Neoadjuvant Chemotherapy with Concurrent Letrozole for Estrogen Receptor-Positive and HER2-Negative Breast Cancer: An Open-Label, Single-Center, Nonrandomized Phase II Study (NeoCHAI)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
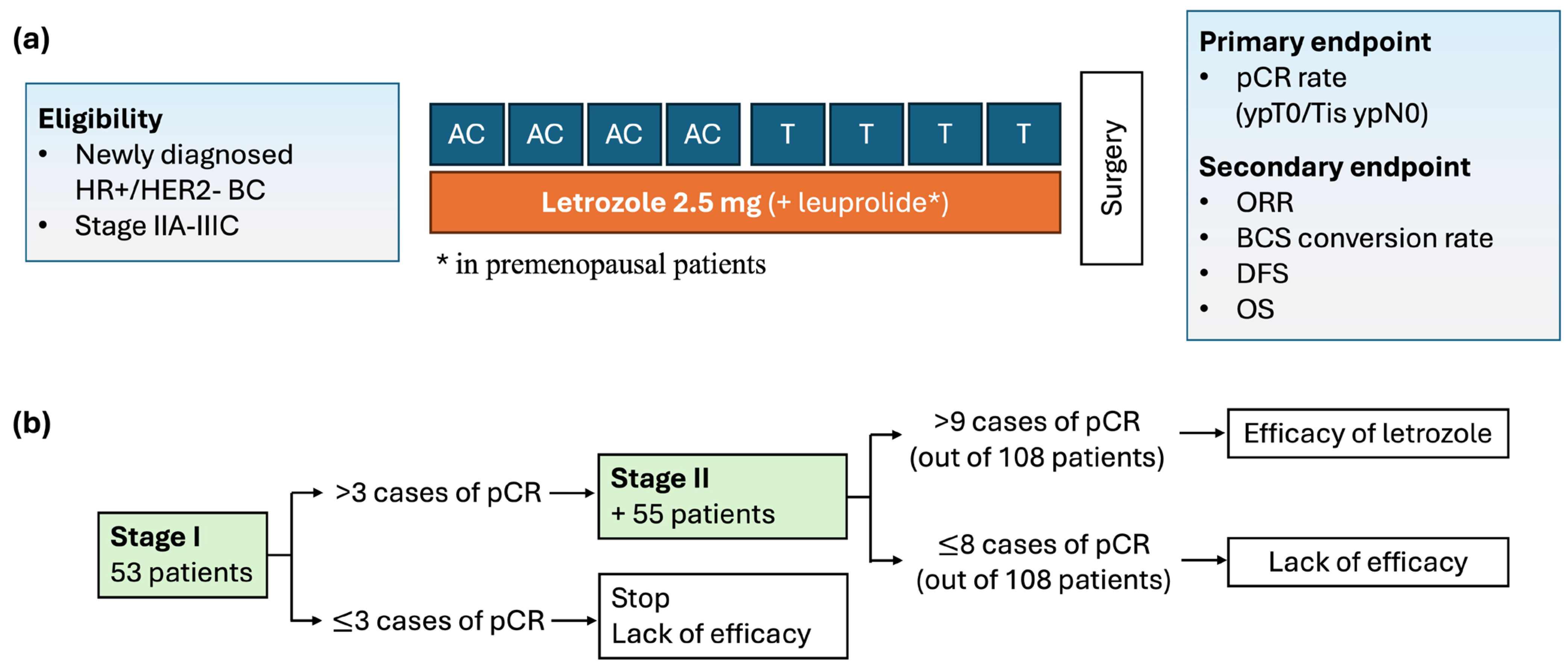
2.1. Study Design and Eligibility
2.2. Treatment and Assessment
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patients and Treatment
3.2. Efficacy
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, T.; Kehm, R.D.; Terry, M.B.; Argov, E.L.; Tehranifar, P. Incidence trends of breast cancer molecular subtypes by age and race/ethnicity in the US from 2010 to 2016. JAMA Netw. Open 2020, 3, e2013226. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Guidelines. Breast Cancer (Version 2, 2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 12 April 2024).
- Cheung, K.L. Endocrine therapy for breast cancer: An overview. Breast 2007, 16, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Chiba, A.; Hoskin, T.L.; Heins, C.N.; Hunt, K.K.; Habermann, E.B.; Boughey, J.C. Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: A National Cancer Data Base study. Ann. Surg. Oncol. 2017, 24, 418–424. [Google Scholar] [CrossRef]
- Albain, K.S.; Barlow, W.E.; Ravdin, P.M.; Farrar, W.B.; Burton, G.V.; Ketchel, S.J.; Cobau, C.D.; Levine, E.G.; Ingle, J.N.; Pritchard, K.I.; et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: A phase 3, open-label, randomised controlled trial. Lancet 2009, 374, 2055–2063. [Google Scholar] [CrossRef]
- Bedognetti, D.; Sertoli, M.R.; Pronzato, P.; Del Mastro, L.; Venturini, M.; Taveggia, P.; Zanardi, E.; Siffredi, G.; Pastorino, S.; Queirolo, P.; et al. Concurrent vs sequential adjuvant chemotherapy and hormone therapy in breast cancer: A multicenter randomized phase III trial. J. Natl. Cancer Inst. 2011, 103, 1529–1539. [Google Scholar] [CrossRef]
- Cocconi, G.; De Lisi, V.; Boni, C.; Mori, P.; Malacarne, P.; Amadori, D.; Giovanelli, E. Chemotherapy versus combination of chemotherapy and endocrine therapy in advanced breast cancer. A prospective randomized study. Cancer 1983, 51, 581–588. [Google Scholar] [CrossRef]
- Lippman, M.E. Efforts to combine endocrine and chemotherapy in the management of breast cancer: Do two and two equal three? Breast Cancer Res. Treat. 1983, 3, 117–127. [Google Scholar] [CrossRef]
- Pritchard, K.I.; Paterson, A.H.; Fine, S.; Paul, N.A.; Zee, B.; Shepherd, L.E.; Abu-Zahra, H.; Ragaz, J.; Knowling, M.; Levine, M.N.; et al. Randomized trial of cyclophosphamide, methotrexate, and fluorouracil chemotherapy added to tamoxifen as adjuvant therapy in postmenopausal women with node-positive estrogen and/or progesterone receptor-positive breast cancer: A report of the National Cancer Institute of Canada Clinical Trials Group. Breast Cancer Site Group. J. Clin. Oncol. 1997, 15, 2302–2311. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Hu, P.; Falkson, G.; Tormey, D.; Abeloff, M. Comparison of chemotherapy with chemohormonal therapy as first-line therapy for metastatic, hormone-sensitive breast cancer: An Eastern Cooperative Oncology Group study. J. Clin. Oncol. 2000, 18, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, K.I.; Paterson, A.H.; Paul, N.A.; Zee, B.; Fine, S.; Pater, J. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J. Clin. Oncol. 1996, 14, 2731–2737. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Baum, M.; Buzdar, A.; Howell, A.; Dowsett, M.; Forbes, J.F.; ATAC/LATTE investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010, 11, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Ruhstaller, T.; Giobbie-Hurder, A.; Colleoni, M.; Jensen, M.B.; Ejlertsen, B.; de Azambuja, E.; Neven, P.; Láng, I.; Jakobsen, E.H.; Gladieff, L.; et al. Adjuvant letrozole and tamoxifen alone or sequentially for postmenopausal women with hormone receptor-positive breast cancer: Long-term follow-up of the BIG 1–98 trial. J. Clin. Oncol. 2019, 37, 105–114. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO Guideline [ASCO guideline]. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Mohammadianpanah, M.; Ashouri, Y.; Hoseini, S.; Amadloo, N.; Talei, A.; Tahmasebi, S.; Nasrolahi, H.; Mosalaei, A.; Omidvari, S.; Ansari, M.; et al. The efficacy and safety of neoadjuvant chemotherapy +/− letrozole in postmenopausal women with locally advanced breast cancer: A randomized phase III clinical trial. Breast Cancer Res. Treat. 2012, 132, 853–861. [Google Scholar] [CrossRef]
- Sugiu, K.; Iwamoto, T.; Kelly, C.M.; Watanabe, N.; Motoki, T.; Ito, M.; Ohtani, S.; Higaki, K.; Imada, T.; Yuasa, T.; et al. Neoadjuvant chemotherapy with or without concurrent hormone therapy in estrogen receptor-positive breast cancer: NACED-randomized multicenter phase II trial. Acta Med. Okayama 2015, 69, 291–299. [Google Scholar] [CrossRef]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Alba, E.; Calvo, L.; Albanell, J.; De la Haba, J.R.; Arcusa Lanza, A.; Chacon, J.I.; Sanchez-Rovira, P.; Plazaola, A.; Lopez Garcia-Asenjo, J.A.; Bermejo, B.; et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: Results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann. Oncol. 2012, 23, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Semiglazov, V.F.; Semiglazov, V.V.; Dashyan, G.A.; Ziltsova, E.K.; Ivanov, V.G.; Bozhok, A.A.; Melnikova, O.A.; Paltuev, R.M.; Kletzel, A.; Berstein, L.M. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 2007, 110, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.D.; Wu, S.Y.; Liu, G.Y.; Wu, J.; Di, G.H.; Hu, Z.; Hou, Y.F.; Chen, C.M.; Fan, L.; Tang, L.C.; et al. Concurrent neoadjuvant chemotherapy and estrogen deprivation in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (CBCSG-036): A randomized, controlled, multicenter trial. Cancer 2019, 125, 2185–2193. [Google Scholar] [CrossRef]
- Eiermann, W.; Pienkowski, T.; Crown, J.; Sadeghi, S.; Martin, M.; Chan, A.; Saleh, M.; Sehdev, S.; Provencher, L.; Semiglazov, V.; et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J. Clin. Oncol. 2011, 29, 3877–3884. [Google Scholar] [CrossRef]
- Sparano, J.A.; Wang, M.; Martino, S.; Jones, V.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W.; Wood, W.C.; Davidson, N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008, 358, 1663–1671. [Google Scholar] [CrossRef]
- Faiz, A.S.; Guo, S.; Kaveney, A.; Philipp, C.S. Risk of venous thromboembolism and endocrine therapy in older women with breast cancer in the United States. Blood Coagul. Fibrinolysis 2021, 32, 373–381. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib as Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, T.Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.J.; et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Cottu, P.; D’Hondt, V.; Dureau, S.; Lerebours, F.; Desmoulins, I.; Heudel, P.E.; Duhoux, F.P.; Levy, C.; Mouret-Reynier, M.A.; Dalenc, F.; et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann. Oncol. 2018, 29, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Puhalla, S.; Wheatley, D.; Ring, A.; Barry, P.; Holcombe, C.; Boileau, J.F.; Provencher, L.; Robidoux, A.; Rimawi, M.; et al. Randomized Phase II Study Evaluating Palbociclib in Addition to Letrozole as Neoadjuvant Therapy in Estrogen Receptor-Positive Early Breast Cancer: PALLET Trial. J. Clin. Oncol. 2019, 37, 178–189. [Google Scholar] [CrossRef]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Muñoz, M.; Paré, L.; González Farré, B.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 33–43. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Press, M.F.; Chan, D.; Fernandez-Abad, M.; Petru, E.; Rostorfer, R.; Guarneri, V.; Huang, C.S.; Barriga, S.; et al. Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR+/HER2− Breast Cancer. Clin. Cancer Res. 2020, 26, 566–580. [Google Scholar] [CrossRef]
- Falo, C.; Azcarate, J.; Fernandez-Gonzalez, S.; Perez, X.; Petit, A.; Perez, H.; Vethencourt, A.; Vazquez, S.; Laplana, M.; Ales, M.; et al. Breast Cancer Patient’s Outcomes after Neoadjuvant Chemotherapy and Surgery at 5 and 10 Years for Stage II-III Disease. Cancers 2024, 16, 2421. [Google Scholar] [CrossRef]
- Choi, J.E.; Kim, Z.; Park, C.S.; Park, E.H.; Lee, S.B.; Lee, S.K.; Choi, Y.J.; Han, J.; Jung, K.W.; Kim, H.J.; et al. Breast Cancer Statistics in Korea, 2019. J. Breast Cancer 2023, 26, 207–220. [Google Scholar] [CrossRef]
- Kan, Z.; Ding, Y.; Kim, J.; Jung, H.H.; Chung, W.; Lal, S.; Cho, S.; Fernandez-Banet, J.; Lee, S.K.; Kim, S.W.; et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat. Commun. 2018, 9, 1725. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total (n = 53) |
|---|---|
| Age (years), median (range) | 49 (33–63) |
| Sex, Female | 53 (100%) |
| Menopausal status | |
| Premenopausal | 32 (60%) |
| Postmenopausal | 21 (40%) |
| Histologic subtype | |
| Invasive ductal carcinoma | 48 (91%) |
| Invasive lobular carcinoma | 5 (9%) |
| Ki-67 | |
| < 14% | 16 (30%) |
| ≥ 14% | 37 (70%) |
| Stage | |
| II | 35 (66%) |
| III | 18 (34%) |
| Node | |
| Negative | 4 (8%) |
| Positive | 49 (93%) |
| Initial surgery plan | |
| Breast-conserving surgery candidate | 39 (74%) |
| Mastectomy candidate | 14 (26%) |
| Total (n = 53) | |
|---|---|
| Chemotherapy | |
| Complete without interruption | 37 (70%) |
| Dose reduction or delay | 14 (26%) |
| Cessation | 2 (4%) |
| Letrozole compliance | |
| ≥ 90% | 49 (93%) |
| < 90%, ≥ 80% | 2 (4%) |
| < 80% | 2 (4%) |
| Overall response rate | |
| Partial response | 44 (83%) |
| Stable disease | 7 (13%) |
| Progressive disease | 2 (4%) |
| Surgery | |
| Breast-conserving surgery | 42 (79%) |
| Mastectomy * | 11 (21%) |
| Pathologic complete response | 2 (4%) |
| Recurrence | |
| Distant | 6 (11%) |
| Local | 0 |
| Total (n = 53) | |
|---|---|
| Neutropenia, grade 3/4 | 21 (40%) |
| Febrile neutropenia | 2 (4%) |
| Myalgia | 17 (32%) |
| Nausea | 10 (19%) |
| Constipation | 9 (17%) |
| Heartburn | 6 (11%) |
| Oral mucositis | 5 (9%) |
| Sensory neuropathy | 5 (9%) |
| Death | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, H.; Sim, S.H.; Kwon, Y.; Lee, E.-G.; Han, J.H.; Jung, S.-Y.; Lee, S.; Kang, H.-S.; Kim, Y.-J.; Kim, T.H.; et al. Neoadjuvant Chemotherapy with Concurrent Letrozole for Estrogen Receptor-Positive and HER2-Negative Breast Cancer: An Open-Label, Single-Center, Nonrandomized Phase II Study (NeoCHAI). Cancers 2024, 16, 3122. https://doi.org/10.3390/cancers16183122
Chae H, Sim SH, Kwon Y, Lee E-G, Han JH, Jung S-Y, Lee S, Kang H-S, Kim Y-J, Kim TH, et al. Neoadjuvant Chemotherapy with Concurrent Letrozole for Estrogen Receptor-Positive and HER2-Negative Breast Cancer: An Open-Label, Single-Center, Nonrandomized Phase II Study (NeoCHAI). Cancers. 2024; 16(18):3122. https://doi.org/10.3390/cancers16183122
Chicago/Turabian StyleChae, Heejung, Sung Hoon Sim, Youngmi Kwon, Eun-Gyeong Lee, Jai Hong Han, So-Youn Jung, Seeyoun Lee, Han-Sung Kang, Yeon-Joo Kim, Tae Hyun Kim, and et al. 2024. "Neoadjuvant Chemotherapy with Concurrent Letrozole for Estrogen Receptor-Positive and HER2-Negative Breast Cancer: An Open-Label, Single-Center, Nonrandomized Phase II Study (NeoCHAI)" Cancers 16, no. 18: 3122. https://doi.org/10.3390/cancers16183122
APA StyleChae, H., Sim, S. H., Kwon, Y., Lee, E.-G., Han, J. H., Jung, S.-Y., Lee, S., Kang, H.-S., Kim, Y.-J., Kim, T. H., & Lee, K. S. (2024). Neoadjuvant Chemotherapy with Concurrent Letrozole for Estrogen Receptor-Positive and HER2-Negative Breast Cancer: An Open-Label, Single-Center, Nonrandomized Phase II Study (NeoCHAI). Cancers, 16(18), 3122. https://doi.org/10.3390/cancers16183122






