; Chen, Y.; Chen, S.; Li, Q.; Cai, R.; et al. In Real Life, Low-Level HER2 Expression May Be Associated With Better Outcome in HER2-Negative Breast Cancer: A Study of the National Cancer Center, China. Front. Oncol. 2022, 11, 774577. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.; et al. De-Escalating and Escalating Treatments for Early-Stage Breast Cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef]
- Shirman, Y.; Lubovsky, S.; Shai, A. HER2-Low Breast Cancer: Current Landscape and Future Prospects. Breast Cancer Targets Ther. 2023, 15, 605. [Google Scholar] [CrossRef] [PubMed]
- Berrino, E.; Annaratone, L.; Bellomo, S.E.; Ferrero, G.; Gagliardi, A.; Bragoni, A.; Grassini, D.; Guarrera, S.; Parlato, C.; Casorzo, L.; et al. Integrative Genomic and Transcriptomic Analyses Illuminate the Ontology of HER2-Low Breast Carcinomas. Genome Med. 2022, 14, 98. [Google Scholar] [CrossRef]
- Giuliano, M.; Trivedi, M.V.; Schiff, R. Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications. Breast Care 2013, 8, 256. [Google Scholar] [CrossRef]
- de Nonneville, A.; Houvenaeghel, G.; Cohen, M.; Sabiani, L.; Bannier, M.; Viret, F.; Gonçalves, A.; Bertucci, F. Pathological Complete Response Rate and Disease-Free Survival after Neoadjuvant Chemotherapy in Patients with HER2-Low and HER2-0 Breast Cancers. Eur. J. Cancer 2022, 176, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, S.H.; Lee, H.J.; Jeong, H.; Jeong, J.H.; Kim, J.E.; Ahn, J.H.; Jung, K.H.; Gong, G.; Kim, H.H.; et al. Pathological Complete Response, Long-Term Outcomes, and Recurrence Patterns in HER2-Low versus HER2-Zero Breast Cancer after Neoadjuvant Chemotherapy. Eur. J. Cancer 2022, 176, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Sahlberg, K.K.; Hongisto, V.; Edgren, H.; Mäkelä, R.; Hellström, K.; Due, E.U.; Moen Vollan, H.K.; Sahlberg, N.; Wolf, M.; Børresen-Dale, A.L.; et al. The HER2 Amplicon Includes Several Genes Required for the Growth and Survival of HER2 Positive Breast Cancer Cells. Mol. Oncol. 2013, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Fiche, M.; Zaman, K.; Wolfer, A.; Lamy, P.J. The HER2 Amplicon in Breast Cancer: Topoisomerase IIA and Beyond. Biochim. Biophys. Acta (BBA) Rev. Cancer 2013, 1836, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Mungamuri, S.K.; Murk, W.; Grumolato, L.; Bernstein, E.; Aaronson, S.A. Chromatin Modifications Sequentially Enhance ErbB2 Expression in ErbB2 Positive Breast Cancers. Cell Rep. 2013, 5, 302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kulak, M.V.; Borcherding, N.; Maina, P.K.; Zhang, W.; Weigel, R.J.; Qi, H.H. A Novel HER2 Gene Body Enhancer Contributes to HER2 Expression. Oncogene 2018, 37, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Jin, Q.; Tayob, N.; Jeselsohn, R.M.; Schnitt, S.J.; Vincuilla, J.; Parker, T.; Tyekucheva, S.; Li, T.; Lin, N.U.; et al. Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol. 2022, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D.S.; Zhao, F.; Chen, N.; Hahn, O.M.; Nanda, R.; Olopade, O.I.; Huo, D.; Howard, F.M. Clinicopathologic Characteristics and Prognosis of ERBB2-Low Breast Cancer Among Patients in the National Cancer Database. JAMA Oncol. 2023, 9, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Ali, S.; Mata, D.G.M.M.; Lohmann, A.E.; Blanchette, P.S. Antibody–Drug Conjugates in Breast Cancer: Ascent to Destiny and Beyond—A 2023 Review. Curr. Oncol. 2023, 30, 6447–6461. [Google Scholar] [CrossRef]
- Tan, R.S.Y.C.; Ong, W.S.; Lee, K.H.; Lim, A.H.; Park, S.; Park, Y.H.; Lin, C.H.; Lu, Y.S.; Ono, M.; Ueno, T.; et al. HER2 Expression, Copy Number Variation and Survival Outcomes in HER2-Low Non-Metastatic Breast Cancer: An International Multicentre Cohort Study and TCGA-METABRIC Analysis. BMC Med. 2022, 20, 105. [Google Scholar] [CrossRef]

| Level | Overall N (%) | |
|---|---|---|
| n | 516 | |
| Age of diagnosis | <50 years | 140 (27.1) |
| ≥50 years | 369 (71.5) | |
| Unknown | 7 (1.4) | |
| Breast cancer histological type | Invasive Ductal Carcinoma of No Special Type (NST) | 516 (100) |
| AJCC clinical stage | I (I, Ia, Ib) | 71 (13.8) |
| II (IIa, IIb) | 224 (43.4) | |
| III (IIIa, IIIb, IIIc) | 199 (38.6) | |
| IV | 13 (2.5) | |
| Unknown | 9 (1.7) | |
| Scarff–Bloom–Richardson | I | 67 (13.2) |
| II | 263 (51.8) | |
| III | 175 (34.4) | |
| Unknown | 3 (0.6) | |
| Tumor size | ≤20 mm | 140 (27.1) |
| 21–49 mm | 191 (37.0) | |
| ≥50 mm | 137 (26.6) | |
| Unknown | 48 (9.3) | |
| Histological invasion | No | 214 (41.5) |
| Yes | 263 (51.0) | |
| Unknown | 39 (7.6) | |
| Lymph node involvement | No | 212 (45.5) |
| Yes | 254 (54.5) | |
| Neoadjuvant treatment | Received | 281 (54.4) |
| Did not receive | 225 (43.6) | |
| Unknown | 10 (1.9) | |
| Type of neoadjuvant therapy | Cytotoxic | 186 (36.0) |
| Hormonal | 20 (3.8) | |
| Cytotoxic + Hormonal | 33 (6.4) | |
| Cytotoxic + Trastuzumab | 42 (8.1) | |
| Did not receive | 225 (43.6) | |
| Unknown | 10 (1.9) | |
| Neoadjuvant treatment response | Complete | 28 (5.4) |
| Stable | 38 (7.4) | |
| Partial | 93 (18.0) | |
| Progression | 43 (8.3) | |
| Unknown | 79 (15.3) | |
| Surgical management | Mastectomy | 262 (50.7) |
| Quadrantectomy | 252 (48.8) | |
| Unknown | 2 (0.4) | |
| Type of adjuvant therapy | Cytotoxic | 81 (15.7) |
| Hormonal | 204 (39.5) | |
| Cytotoxic + Hormonal | 100 (19.4) | |
| Trastuzumab + Cytotoxic and/or Hormonal | 65 (12.6) | |
| Did not receive | 17 (3.3) | |
| Unknown | 49 (9.5) | |
| Radiotherapy | Received | 424 (82.2) |
| Did not receive | 57 (11.0) | |
| Unknown | 35 (6.8) | |
| 5-year clinical recurrence | No | 330 (64.0) |
| Yes | 118 (22.9) | |
| Unknown | 68 (13.2) | |
| 5-year vital state | Alive | 389 (75.4) |
| Deceased | 100 (19.4) | |
| Unknown | 27 (5.2) | |
| Ki67 status | High (≥20%) | 277 (53.7) |
| Low (<20%) | 239 (46.3) | |
| HER2 status | Negative (0+) | 325 (63.0) |
| Low (1+/2+) | 97 (18.8) | |
| Positive (3+) | 94 (18.2) | |
| Intrinsic subtype | ER+/HER2− (luminal A-like) | 166 (32.2) |
| ER+/HER2− (luminal B-like) | 166 (32.2) | |
| ER+/HER2+ | 56 (10.9) | |
| ER−/HER2+ | 38 (7.4) | |
| ER−/HER2− | 69 (13.4) | |
| Not classifiable | 21 (4.1) |
| HER2 Category | Low N (%) | Negative N (%) | p Value | Low N (%) | Positive N (%) | p Value | |
|---|---|---|---|---|---|---|---|
| n | 97 | 325 | 97 | 94 | |||
| Age of diagnosis | <50 years | 20 (20.6) | 83 (25.5) | 0.392 | 20 (20.6) | 37 (39.4) | 0.008 |
| ≥50 years | 77 (79.4) | 242 (74.5) | 77 (79.4) | 57 (60.6) | |||
| AJCC clinical stage | I | 15 (15.5) | 47 (14.6) | 0.977 | 15 (15.5) | 9 (10.2) | 0.390 |
| II | 43 (44.3) | 145 (45.0) | 43 (44.3) | 36 (40.9) | |||
| III/IV | 39 (40.2) | 130 (40.4) | 39 (40.2) | 43 (48.9) | |||
| Scarff–Bloom–Richardson | I | 13 (13.4) | 43 (13.6) | 0.612 | 13 (13.4) | 11 (12.0) | 0.015 |
| II | 57 (58.8) | 169 (53.5) | 57 (58.8) | 37 (40.2) | |||
| III | 27 (27.8) | 104 (32.9) | 27 (27.8) | 44 (47.8) | |||
| Tumor size | ≤20 mm | 24 (24.7) | 102 (31.4) | 0.175 | 24 (24.7) | 18 (19.1) | 0.633 |
| 21–49 mm | 42 (43.3) | 108 (33.2) | 31 (32.0) | 31 (33.0) | |||
| ≥50 mm | 31 (32.0) | 115 (35.4) | 42 (43.3) | 45 (47.9) | |||
| Histological invasion | No | 40 (44.9) | 132 (44.3) | 1.000 | 40 (44.9) | 42 (46.7) | 0.935 |
| Yes | 49 (55.1) | 166 (55.7) | 49 (55.1) | 48 (53.3) | |||
| Lymph node involvement | No | 42 (44.2) | 135 (46.9) | 0.739 | 42 (44.2) | 35 (42.2) | 0.902 |
| Yes | 53 (55.8) | 153 (53.1) | 53 (55.8) | 48 (57.8) | |||
| Neoadjuvant treatment | Received | 55 (57.3) | 174 (54.5) | 0.721 | 55 (57.3) | 52 (57.1) | 1.000 |
| Did not receive | 41 (42.7) | 145 (45.5) | 41 (42.7) | 39 (42.9) | |||
| Type of neoadjuvant therapy | Cytotoxic | 39 (70.9) | 126 (72.4) | 0.053 | 39 (70.9) | 21 (40.4) | <0.001 |
| Hormonal | 4 (7.3) | 15 (8.6) | 4 (7.3) | 1 (1.9) | |||
| Cytotoxic + Hormonal | 5 (9.1) | 27 (15.5) | 5 (9.1) | 1 (1.9) | |||
| Cytotoxic + Trastuzumab | 7 (12.7) | 6 (3.4) | 7 (12.7) | 29 (55.8) | |||
| Neoadjuvant treatment response * | Complete | 7 (17.9) | 14 (11.1) | 0.032 | 7 (17.9) | 7 (18.9) | 0.085 |
| Stable | 5 (12.8) | 30 (23.8) | 5 (12.8) | 3 (8.1) | |||
| Partial | 24 (61.5) | 53 (42.1) | 24 (61.5) | 16 (43.2) | |||
| Progression | 3 (7.7) | 29 (23.0) | 3 (7.7) | 11 (29.7) | |||
| Surgical management | Mastectomy | 49 (50.5) | 157 (48.6) | 0.831 | 49 (50.5) | 56 (59.6) | 0.266 |
| Quadrantectomy | 48 (49.5) | 166 (51.4) | 48 (49.5) | 38 (40.4) | |||
| Type of adjuvant therapy | Cytotoxic | 7 (7.8) | 55 (19.5) | 0.008 | 7 (7.8) | 19 (24.4) | <0.001 |
| Hormonal | 53 (58.9) | 140 (49.6) | 53 (58.9) | 11 (14.1) | |||
| Cytotoxic + Hormonal | 21 (23.3) | 76 (27.0) | 21 (23.3) | 3 (3.8) | |||
| Trastuzumab + Cytotoxic and/or Hormonal | 9 (10.0) | 11 (3.9) | 9 (10.0) | 45 (57.7) | |||
| Radiotherapy | Received | 83 (92.2) | 264 (86.3) | 0.185 | 83 (92.2) | 77 (90.6) | 0.908 |
| Did not receive | 7 (7.8) | 42 (13.7) | 7 (7.8) | 8 (9.4) | |||
| Ki67 status | High (≥20%) | 48 (49.5) | 153 (47.1) | 0.764 | 48 (49.5) | 76 (80.9) | <0.001 |
| Low (<20%) | 49 (50.5) | 172 (52.9) | 49 (50.5) | 18 (19.1) | |||
| ER status | Negative | 10 (10.3) | 66 (20.3) | 0.036 | 10 (10.3) | 38 (40.4) | <0.001 |
| Positive | 87 (89.7) | 259 (79.7) | 87 (89.7) | 56 (59.6) | |||
| PR status | Negative | 18 (18.6) | 94 (28.9) | 0.058 | 18 (18.6) | 49 (52.1) | <0.001 |
| Positive | 79 (81.4) | 231 (71.1) | 79 (81.4) | 45 (47.9) | |||
| Intrinsic subtype | ER+/HER2− (luminal A-like) | 26 (34.2) | 140 (43.1) | <0.001 | 26 (34.2) | 0 (0.0) | <0.001 |
| ER+/HER2− (luminal B-like) | 47 (61.8) | 119 (36.6) | 47 (61.8) | 0 (0.0) | |||
| ER−/HER2− | 3 (3.9) | 66 (20.3) | 3 (3.9) | 0 (0.0) | |||
| ER+/HER2+ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 56 (59.6) | |||
| ER−/HER2+ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 38 (40.4) |
| Model | Univariate | Multivariate * | ||
|---|---|---|---|---|
| HER2-low vs. HER2-positive | ||||
| Variable | HR (95% CI) | p value | HR (95% CI) | p value |
| HER2 status | ||||
| Positive | 1.00 | 0.098 | 1.00 | 0.403 |
| Low | 0.53 (0.25–1.12) | 0.69 (0.30–1.61) | ||
| ER status | ||||
| Negative | 1.00 | 0.0027 | 1.00 | 0.180 |
| Positive | 0.32 (0.15–0.67) | 0.54 (0.22–1.32) | ||
| Clinical stage | ||||
| I | 1.00 | 1.00 | ||
| II | 1.91 (0.23–15.9) | 0.547 | 2.1 (0.25–17.7) | 0.491 |
| III/IV | 6.57 (0.88–48.9) | 0.065 | 5.7 (0.76–42.7) | 0.089 |
| HER2-low vs. HER2-negative | ||||
| Variable | HR (95% CI) | p value | HR (95% CI) | p value |
| HER2 status | ||||
| Negative | 1.00 | 0.028 | 1.00 | 0.092 |
| Low | 0.49 (0.26–0.92) | 0.57 (0.30–1.09) | ||
| ER status | ||||
| Negative | 1.00 | <0.001 | 1.00 | <0.001 |
| Positive | 0.23 (0.15–0.36) | 0.26 (0.16–0.41) | ||
| Clinical stage | ||||
| I | 1.00 | 1.00 | ||
| II | 1.88 (0.65–5.39) | 0.238 | 1.84 (0.64–5.28) | 0.255 |
| III/IV | 4.43 (1.6–12.2) | 0.004 | 3.96 (1.43–10.9) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey-Vargas, L.; Bejarano-Rivera, L.M.; Ballen, D.F.; Serrano-Gómez, S.J. Characterization of HER2-Low Breast Tumors among a Cohort of Colombian Women. Cancers 2024, 16, 3141. https://doi.org/10.3390/cancers16183141
Rey-Vargas L, Bejarano-Rivera LM, Ballen DF, Serrano-Gómez SJ. Characterization of HER2-Low Breast Tumors among a Cohort of Colombian Women. Cancers. 2024; 16(18):3141. https://doi.org/10.3390/cancers16183141
Chicago/Turabian StyleRey-Vargas, Laura, Lina María Bejarano-Rivera, Diego Felipe Ballen, and Silvia J. Serrano-Gómez. 2024. "Characterization of HER2-Low Breast Tumors among a Cohort of Colombian Women" Cancers 16, no. 18: 3141. https://doi.org/10.3390/cancers16183141
APA StyleRey-Vargas, L., Bejarano-Rivera, L. M., Ballen, D. F., & Serrano-Gómez, S. J. (2024). Characterization of HER2-Low Breast Tumors among a Cohort of Colombian Women. Cancers, 16(18), 3141. https://doi.org/10.3390/cancers16183141






